Abstract
A significant portion of our risk for dementia in old age is associated with lifestyle factors (diet, exercise, and cardiovascular health) that are modifiable, at least in principle. One such risk factor – high homocysteine levels in the blood – is known to increase risk for Alzheimer’s disease and vascular disorders. Here we set out to understand how homocysteine levels relate to 3D surface-based maps of cortical gray matter distribution (thickness, volume, surface area) computed from brain MRI in 803 elderly subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset. Individuals with higher plasma levels of homocysteine had lower gray matter thickness in bilateral frontal, parietal, occipital and right temporal regions; and lower gray matter volumes in left frontal, parietal, temporal, and occipital regions, after controlling for diagnosis, age, and sex, and after correcting for multiple comparisons. No significant within-group associations were found in cognitively healthy people, mild cognitive impairment, or Alzheimer’s disease. These regional differences in gray matter structure may be useful biomarkers to assess the effectiveness of interventions, such as vitamin B supplements, that aim to prevent homocysteine-related brain atrophy by normalizing homocysteine levels.
Keywords: Alzheimer’s disease, MRI, cortical, gray matter, atrophy, thickness, volume, surface area, brain structure, homocysteine, folate, vitamin B, ADNI
1. Introduction
The quest to avert the societal crisis of Alzheimer’s disease (AD) involves understanding modifiable risk factors for dementia and how they affect the brain. The core pathology of AD (plaques and tangles) is challenging to treat, but there is a multitude of known risk factors that are modifiable (at least in principle), and contribute to dementia risk. Many recent studies have established consistent links between cognition and brain integrity with an individual’s physical activity (Erickson et al., 2010, Raji et al., 2010), body mass index and its genetic determinants (Ho et al., 2010, Kerwin et al., 2011, Kerwin et al., 2010, Raji et al., 2010), blood levels of the stress-related hormone cortisol (Rajagopalan, 2013), the fat-mass related hormone leptin (Rajagopalan et al., 2013b), and biomarkers of kidney health, such as creatinine and cystatin C (Rajagopalan et al., 2013a). One factor in the blood that is perhaps less studied, in terms of detailed mapping of the brain’s cortex, is homocysteine; although recent evidence has connected it to both brain atrophy and dementia risk (Rajagopalan et al., 2011, Smith, 2008, Wald et al., 2011).
An abnormally high level of homocysteine in the blood is a major cardiovascular risk factor (Bostom et al., 1999, Bots et al., 1997, Perry et al., 1995, Selhub et al., 1995), and there is mounting evidence that poor cardiovascular health is associated with brain atrophy and increased risk for developing Alzheimer’s dementia (Breteler, 2000, Gustafson et al., 2004, Leritz et al., 2011, Muqtadar et al., 2012, Oulhaj et al., 2010, Salat et al., 2012, Swan et al., 1998). High blood levels of homocysteine are also a known risk factor for the development of poor cognition and dementia, including Alzheimer’s disease (Lehmann et al., 1999, Morris et al., 2001, Riggs et al., 1996). In fact, the adjusted relative risk of dementia is 40% higher for every standard deviation increase in homocysteine (log transformed, as is standard) (Seshadri et al., 2002).
Mechanistically, elevated homocysteine levels promote neurotoxicity by altering synaptic function (Lipton et al., 1997) and inducing DNA damage in neurons (Kruman et al., 2000). The neurotoxic effects could explain the association between elevated homocysteine levels and dementia risk (Seshadri et al., 2002), white matter (WM) deterioration (Rajagopalan et al., 2011), and, as evaluated in the current study, altered profiles of cortical gray matter (GM) thickness and volume.
The link between homocysteine and dementia, along with the known mechanisms for homocysteine induced neurotoxicity, suggest that elevated homocysteine levels in the elderly might also be associated with brain atrophy detectable on MRI. Previous imaging literature shows that elevated homocysteine is associated with hippocampal atrophy in cognitively normal elderly people (den Heijer et al., 2003, Williams et al., 2002) and with faster rates of medial temporal lobe atrophy in AD (Clarke et al., 1998a). Our previous study of 732 elderly Caucasians from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort found that higher levels of homocysteine were significantly associated with lower regional WM volumes in large frontal and parietal regions (Rajagopalan et al., 2011). That study used whole-brain tensor-based morphometry (TBM) to show statistically significantly lower GM and WM associated with higher levels of homocysteine after controlling for the effects of sex, age, and dementia diagnosis (AD or MCI) and correcting for multiple comparisons (false discovery rate). That analysis showed significant homocysteine effects in the smaller sample of MCI individuals separately (N=356), suggesting that homocysteine-related WM atrophy may be detectable early in the disease; although the lack of significant results in the separate AD or control groups could also be attributed to poorer statistical power (healthy controls, N=203; AD, N=173). In another prior study, increased cortical atrophy was associated with higher levels of homocysteine in a non-demented elderly sample (den Heijer et al., 2003). That study measured global cortical atrophy on a 15-point scale. Here we analyzed cortical gray matter locally at each point on the brain surface.
Importantly, the areas where WM volumes were lower in individuals with higher homocysteine levels appear to correspond with cortical GM regions that degenerate in AD (Apostolova et al., 2007b, Pievani et al., 2009, Salat et al., 1999, Serra et al., 2010, Thompson et al., 2003, Toga and Thompson, 2013). While the TBM method (used in Rajagopalan et al. 2011) is very sensitive to differences in subcortical WM and GM structures in the brain (Hua et al., 2013, Leow et al., 2005), it is not optimized for detecting effects on the thin strip of GM that makes up the cortical mantle. In TBM studies, it is challenging to perform accurate cortical morphometry, even with very large sample sizes, without the use of explicit cortical surface models that adapt to the geometry of the highly folded cortical surface. Without such models, a 3D volumetric nonlinear registration is not usually able to reliably register the cortical folds since it lacks a reliable feature set to accurately match this complex geometry. With this in mind, we decided to use a surface-based morphometry approach for the current study, as cortical mapping has been informative in many studies of aging and AD (Apostolova et al., 2007a, Apostolova et al., 2007b, Apostolova and Thompson, 2007, Frisoni et al., 2009, Prestia et al., 2010, Thompson et al., 2003). To examine associations between homocysteine levels and cortical GM more precisely, and to accurately localize the associated cortical regions, we analyzed cortical GM thickness, volume, and surface area, in the ADNI sample of N=803 elderly individuals.
