Abstract
Misexpression of developmental transcription factors occurs often in human cancers, where embryonic programs may be reinstated in a context that promotes or sustains malignant development. In this study, we report the involvement of the kidney development transcription factor Six2 in the metastatic progression of human breast cancer. We found that Six2 promoted breast cancer metastasis by a novel mechanism involving both transcriptional and epigenetic regulation of E-cadherin. Downregulation of E-cadherin by Six2 was necessary for its ability to increase soft agar growth and in vivo metastasis in an immune competent mouse model of breast cancer. Mechanistic investigations showed that Six2 represses E-cadherin expression by upregulating Zeb2, in part through a microRNA-mediated mechanism, and by stimulating promoter methylation of the E-cadherin gene (Cdh1). Clinically, SIX2 expression correlated inversely with CDH1 expression in human breast cancer specimens, corroborating the disease relevance of their interaction. Our findings establish Six2 as a regulator of metastasis in human breast cancers and demonstrate an epigenetic function for SIX family transcription factors in metastatic progression through the regulation of E-cadherin.
Keywords: Six2, E-cadherin, breast cancer, metastasis
Introduction
Homeobox genes encode transcription factors that serve as master regulators of embryonic development, where they participate in all aspects of growth and differentiation. Because gene expression programs and cellular processes that are utilized in embryogenesis are often reinstated in tumors, it is not surprising that misexpression of homeobox genes has been implicated in many aspects of tumor progression, both in solid tumors and hematologic malignancies. The SIX family of homeobox genes is comprised of 6 members (SIX1-SIX6), of which SIX1 is most studied for its roles in tumor initiation and progression (1–4). In contrast, little is known about the role of the other SIX family members in tumor onset or progression. Among the SIX family members, SIX1 and SIX2 share the most homology across their homeodomain (DNA binding) and Six domain (cofactor binding) regions, suggesting that they may play similar roles in tumor progression. We recently demonstrated that Six1 knockdown (KD) decreases metastasis in a murine mammary cancer model, but that Six2 is upregulated in the rare Six1KD cells that are still capable of metastasizing (4). These data suggest that Six1 and Six2 may compensate for each other during tumor progression. Few studies have implicated Six2 in cancer (5, 6). Instead, it has been primarily associated with kidney development, where it is important in maintaining mesenchymal progenitor populations and suppressing premature nephrogenesis (7, 8). Although a pro-proliferative and pro-migratory function for Six2 was reported in the pathogenesis of renal clear cell carcinomas and nephroblastomas (6), the mechanism by which Six2 contributes to tumor progression remains unknown.
During cancer progression, loss of cell-cell adhesion is an important step associated with tumor invasion and metastases, and this is frequently accompanied by downregulation of the epithelial molecule E-cadherin. E-cadherin belongs to the cadherin family of proteins that form junctions with neighboring cells, thus playing an important role in the maintenance of epithelial polarization. Numerous studies have demonstrated that E-cadherin functions as a tumor suppressor. Reduced expression of E-cadherin is observed in many epithelial cancers compared to normal tissue, and expression of E-cadherin in tumor cells reduces invasion in vitro and in vivo (9–11). In addition to destabilizing adhesion junctions, loss of E-cadherin also affects cell cycle regulation, cell survival, anoikis resistance, angiogenesis, and colonization at secondary sites, likely through its release of β-catenin and subsequent activation of Wnt signaling, as well as through altering integrin mediated signaling (12–15). Together, numerous studies demonstrate that E-cadherin loss contributes to different stages of the metastatic cascade, which suggest that restoration of E-cadherin expression may allow for suppression of tumor malignancy.
Because E-cadherin loss is frequently associated with metastasis in mouse models and with poor prognosis in human cancers (16, 17), many studies have focused on understanding the mechanism by which E-cadherin is inactivated in human tumors. Mutations of the E-cadherin gene (Cdh1) are found in cancers such as lobular breast cancer and gastric cancer (18). However, in the vast majority of cancers, repression of E-cadherin occurs through transcriptional or epigenetic control mechanisms. Transcription factors, such as those belonging to the Snail and Zeb families, are up-regulated in many cancers, resulting in silencing of E-cadherin through binding to its promoter and recruiting histone modifiers or transcriptional co-repressors (19). Loss of E-cadherin is also commonly accompanied by promoter hyper-methylation (20); suggesting that epigenetic regulation of E-cadherin is an important mechanism for silencing the gene. In addition, recent studies have shown that E-cadherin can be posttranscriptionally regulated by microRNAs (directly or indirectly through transcriptional repressors) (21–23) or posttranslationally regulated through proteasome-mediated degradation (24, 25). Due to the critical role of E-cadherin loss in cancer progression, elucidating the mechanisms by which its expression is controlled may provide novel means to inhibit its downregulation.
In this study, we demonstrate that Six2 is a novel regulator of breast cancer metastasis via its ability to downregulate E-cadherin. We further show that Six2 downregulates E-cadherin via two mechanisms: 1) Upregulation of Zeb2, which is known to directly repress E-cadherin, and 2) through E-cadherin promoter methylation. Importantly, we observe an inverse correlation between CDH1 and SIX2 in human breast cancer, underscoring the relevance of Six2-E-cadherin regulation in the human disease.
Materials and Methods
Cell lines
The 4TO7 and 66c14 mammary carcinoma cell lines were generously provided by Dr. Fred Miller (26). The HMLE cell line was generously provided by Dr. Robert Weinberg (Massachusetts Institute of Technology). Stable knockdown of Six2 was achieved in 66c14 cells using two different shRNAs (Clone ID: V3LMM_459347 and Clone ID: V2LMM_83091, Open Biosystems) and lentivirus delivery. The mouse Six2 cDNA taken from CMV-sport6 (Open Biosystems) and cloned into a pcDNA3.1-hygromycin vector and transfected into 4TO7 cells, after which stably transfected cells were selected. ShRNA targeting mouse Zeb2 were purchased from The Functional Genomics Shared Resource from the University of Colorado Cancer Center. Stable cells were selected either with puromycin (2.5ug/ml) or hygromycin (400ug/ml).
Real time PCR analysis
cDNA was made using the iScript cDNA synthesis kit, and PCR was performed using real time PCR master mix (Bio-Rad) for SYBR green or the Taqman assay according to the manufacturer’s recommended protocols. Primers used: Six2 F: GCCAAGGAAAGGGAGAACAGC; Six2R: GCGTCTTCTCATCCTCGGAAC; Six2 probe: FAM/ACCGACTTGCCACTGCCATTGAGCG. Cdh1 F: GGTGTGGGTCAGGAAATCAC; Cdh1 R:TGTCCCTCCAAATCCGATAC.
Soft agar assay
2ml of 0.6 % base agar was added to the wells of a 6-well plate and allowed to solidify for 30 mins. 4TO7-pcDNA and 4TO7-Six2 cells were trypsinized and the same number of cells (0.1*103) were suspended in 0.4% top agar and plated on top of the bottom agar. Two weeks after plating, 0.01% nitroblue tetrazolium was used to stain the colonies and pictures were taken to quantitate colony number.
Western blots
Whole cell lysates were collected using RIPA buffer. Antibodies against Six2 (1:800, Novus), E-cadherin (1:2000, cell signaling) and Zeb2 (1:1000, BD) were used for Western blotting.
Histology and immunofluorescence
Tumors and lungs from animals were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections and sections were stained with H&E. Mitotic and apoptotic cells of tumors were counted in a blinded manner by a collaborating pathologist (PJ). Primary antibodies against Lyve-1 (1:200, Angiobio) and MECA32 (1:50, BD) were used for immunofluorescence staining of tumors, and pictures were taken and quantified as previously described (4).