We hypothesized that lower cortical GM measures in frontal, parietal, and temporal regions – areas implicated in AD studies using MRI – would be significantly associated with higher levels of homocysteine, even after controlling for age, sex, and diagnosis. We were especially interested in knowing whether the homocysteine-associated atrophy pattern was essentially uniform across the brain, or whether it was detectable primarily in areas traditionally considered as vulnerable to AD-related cortical thinning.
2. Methods
2.1. Study population
We analyzed brain MRI scans from 803 elderly individuals (AD: N=186, MCI: N=392, healthy elderly controls: N=225) who received a 1.5T anatomical brain MRI scan, blood draw, and neuropsychological battery as part of the ADNI study. Our subject sample included only subjects listed in the standard set of baseline scans obtained during the ADNI1 phase of data collection, which was created to promote rigor and meaningful comparability across the large number of ADNI studies (Wyman et al., 2012). Fourteen subjects from the standard set were excluded from our study because they did not have homocysteine data (six subjects) or their cortical GM surfaces did not pass QC (eight subjects). ADNI uses vendor-specific scanning protocols to minimize differences between scanner type (Jack et al., 2008).
ADNI was launched in 2004 by the National Institute of Health, the Food and Drug Administration, private pharmaceutical companies, and non-profit organizations to identify and evaluate biomarkers of AD for use in multisite studies. All ADNI data are publicly available online. The study was conducted according to the Good Clinical Practice guidelines, the Declaration of Helsinki, and the US 21 CFR Part 50–Protection of Human Subjects, and Part 56–Institutional Review Boards. Written informed consent was obtained from all participants in advance.
Blood plasma samples were collected before breakfast on the morning of the baseline MRI scans, after an overnight fast in order to avoid inaccuracies due to recent consumption of certain foods. Total homocysteine levels were measured in a sample of blood plasma taken from each subject using a validated enzyme immunoassay (Shaw, 2008).
All individuals also underwent a thorough clinical and cognitive evaluation close to the time of the MRI scan acquisition, including a diagnosis of probable AD, MCI, or cognitively normal. Inclusion and exclusion criteria are detailed in the ADNI protocol (Mueller et al., 2005).
We analyzed all N=803 ADNI individuals who had plasma homocysteine levels assessed at the time of their baseline MRI scans. All MRI scans underwent quality control after processing with the FreeSurfer software (v5.0.0) (Fischl and Dale, 2000) for cortical GM extraction (Table 1) by visually inspecting the cortical surfaces from multiple points of view. Only subjects that passed quality control were included in the study. A one-way ANOVA and separate one-tailed two-sample t-tests with unequal variance were used to statistically compare homocysteine levels across AD, MCI, and healthy elderly control groups (using p=0.05 as a significance criterion).
Table 1.
Demographic characteristics of N=803 individuals analyzed in this study.
| AD | MCI | Controls | All Individuals | |
|---|---|---|---|---|
| Sample Size (n) | 186 | 392 | 225 | 803 |
| Sex (women/men) | 89/97 | 141/251 | 108/117 | 338/465 |
| Age (years) | 75.45 ± 6.84 | 75.39 ± 7.60 | 75.33 ± 7.67 | 75.30 ± 6.84 |
| Plasma Homocysteine (μM) | 10.77 ± 3.32 * | 10.62 ± 2.82 * | 9.94 ± 2.80 | 10.46 ± 2.95 |
Selected demographic information for the study participants (mean ± standard deviation). An asterisk denotes a significant difference relative to the control group.
2.2. Image acquisition and processing
High-resolution structural brain MRI scans were acquired on 1.5T scanners from General Electric (Milwaukee, Wisconsin, USA), Siemens (Germany), or Philips (The Netherlands) with a standardized MRI protocol (Jack et al., 2008). Each scan involved a three-dimensional sagittal magnetization-prepared rapid gradient-echo sequence with the following parameters: repetition time (2400ms), flip angle (8°), inversion time (1000 ms), 24-cm field of view, a 192×192×166 acquisition matrix, a voxel size of 1.25×1.25×1.2 mm3, later reconstructed to 1 mm isotropic voxels.
2.3. FreeSurfer cortical GM analysis
Cortical reconstruction and GM segmentation was performed with the FreeSurfer image analysis suite (v5.0.0), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Technical details of these procedures have been described previously (Dale et al., 1999, Fischl and Dale, 2000). Briefly, the processing includes motion correction and averaging of each subject’s volumetric T1-weighted MRI brain image, removal of non-brain tissue, intensity normalization, tessellation of cortical GM/WM boundary, automated topology correction and surface deformation along intensity gradients to optimally define cortical surface borders, registration to a spherical atlas using individual cortical folding patterns to align cortical anatomy across subjects, and creation of 3D maps of GM (as measured with thickness, volume, and surface area) at each point on the cortical surface. After processing, images are in an isotropic space of 256 voxels along each axis (x, y, and z) with a final voxel size of 1 mm3. GM thickness was calculated as the average of the distance from the GM/cerebrospinal fluid boundary to the GM/WM surface, and vice versa, at each vertex on the cortical surface. The GM surface area at each vertex was defined as the average of the areas of triangles that include that vertex (clearly, “surface area” is not defined at a point, but this measure is proportional to the area of a cortical region, when averaged over that region). The GM volume was obtained by multiplying the GM thickness by the area of the surface layer equidistant between the inner and outer cortical surfaces.
2.4. Statistical Analysis
Statistical tests were conducted using the general linear model (GLM) analysis tools in FreeSurfer (mri_glmfit, v5.0.0). Smoothing was applied separately to each subject’s 3D cortical surface map for GM thickness, volume, and surface area (kernel radius= 25 mm full width half maximum). We chose a large smoothing kernel based on the past literature showing that the size of regions affected by brain atrophy in this population is generally quite expansive (Buckner et al., 2005, Serra et al., 2010).
We tested a series of three step-wise GLM models for total homocysteine levels on cortical GM: (1) controlling for effects of sex and age in all subjects; (2) controlling for effects of sex, age, and diagnosis (probable AD, MCI, or healthy elderly control) in all 11 subjects; and (3) controlling for sex and age in AD, MCI and healthy elderly control groups, separately. To control for false positives, we enforced a standard false discovery rate (FDR) correction (Benjamini and Hochberg, 1995, Genovese et al., 2002) for multiple statistical comparisons across vertices in the entire left and right cortical surfaces, using the conventionally accepted false positive rate of 5% (q=0.05).