Animal studies
Female Balb/c mice (6- to 8-weeks, NCI) were purchased for orthotopic/tail vein injection using 66c14/4TO7 mammary carcinoma cell lines. For orthotopic experiments, 1 × 106 cells in 100 μl of DMEM medium were injected into the 4th mammary fat pad. Tumor growth was measured using calipers bi-weekly. For tail vein inoculation, 1 × 105 cells in 100 μl of DMEM medium were injected. The 4TO7 and 66c14 mammary carcinoma cell lines were tagged with luciferase for in vivo imaging. In vivo detection of metastases was similar to that described previously (3, 4). Animal work was performed on an approved Institutional Animal Care and Use Protocol at the University of Colorado Anschutz Medical Campus.
Microarray and statistic analysis
Microarray datasets from breast tumors were retrieved from the Oncomine web site (https://www.oncomine.com). Microarray analysis for 4TO7-pcDNA and 4TO7-Six2 cells was performed using the Affymetrix MoGene 1.1 ST GeneChip by the Genomics and Microarray Shared Resource of the University of Colorado Cancer Center. The heatmap is standardized such that mean = 0, standard deviation = 1, where the red, blue and white color scale represents the expression of a gene above, below and equal, respectively, to the mean expression of that probe across all samples. All microarray data has been deposited in the NCBI GEO database (accession number GSE57678). Kaplan-Meier analyses in figures 1C–D were retrieved from GOBO (http://co.bmc.lu.se/gobo). Statistical analysis was performed using 2-tailed t test for comparing two groups. One-way ANOVA with Tukey posttests was performed for comparing more than three groups. Log-rank (Mantel-Cox) Test was used for survival analysis in the animals. Pearson r test was used to analyze correlation of gene expression retrieved from Oncomine datasets. GraphPad Prism 5 was used to perform above mentioned analyses. Error bars represent the standard error of the mean from three independent experiments. Asterisks denote significant difference from control group *, P<0.05; **, P<0.01; ***, P<0.001.
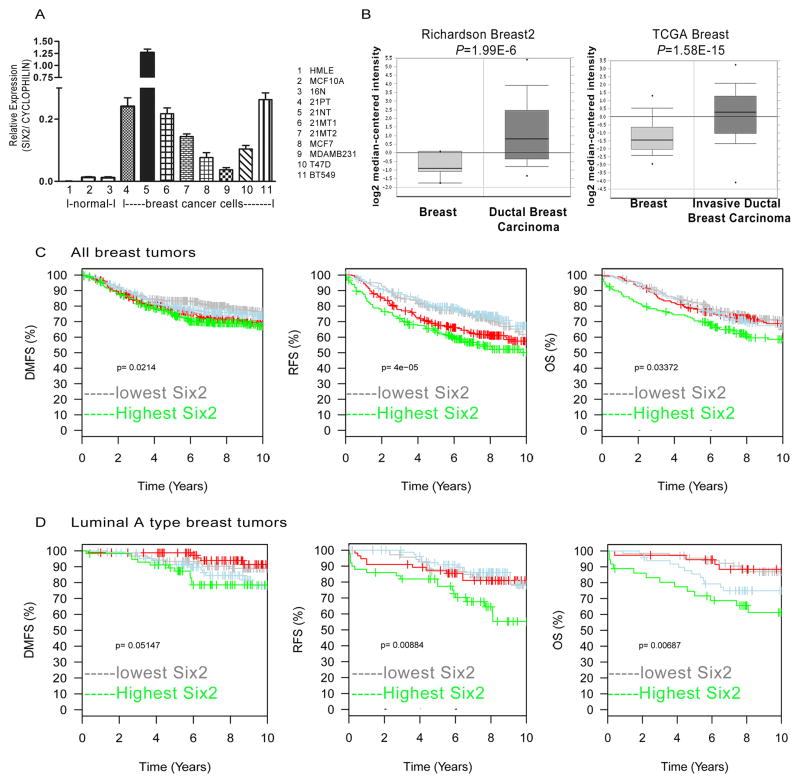
Fig 1. Increased expression of SIX2 in human breast cancers correlates with poor prognosis.