To visualize the distribution of homocysteine levels and GM measures in our data, we calculated mean GM thickness or total GM volume or area in regions where we found significant associations between homocysteine levels and GM measures, after controlling for age, sex, and dementia.
3. Results
3.1. Homocysteine levels across AD, MCI, and healthy elderly controls
A one-way ANOVA showed that homocysteine levels were significantly different across the AD, MCI, and healthy elderly controls (p=0.006, F=5.08, dof=2). Separate one-tailed two sample t-tests with unequal variance showed that AD (p=0.004) and MCI (p=0.002) groups each had significantly higher levels of homocysteine compared to healthy elderly controls (AD: 10.77 ± 3.32 μM, MCI: 10.62 ± 2.82 μM, healthy elderly controls: 9.94 ± 2.80 μM), as expected. Homocysteine levels did not differ significantly between the AD and MCI groups (p=0.295).
3.2. Homocysteine and cortical GM thickness
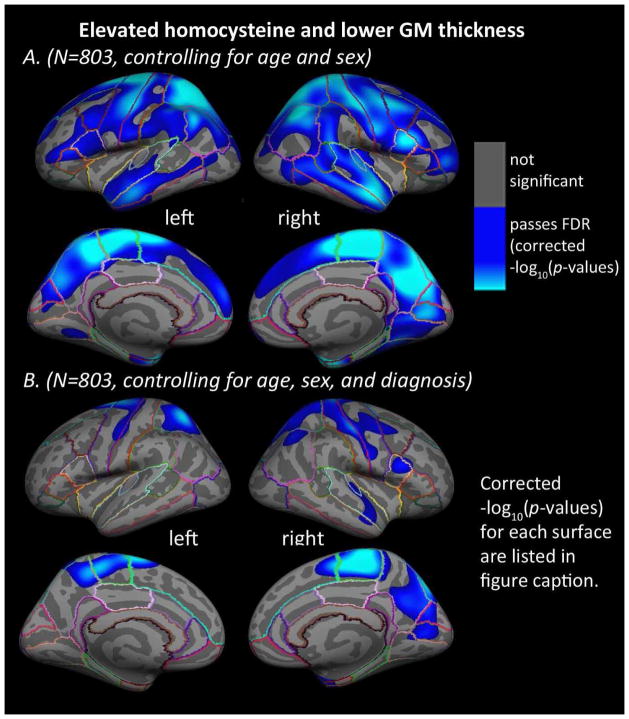
In the entire sample, higher levels of plasma homocysteine were significantly associated with thinner GM in large bilateral regions including frontal, parietal, temporal, and occipital cortex (Figure 1A), after controlling for age and sex. Results were similar, although not identical, between the left and right hemispheres. Adding diagnosis (AD, MCI, or control) to the regressors of non-interest, produced a map showing significant associations between higher levels of homocysteine and thinner cortical GM in the bilateral superior frontal gyrus, paracentral gyrus, precuneus, precentral gyrus, postcentral gyrus, and superior parietal cortex, along with right entorhinal, cuneus, pericalcarine cortex, middle temporal gyrus, superior temporal gyrus, inferior parietal cortex, pars opercularis, and caudal middle frontal gyrus (Figure 1B). No significant associations were found between homocysteine levels and cortical GM thickness in the AD, MCI, or healthy elderly control groups separately, after controlling for age and sex. All surface-based statistical results underwent correction for multiple comparisons using FDR (5% false positive rate, q=0.05). No areas of significant positive associations between higher homocysteine and greater cortical GM thickness were found.
Figure 1. Homocysteine and cortical GM thickness.
Whole-brain 3D maps show significant associations between homocysteine levels in the blood and cortical gray matter (GM) thickness in the left and right hemispheres for all N=803 subjects, (A) after controlling for age and sex (left: −log10(p-value)= 1.597–3.855, right: −log10(p-value)=1.499–3.757, FDR corrected), and (B) after controlling for age, sex, and diagnosis (AD, MCI, or healthy elderly) (left: −log10(p-value)= 2.321–4.579, right: −log10(p-value)=1.996–4.253, FDR corrected at q=0.05). Results were corrected for multiple comparisons by thresholding at a p=0.05 false discovery rate (FDR) threshold across the entire brain hemisphere. Blue areas represent points on the cortical surface where p-values passed the corrected significance threshold for a negative relationship between homocysteine levels and cortical thickness values (higher levels of homocysteine associated with lower cortical GM thickness). No areas of significant positive associations were found, in a post hoc test, after appropriate FDR correction.
3.3. Homocysteine and cortical GM volume
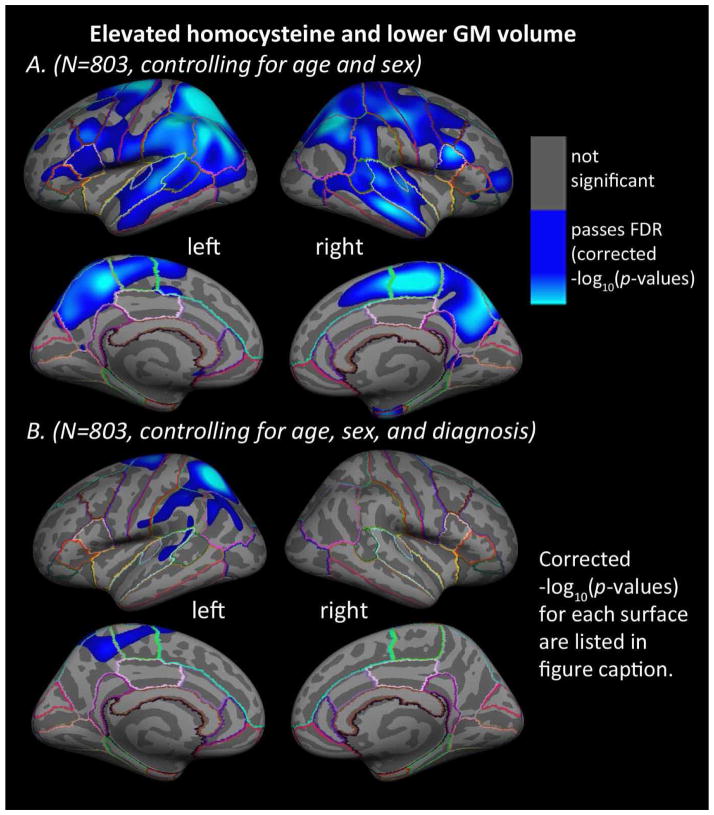
In the entire sample, higher levels of plasma homocysteine were significantly associated with lower cortical GM volumes in large bilateral regions including frontal, parietal, temporal, and occipital lobes after controlling for age and sex (Figure 2A). Results were similar, but not identical, between the left and right hemispheres and covered a slightly smaller area of the brain surface compared to the cortical thickness results. Adding diagnosis (AD, MCI, or control) to the regressors of non-interest, produced a map showing significant associations between higher levels of homocysteine and lower cortical GM volumes in the left superior frontal gyrus, paracentral gyrus, precuneus, superior temporal gyrus, superior precentral gyrus, inferior postcentral gyrus, supramarginal gyrus and superior and inferior parietal cortex (no significant regions were found in the right hemisphere for cortical GM volume) (Figure 2B). No significant associations were found between homocysteine levels and cortical GM volumes in the AD, MCI, or healthy elderly control groups separately, after controlling for age and sex. All vertex-wise statistical results on the surfaces were corrected for multiple comparisons using standard FDR (5% false positive rate, q=0.05). No areas of significant positive associations between homocysteine and cortical GM volume were found.