(A) SIX2 mRNA expression was determined by real-time PCR and normalized to CYCLOPHILIN in three normal mammary epithelial cell lines and eight human breast cancer cell lines. (B) SIX2 mRNA expression in human breast cancers compared to normal breast tissue in Richardson breast2 and TCGA breast data sets from Oncomine. Oncomine™ (Compendia Bioscience, Ann Arbor, MI) was used for analysis and visualization. (C) Top quartile SIX2 expression predicts poor prognosis in human breast cancers. Kaplan-Meier curves show that SIX2 expression correlates with distant metastasis free (DMFS), relapse free survival (RFS), and overall survival (OS). (D) Top quartile SIX2 expression predicts poor prognosis in luminal A breast cancer. Kaplan-Meier curves demonstrate that SIX2 expression correlates with distant metastasis free survival (DMFS), relapse free survival (RFS) and overall survival (OS) in luminal A tumors by HU-gene expression subtype (45). Data was extracted from the GOBO website (http://co.bmc.lu.se/gobo).
Results
SIX2 expression is increased in human breast cancer and correlates with poor prognosis
Our previous studies, focused on Six1, demonstrated that Six1 knockdown (KD) dramatically decreased metastasis. However, we found that some Six1 KD cells were still capable of metastasizing, and that these cells had increased expression of Six2. These data suggested that Six2 could compensate for Six1 loss (4), and prompted us to examine SIX2 levels in human breast cancer. Thus, to determine if SIX2 is overexpressed in breast cancers, we first examined its expression in normal and breast cancer cell lines, where we found a 4.3-fold to 144.6-fold increase in SIX2 expression in breast cancer cells when compared to non-transformed mammary epithelial cells (Fig. 1A). Examination of four independent Oncomine databases (Fig. 1B and Supplemental Fig. 1A–B) demonstrated that SIX2 expression is also higher in human breast carcinomas than in normal breast tissue. Analysis of SIX2 in the TCGA dataset showed that SIX2 is more highly expressed in breast tumors of higher metastatic stage, as well as in tumors from patients that died within 5 years (Supplemental Fig. 1C). Using the Gene Expression-Based Outcome for Breast Cancer Online tool (GOBO; http://co.bmc.lu.se/gobo), which contains expression data from 1881 breast tumor samples encompassing 11 public microarray data sets (27), we stratified tumors based on SIX2 levels and performed Kaplan-Meier analyses. We found that patients whose tumors expressed the highest levels of SIX2 (in the top quartile) had significantly worsened distant metastasis free survival (DMFS), worsened relapse free survival (RFS), and worsened overall survival (OS) (Fig. 1C). Interestingly, SIX2 expression correlates with worsened prognosis in luminal A breast cancers, but not in other breast cancer subtypes (basal-like, HER2, luminal B, and normal-like) (Fig. 1D; data not shown). We recently reported that SIX1 expression predicts adverse outcomes particularly in luminal B types of breast cancer (28); thus, these data suggest that SIX2 may contribute to disease progression in a different breast cancer subtype than SIX1.