Figure 2. Homocysteine levels are related to regional cortical GM volumes.
Whole brain 3D maps of significant associations between homocysteine levels and regional cortical gray matter (GM) volumes in the left and right hemispheres of all N=803 subjects, (A) after controlling for age and sex (left: −log10(p-value)=1.673–3.930, right: −log10(p-value)=1.663–3.920, FDR corrected) and (B) after controlling for age, sex, and diagnosis (AD, MCI, or healthy elderly) (left: −log10(p-value)=2.204–4.462, right: not significant, FDR corrected). Results were corrected for multiple comparisons by thresholding at a p=0.05 false discovery rate (FDR) threshold across the entire brain surface. Blue areas represent points on the cortical surface where p-values passed the corrected significance threshold for a negative relationship between homocysteine levels and cortical GM volumes (higher levels of homocysteine associated with lower cortical GM volumes). No areas of significant positive associations were found.
3.4. Homocysteine and cortical GM surface area
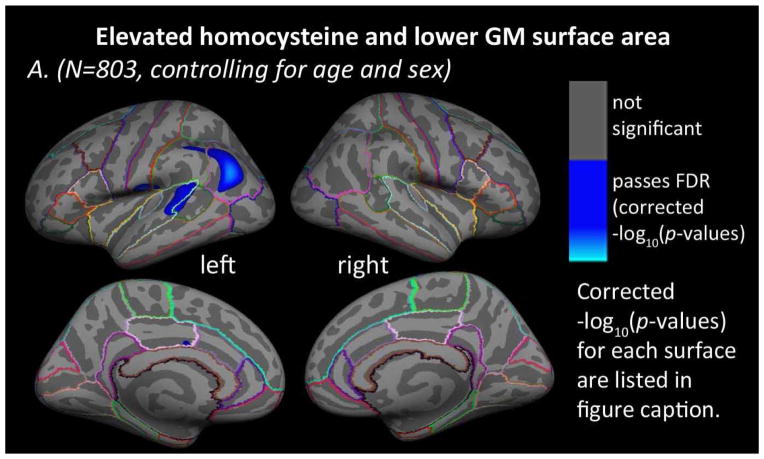
In the entire sample, higher levels of plasma homocysteine were significantly associated with lower cortical GM surface areas in left superior temporal, supramarginal, and inferior parietal cortices after controlling for age and sex (Figure 3). No significant regions were found in the right hemisphere for cortical GM surface area. Adding diagnosis (AD, MCI, or control) to the regressors of non-interest did not produce any regions of significant association. No significant associations were found between homocysteine levels and cortical GM surface area in the AD, MCI, or healthy elderly control groups separately, after controlling for age and sex. All statistical results passed correction for multiple comparisons using FDR (5% false positive rate, q=0.05). No areas of significant positive associations between homocysteine and cortical GM surface area were found.
Figure 3. Homocysteine and cortical GM surface area.
Whole brain 3D maps of significant associations between homocysteine levels and cortical gray matter (GM) surface area in the left and right hemispheres of all N=803 subjects, (A) after controlling for age and sex (left: −log10(p-value)=2.763–5.021, right: not significant, FDR corrected) and (B) after controlling for age, sex, and diagnosis (AD, MCI, or healthy elderly) (left and right: not significant, FDR corrected). Results were corrected for multiple comparisons by thresholding at a p=0.05 false discovery rate (FDR) threshold across the entire brain surface. Blue areas represent points on the cortical surface where t-values passed the corrected significance threshold for a negative relationship between homocysteine levels and cortical GM surface area (higher levels of homocysteine associated with lower cortical GM surface area). No areas of significant positive associations were found.
3.5. Homocysteine levels and GM measures plotted for each subject
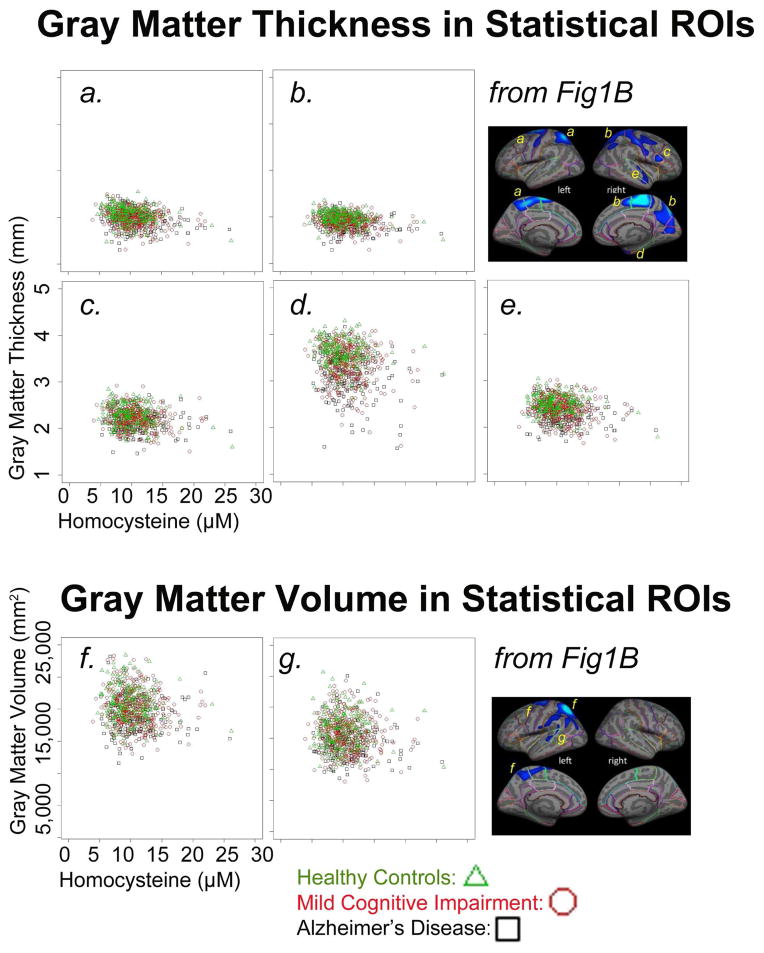
Figure 4 plots individual homocysteine levels against GM measures from regions that were significantly associated with homocysteine levels, after controlling for age, sex, and dementia. In all plots, the healthy controls (green triangles) have generally higher GM values compared to the MCI (red circles) or AD (black squares) groups. Also, the MCI and AD groups show a larger range of homocysteine values. Notably the MCI and AD groups dominate the higher end of the homocysteine measurements, with very few or no controls with higher homocysteine values.
Figure 4. Homocysteine levels and GM measures plotted for each subject.
Scatter plots show homocysteine levels and mean cortical gray matter (GM) thickness (A, mm) or total cortical GM volume (B, mm2) for all N=803 subjects in statistical regions of interest. Data is shown for regions with a significant association between homocysteine levels (μM) and cortical GM measures in the whole brain, after controlling for age, sex, and dementia, FDR corrected (panel B in Figures 1 and 2). Healthy control subjects are shown with green triangles, subjects with mild cognitive impairment are shown with red circles, and subjects with Alzheimer’s disease are shown with black squares.
4. Discussion
Our results show that elevated levels of homocysteine are associated with regional GM reductions in the cortex of elderly individuals, irrespective of age, sex, and dementia diagnosis. As expected, AD and MCI groups had significantly elevated homocysteine levels compared to healthy elderly controls. The lack of significant difference between AD and MCI groups may suggest that while elevated homocysteine levels at any point in time confer an increased risk for dementia (Seshadri et al., 2002), they may not worsen substantially after the MCI stage. A longitudinal investigation of homocysteine levels taken at multiple time points is needed before we can make this statement conclusively. To our knowledge, no such longitudinal report exists for cortical GM, which would be a helpful contribution to the field.
This new cortical mapping study complements prior studies associating high levels of homocysteine with overall brain volume reduction, WM atrophy rate, baseline volume and atrophy rate of the hippocampus and medial temporal lobe (Clarke et al., 1998a, den Heijer et al., 2003, Douaud et al., 2013, Firbank et al., 2010, Williams et al., 2002), lower tissue volumes in frontal and parietal WM (Rajagopalan et al., 2011), greater cortical atrophy (den Heijer et al., 2003), longitudinal ventricular volume enlargement in elderly individuals with arteriosclerotic disease (Jochemsen et al., 2012), lower whole brain GM volume in non-demented elderly (Whalley et al., 2003) in humans and with lower GM volume and density in prefrontal cortices and striatum in rhesus monkeys (Willette et al., 2012). Our prior study in an almost entirely overlapping subject population – but with a different method, TBM – found WM tissue contraction (Rajagopalan et al., 2011) in areas that structurally connect the GM regions we see here in the cortex.
Our results also complement a recently published randomized double-blind placebo-controlled trial of folic acid, which slowed the acceleration of brain atrophy in elderly individuals with MCI significantly over a two-year period in medial temporal areas (Smith et al., 2010). These longitudinal voxel based morphometry (VBM) results are most similar to our cross-sectional results in cortical GM thickness, which found significant associations with less brain tissue in the bilateral precuneus, the right superior frontal gyrus, entorhinal cortex, middle temporal gyrus, inferior parietal gyrus and middle frontal gyrus. There were several overlapping regions between their longitudinal VBM results and our results for cross-sectional cortical GM volume and area (supramarginal gyrus and inferior parietal cortex for GM area and superior frontal gyrus, precuneus, supramarginal gyrus, and inferior parietal cortex for GM volume); however, the VBM effects were predominantly detected in the right hemisphere, while our cortical GM volume and area results were detected in the left hemisphere. The VBM also found effects in the cerebellum, which we did not analyze in this cortical GM study. Our previous TBM study did not detect any significant effects in the cerebellum (Rajagopalan et al., 2011).
Cortical GM structures underlie a wide range of cognitive functions that are susceptible to age- and disease-related degeneration (Dickerson et al., 2009a, Dickerson et al., 2009b, Thompson et al., 2004, Wolk et al., 2010). Our results show relative reductions in cortical GM in regions consistently implicated in AD by a convergence of molecular, structural, and functional neuroimaging data, specifically posterior cortical regions including the posterior cingulate, retrosplenial, and lateral parietal cortex. These areas are clearly outlined in Figure 6 of (Buckner et al., 2005). Broadly, these areas are implicated in memory networks (Sperling et al., 2010) and default mode network activity (Wu et al., 2011) and are susceptible to amyloid deposition, cortical atrophy, and metabolic disruption in AD (Alexander et al., 2012, Reiman and Jagust, 2012, Wolf et al., 2013). It makes sense that we might also detect homocysteine-related brain differences in these areas, as elevated homocysteine levels are associated with increased risk for AD and cognitive decline similar to that found in AD. Perhaps surprisingly, we did not see associations in parahippocampal cortical regions, where we might have expected to see significant associations (Douaud et al., 2013). Compared to models that did not control for diagnostic group, removing the contribution of diagnosis status resulted in a dramatic decrease in the extent of brain regions associated with homocysteine levels. Since homocysteine levels were also significantly higher in the MCI and AD groups, this may simply mean that dementia status and elevated homocysteine are tightly linked in our data. It could be that homocysteine-related atrophy promotes dementia, in which case it is not surprising that controlling for dementia status reduces the effect. It does also suggest that the associations with homocysteine level are not independent of the disease effects on the brain, as expected.
Elevated homocysteine is also associated with poor cardiovascular health (Bostom et al., 1999, Bots et al., 1997, Breteler, 2000, Marti-Carvajal et al., 2013, Selhub et al., 1995), which in turn is associated with cortical atrophy (Erickson et al., 2010, Gustafson et al., 2004, Ho et al., 2010, Swan et al., 1998). Cardiovascular risk factors are associated with reduced gray matter in temporal (Gustafson et al., 2004, Raji et al., 2010) and frontal areas (Celle et al., 2012, Krishnadas et al., 2013, Marques-Iturria et al., 2013, Raji et al., 2010), but not in the occipital lobes. This suggests that the associations we found between homocysteine levels and cortical GM in frontal and temporal areas may be partly due to to cardiovascular effects, whereas the findings in occipital and parietal gray matter may represent a process more specific to homocysteine levels.