Knockdown of Six2 decreases distant metastasis in an orthotopic mammary carcinoma model
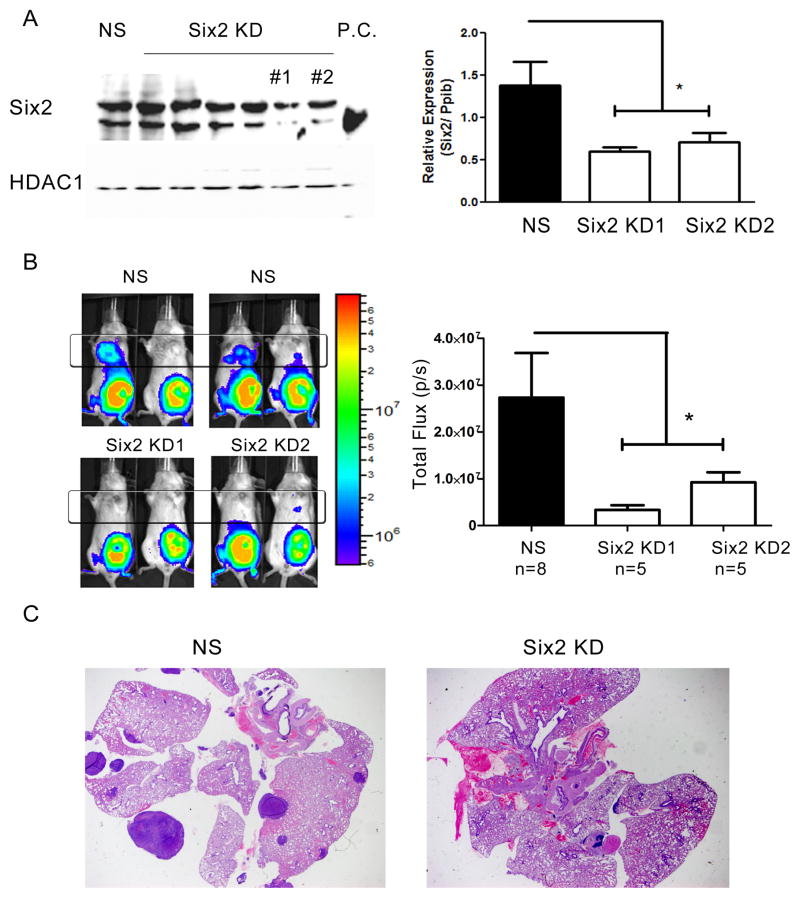
As outlined above, we previously demonstrated that Six1 KD in 66cl4 mouse mammary carcinoma cells decreases their ability to metastasize in an immune competent model of mammary carcinoma, but that some Six1 KD cells are still capable of metastasizing, having upregulated Six2 in vivo (4). We thus investigated whether Six2 mediates metastasis in the same 66cl4 metastasis model. Knockdown of Six2 was performed using 6 different shRNA constructs, two of which efficiently knocked down Six2 protein levels (Fig. 2A). The two efficient knockdowns were confirmed at the RNA level, demonstrating a reduction in Six2 expression to 53% of its original level (Fig. 2A). To determine whether Six2 KD affects metastasis, 66cl4-NS (non-silencing) and 66cl4-Six2 KD cells were tagged with luciferase and injected into the 4th mammary fat pad of immune competent Balb/c mice. Fifty days after injection, we observed an ~ 85% decrease in lung metastases in 66cl4-Six2 KD-bearing animals compared to 66cl4-NS control animals (Fig. 2B). The decrease in metastasis was further confirmed by examining the histology of the lungs in tumor bearing mice (Fig. 2C). These data demonstrate that Six2, like Six1, is necessary to mediate distant metastasis in the 66cl4 orthotopic mouse mammary carcinoma model.
Fig 2. Six2 KD decreases metastasis in the 66cl4 mammary carcinoma models.
(A) 6 different shRNA against Six2 was used to knockdown Six2 in the 66cl4 cells and the most efficient Six2 knockdown cells (KD#1 and KD#2) were used for subsequent studies. Six2 expression was determined using Western blotting (left) and real-time PCR (right) in control (NS, non-silencing) and Six2 KD cells. P.C. stands for positive control, and demonstrates where the Six2 specific band runs. (B) Representative bioluminescent imaging of Balb/c mice injected with control (NS) or Six2 KD cells into the 4th mammary fat pad (left). Quantitation of distant luminescent signal, likely in lungs (boxed region) reveals a significant decrease in metastasis when Six2 is knocked down (right). (C) Histological confirmation of lung metastasis by H& E staining from control and Six2 KD.