As with other studies of ADNI and other large cohorts, the relatively large sample size of N=803 makes this a statistically well-powered study. We were able to perform a GLM analysis of homocysteine levels on GM thickness, volume, and surface area at each point on the cortical surface, after controlling for confounding factors. A prior study using more global measures such as total brain, ventricular, and cortical volume (Jochemsen et al., 2012) did not detect significant associations between homocysteine and regional GM measures, even in a large sample of N=663. The detailed 3D pattern of cortical GM atrophy we identified here is an important signature of homocysteine-related brain atrophy that may be missed by more global summary statistics. One prior study, that assessed GM at each voxel in the brains of adults with cardiovascular disease, found widespread regions of lower GM volume associated with higher homocysteine; even so, only small subcortical areas and no cortical GM regions remained significant after controlling for age, sex, and other measures of health status (Ford et al., 2012). This study was relatively large, with N=150; however, our results suggest that cortical studies of homocysteine effects on the brain may require even larger sample sizes, especially to control for the many confounding factors known to affect brain structure.
A large sample size is beneficial for cortical GM studies, as effect sizes tend to be small (cortical thickness differences between AD and elderly controls may range up to 0.20 mm) (Becker et al., 2011, McGinnis et al., 2011). Measurements may also be noisy due to the complexity of the cortical surface, especially in atrophied elderly brains. Sample size estimates suggest that 50 subjects per group are needed to detect a 0.25 mm group difference (Pardoe et al., 2012), which would be considered a large degree of cortical atrophy. In a study such as ours, where associations with blood measures are subtle, a large sample size may be crucial. This may explain why we found significant associations between homocysteine levels and cortical GM in this large sample, but several prior studies of smaller datasets using different segmentation methods, did not find significant cortical associations.
One difference between the cortical GM results presented here and our previous TBM study is that significant relationships were found in frontoparietal WM for the MCI group, whereas we did not find any significant associations for cortical GM in the MCI group, considered separately. We may have lacked power to detect cortical differences in this region (N=392 MCI for this analysis), or they may not be present.
Higher levels of homocysteine and lower levels of folate and B vitamins are found in people with Alzheimer’s disease (AD) (Clarke et al., 1998b). As a result, there has been much interest in testing dietary vitamin B supplements (including folate) that reduce homocysteine to normal levels (Clarke et al., 2005) and to see if this can thereby prevent or limit the related negative cardiovascular and neurological effects. A meta-analysis failed to show that homocysteine lowering interventions were effective against preventing cardiovascular events (Marti-Carvajal et al., 2013), but the results for alleviating dementia have been more supportive. A randomized, double-blind controlled trial was performed with a high dose of folate and vitamin B supplements, given over the course of two years, to MCI individuals over the age of 70 years (de Jager et al., 2012, Smith et al., 2010). Treatment slowed the accelerated brain atrophy and cognitive decline that is typically seen at this stage of the disease. Another study found that adults with greater intake of B vitamins (B6 and B12) had greater GM volumes in medial and lateral frontal, parietal, and temporal cortical regions (Erickson et al., 2008). This readily accessible treatment therefore has the potential, at least in principle, to reduce risk for cognitive impairment and dementia associated with high homocysteine levels. To understand treatment effects, we also need to better understand the specific neural correlates of homocysteine levels.
Elevated homocysteine levels are neurotoxic and are linked with cardiovascular dysfunction, cognitive decline, increased dementia risk, and brain atrophy. This large study identifies a detailed 3D pattern of lower cortical GM thickness, volume, and surface area associated with elevated homocysteine in the elderly. This cortical signature, along with lower subcortical brain volumes, may offer a more comprehensive set of biomarkers to identify brain atrophy associated with high levels of homocysteine. This has important clinical implications in trials of homocysteine lowering interventions such as folate and B vitamins. These cortical and subcortical biomarkers could be useful to assess the effectiveness of interventions that aim to prevent or slow homocysteine-related brain degeneration and dementia.
Acknowledgments
This research was also supported by a National Defense Science and Engineering Graduate (NDSEG) Fellowship (32 CFR 168a), to S.K.M., from the DoD, and Air Force Office of Scientific Research. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org/). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. Additional support was provided by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Footnotes
Disclosure Statement: The authors have no potential financial or personal conflicts of interest including relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Bergfield KL, Chen KW, Reiman EM, Hanson KD, Lin L, Bandy D, Caselli RJ, Moeller JR. Gray matter network associated with risk for Alzheimer's disease in young to middle-aged adults. Neurobiol Aging. 2012;33(12):2723–32. doi: 10.1016/j.neurobiolaging.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM. Structural correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn. 2007a;24(2):91–7. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Steiner CA, Akopyan GG, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Arch Neurol-Chicago. 2007b;64(10):1489–95. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Thompson PM. Brain mapping as a tool to study neurodegeneration. Neurotherapeutics. 2007;4(3):387–400. doi: 10.1016/j.nurt.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69(6):1032–42. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- Bostom AG, Silbershatz H, Rosenberg IH, Selhub J, D'Agostino RB, Wolf PA, Jacques PF, Wilson PWF. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med. 1999;159(10):1077–80. doi: 10.1001/archinte.159.10.1077. [DOI] [PubMed] [Google Scholar]
- Bots ML, Launer LJ, Lindemans J, Hofman A, Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997;242(4):339–47. doi: 10.1046/j.1365-2796.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- Breteler MMB. Vascular risk factors for Alzheimer's disease: An epidemiologic perspective. Neurobiol Aging. 2000;21(2):153–60. doi: 10.1016/S0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–17. doi: 10.1523/Jneurosci.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celle S, Annweiler C, Pichot V, Bartha R, Barthelemy JC, Roche F, Beauchet O. Association Between Ambulatory 24-Hour Blood Pressure Levels and Brain Volume Reduction A Cross-Sectional Elderly Population-Based Study. Hypertension. 2012;60(5):1324. doi: 10.1161/Hypertensionaha.112.193409. [DOI] [PubMed] [Google Scholar]
- Clarke R, Frost C, Sherliker P, Lewington S, Collins R, Brattstrom L, Brouwer I, van Dusseldorp M, Steegers-Theunissen RPM, Cuskelly G, Ward M, McNulty H, Scott J, den Heijer M, Blom H, van der Put N, Shorah CJ, Malinow MR, McMahon M, Tobert J, Kush D, Joosten E, Riezier R, Pietrzik K, Dierkes J, Bronstrup A, Jacques P, Mason J, Rosenberg I, Thambyrajah J, Landray M, Townend J, Wheeler D, Ubbink J, van Oort F, Melse-Boonstra A, Verhoef P, Woodside JV, Yarnell J, Young IS, Evans AE, Wald D, Law M, Wald N, CHLT Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82(4):806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol-Chicago. 1998a;55(11):1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B-12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol-Chicago. 1998b;55(11):1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis - I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126(Pt 1):170–5. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009a;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009b;30(3):432–40. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. P Natl Acad Sci USA. 2013;110(23):9523–8. doi: 10.1073/Pnas.1301816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood The Cardiovascular Health Study. Neurology. 2010;75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Suever BL, Prakash RS, Colcombe SJ, McAuley E, Kramer AF. Greater intake of vitamins B6 and B12 spares gray matter in healthy elderly: A voxel-based morphometry study. Brain Res. 2008;1199:20–6. doi: 10.1016/j.brainres.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank MJ, Narayan SK, Saxby BK, Ford GA, O'Brien JT. Homocysteine is associated with hippocampal and white matter atrophy in older subjects with mild hypertension. Int Psychogeriatr. 2010;22(5):804–11. doi: 10.1017/S1041610210000499. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sci USA. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AH, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L, Almeida OP. Homocysteine, grey matter and cognitive function in adults with cardiovascular disease. Plos One. 2012;7(3):e33345. doi: 10.1371/journal.pone.0033345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Prestia A, Rasser PE, Bonetti M, Thompson PM. In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer's disease. J Neurol. 2009;256(6):916–24. doi: 10.1007/s00415-009-5040-7. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876–81. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, DeCarli CS, DeChairo BM, Potkin SG, Jack CR, Weiner MW, Raji CA, Lopez OL, Becker JT, Carmichael OT, Thompson PM Neuroimaging AsD. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. P Natl Acad Sci USA. 2010;107(18):8404–9. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Ching CR, Boyle CP, Rajagopalan P, Gutman BA, Leow AD, Toga AW, Jack CR, Jr, Harvey D, Weiner MW, Thompson PM. Unbiased tensorbased morphometry: improved robustness and sample size estimates for Alzheimer's disease clinical trials. Neuroimage. 2013;66:648–61. doi: 10.1016/j.neuroimage.2012.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, JLW, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen HM, Kloppenborg RP, de Groot LC, Kampman E, Mali WP, van der Graaf Y, Geerlings MI. Homocysteine, progression of ventricular enlargement, and cognitive decline. The Second Manifestations of ARTerial disease-Magnetic Resonance study. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Kerwin DR, Gaussoin SA, Chlebowski RT, Kuller LH, Vitolins M, Coker LH, Kotchen JM, Nicklas BJ, Wassertheil-Smoller S, Hoffmann RG, Espeland MA, Memory WHI. Interaction Between Body Mass Index and Central Adiposity and Risk of Incident Cognitive Impairment and Dementia: Results from the Women's Health Initiative Memory Study. J Am Geriatr Soc. 2011;59(1):107–12. doi: 10.1111/j.1532-5415.2010.03219.x. [DOI] [PubMed] [Google Scholar]
- Kerwin DR, Zhang YH, Kotchen JM, Espeland MA, Van Horn L, McTigue KM, Robinson JG, Powell L, Kooperberg C, Coker LH, Hoffmann R. The Cross-Sectional Relationship Between Body Mass Index, Waist-Hip Ratio, and Cognitive Performance in Postmenopausal Women Enrolled in the Women's Health Initiative. J Am Geriatr Soc. 2010;58(8):1427–32. doi: 10.1111/j.1532-5415.2010.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, McLean J, Batty DG, Burns H, Deans KA, Ford I, McConnachie A, McGinty A, McLean JS, Millar K, Sattar N, Shiels PG, Velupillai YN, Packard CJ, Cavanagh J. Cardio-metabolic risk factors and cortical thickness in a neurologically healthy male population: Results from the psychological, social and biological determinants of ill health (pSoBid) study. Neuroimage Clin. 2013;2:646–57. doi: 10.1016/j.nicl.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo ZH, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20(18):6920–6. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Gottfries CG, Regland B. Identification of cognitive impairment in the elderly: Homocysteine is an early marker. Dement Geriatr Cogn. 1999;10(1):12–20. doi: 10.1159/000017092. [DOI] [PubMed] [Google Scholar]
- Leow A, Huang SC, Geng A, Becker J, Davis S, Toga A, Thompson P. Inverse consistent mapping in 3D deformable image registration: Its construction and statistical properties. Lect Notes Comput Sc. 2005;3565:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54(4):2659–71. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, DEmilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. P Natl Acad Sci USA. 1997;94(11):5923–8. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Iturria I, Pueyo R, Garolera M, Segura B, Junque C, Garcia-Garcia I, Jose Sender-Palacios M, Vernet-Vernet M, Narberhaus A, Ariza M, Jurado MA. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res. 2013;214(2):109–15. doi: 10.1016/j.pscychresns.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Marti-Carvajal AJ, Sola I, Lathyris D, Karakitsiou DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Db Syst Rev. 2013;(1) doi: 10.1002/14651858.CD006612.pub3. [DOI] [PubMed] [Google Scholar]
- McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-Related Changes in the Thickness of Cortical Zones in Humans. Brain Topogr. 2011;24(3–4):279–91. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MS, Jacques PF, Rosenberg IH, Selhub J. Hyperhomocysteinemia associated with poor recall in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2001;73(5):927–33. doi: 10.1093/ajcn/73.5.927. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1(1):55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muqtadar H, Testai FD, Gorelick PB. The dementia of cardiac disease. Curr Cardiol Rep. 2012;14(6):732–40. doi: 10.1007/s11886-012-0304-8. [DOI] [PubMed] [Google Scholar]
- Oulhaj A, Refsum H, Beaumont H, Williams J, King E, Jacoby R, Smith AD. Homocysteine as a predictor of cognitive decline in Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25(1):82–90. doi: 10.1002/Gps.2303. [DOI] [PubMed] [Google Scholar]
- Pardoe HR, Abbott DF, Jackson GD. Sample size estimates for well-powered crosssectional cortical thickness studies. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective-Study of Serum Total Homocysteine Concentration and Risk of Stroke in Middle-Aged British Men. Lancet. 1995;346(8987):1395–8. doi: 10.1016/S0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- Pievani M, Rasser PE, Galluzzi S, Benussi L, Ghidoni R, Sabattoli F, Bonetti M, Binetti G, Thompson PM, Frisoni GB. Mapping the effect of APOE epsilon 4 on gray matter loss in Alzheimer's disease in vivo. Neuroimage. 2009;45(4):1090–8. doi: 10.1016/j.neuroimage.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestia A, Drago V, Rasser PE, Bonetti M, Thompson PM, Frisoni GB. Cortical Changes in Incipient Alzheimer's Disease. J Alzheimers Dis. 2010;22(4):1339–49. doi: 10.3233/Jad-2010-101191. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Gutman B, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM for the Alzheimer’s Disease Neuroimaging Initiative. Stress hormone, cortisol, is associated with 3D patterns of accelerated brain tissue loss in the elderly. 2013. [Google Scholar]
- Rajagopalan P, Hua X, Toga AW, Jack CR, Weiner MW, Thompson PM, Initia ADN. Homocysteine effects on brain volumes mapped in 732 elderly individuals. Neuroreport. 2011;22(8):391–5. doi: 10.1097/Wnr.0b013e328346bf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan P, Refsum H, Hua X, Toga AW, Jack CR, Weiner MW, Thompson PM Initi A.s.D.N. Mapping creatinine- and cystatin C-related white matter brain deficits in the elderly. Neurobiol Aging. 2013a;34(4):1221–30. doi: 10.1016/j.neurobiolaging.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan P, Toga AW, Jack CR, Weiner MW, Thompson PM Initi A.s.D.N. Fat-mass-related hormone, plasma leptin, predicts brain volumes in the elderly. Neuroreport. 2013b;24(2):58–62. doi: 10.1097/Wnr.0b013e32835c5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain Structure and Obesity. Hum Brain Mapp. 2010;31(3):353–64. doi: 10.1002/Hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Jagust WJ. Brain imaging in the study of Alzheimer's disease. Neuroimage. 2012;61(2):505–16. doi: 10.1016/j.neuroimage.2011.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs KM, Spiro A, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the normative aging study. Am J Clin Nutr. 1996;63(3):306–14. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol-Chicago. 1999;56(3):338–44. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, McGlinchey RE, Milberg WP. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59(1):181–92. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Bostom AG, Dagostino RB, Wilson PWF, Belanger AJ, Oleary DH, Wolf PA, Schaefer EJ, Rosenberg IH. Association between Plasma Homocysteine Concentrations and Extracranial Carotid-Artery Stenosis. New Engl J Med. 1995;332(5):286–91. doi: 10.1056/Nejm199502023320502. [DOI] [PubMed] [Google Scholar]
- Serra L, Cercignani M, Lenzi D, Perri R, Fadda L, Caltagirone C, Macaluso E, Bozzali M. Grey and White Matter Changes at Different Stages of Alzheimer's Disease. J Alzheimers Dis. 2010;19(1):147–59. doi: 10.3233/Jad-2010-1223. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PWF, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. New Engl J Med. 2002;346(7):476–83. doi: 10.1056/Nejmoa011613. [DOI] [PubMed] [Google Scholar]
- Shaw LM. PENN biomarker core of the Alzheimer's disease Neuroimaging Initiative. Neurosignals. 2008;16(1):19–23. doi: 10.1159/000109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD. The worldwide challenge of the dementias: A role for B vitamins and homocysteine? Food Nutr Bull. 2008;29(2):S143–S72. doi: 10.1177/15648265080292S119. [DOI] [PubMed] [Google Scholar]
- Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. Plos One. 2010;5(9) doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional Alterations in Memory Networks in Early Alzheimer's Disease. Neuromol Med. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–93. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang YL, van Erp TGM, Cannon TD, Toga AW. Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neuroimage. 2004;23:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Connectomics sheds new light on Alzheimer's disease. Biol Psychiatry. 2013;73(5):390–2. doi: 10.1016/j.biopsych.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: Metaanalysis of eight cohort studies including 8669 participants. Alzheimers & Dementia. 2011;7(4):412–7. doi: 10.1016/J.Jalz.2010.08.234. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Staff RT, Murray AD, Duthie SJ, Collins AR, Lemmon HA, Starr JM, Deary IJ. Plasma vitamin C, cholesterol and homocysteine are associated with grey matter volume determined by MRI in non-demented old people. Neurosci Lett. 2003;341(3):173–6. doi: 10.1016/s0304-3940(02)01452-0. [DOI] [PubMed] [Google Scholar]
- Willette AA, Gallagher C, Bendlin BB, McLaren DG, Kastman EK, Canu E, Kosmatka KJ, Field AS, Alexander AL, Colman RJ, Voytko MLL, Weindruch RH, Coe CL, Johnson SC. Homocysteine, neural atrophy, and the effect of caloric restriction in rhesus monkeys. Neurobiol Aging. 2012;33(4):670–80. doi: 10.1016/j.neurobiolaging.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Pereira EA, Budge MM, Bradley KM. Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing. 2002;31(6):440–4. doi: 10.1093/ageing/31.6.440. [DOI] [PubMed] [Google Scholar]
- Wolf AB, Caselli RJ, Reiman EM, Valla J. APOE and neuroenergetics: an emerging paradigm in Alzheimer's disease. Neurobiol Aging. 2013;34(4):1007–17. doi: 10.1016/j.neurobiolaging.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC Neuroimaging A.s.D. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. P Natl Acad Sci USA. 2010;107(22):10256–61. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li R, Fleisher AS, Reiman EM, Guan XT, Zhang YM, Chen KW, Yao L. Altered Default Mode Network Connectivity in Alzheimer's Disease-A Resting Functional MRI and Bayesian Network Study. Hum Brain Mapp. 2011;32(11):1868–81. doi: 10.1002/Hbm.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman BT, Harvey DJ, Crawford K, Bernstein MA, Carmichael O, Cole PE, Crane PK, Decarli C, Fox NC, Gunter JL, Hill D, Killiany RJ, Pachai C, Schwarz AJ, Schuff N, Senjem ML, Suhy J, Thompson PM, Weiner M, Jack CR., Jr Standardization of analysis sets for reporting results from ADNI MRI data. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]