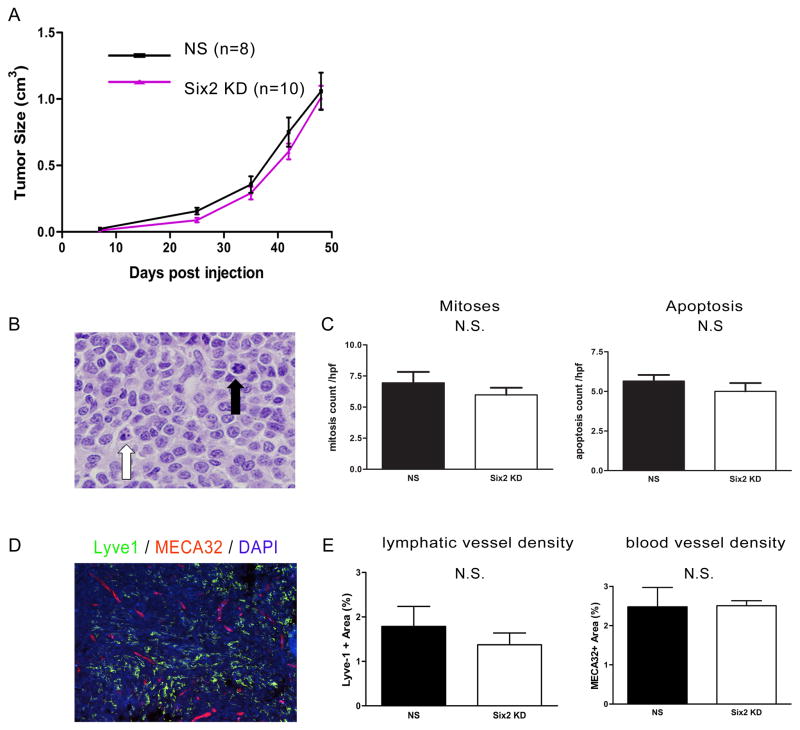
Six2 knockdown does not affect the growth of primary tumors
Proliferation and apoptosis are two important hallmarks of tumor progression and primary tumor size is known to correlate with the risk of metastatic dissemination. We thus first assessed whether Six2 knockdown altered proliferation of 66cl4 cells. We observed no differences in BrdU incorporation between NS and Six2 KD cells in vitro (Supplemental Fig. 2). Furthermore, in contrast to Six1 KD in this model, which drastically decreases the size of primary tumors (4), Six2 KD in 66cl4 cells did not affect primary tumor growth/size compared to 66cl4-NS control tumors (Fig. 3A). As expected, no differences in mitosis or apoptosis were seen in the primary tumors, when scored in a blinded manner (Fig. 3B–C). These data suggest that Six1 and Six2 are not entirely redundant, in that Six1 KD can inhibit primary tumor growth in this orthotopic 66cl4 mammary tumor model, whereas Six2 KD cannot.
Fig 3. Six2 KD does not affect primary tumor growth.
(A) Primary tumor size in 66cl4 control (NS) injected mice and Six2 KD injected mice (two different shRNA lines were used and combined in the figure) tumors. Tumor size in the animals was measured using calipers, and calculated according to the formula V=1/2(W)(W)(L). (B) Representative histology showing cells undergoing apoptosis or mitosis. Black arrowhead: mitotic cell; white arrowhead: apoptotic cell. (C) Quantification of mitotic and apoptotic cells from 66cl4-NS and 66cl4-Six2 KD tumors. Mitotic and apoptotic cells were counted under ten high power fields per H&E section. Four control and five Six2 KD tumors were counted. (D) Representative picture showing lymphatic vessels using Lyve-1 staining and blood vessels using MECA-32 staining in a 66cl4 tumor. (E) Slidebook software was used to quantify lymphatic or blood vessels in four 66cl4-NS and four 66cl4-Six2 KD tumors. N.S. stands for no statistic significance.
For tumor cells to reach secondary sites, they must either enter the bloodstream or the lymphatic system. We recently demonstrated that Six1 leads to increased tumor-associated lymphangiogenesis and distant metastasis through regulating VEGF-C transcription in the 66cl4 mammary carcinoma model (4). Thus, we examined whether Six2KD also influences lymphangiogenesis to enhance metastastic dissemination. However, we observed no difference in VEGF-C expression between control and Six2 KD tumors (Supplemental Fig. 2B), suggesting that unlike Six1, Six2 is not a major regulator of VEGF-C. We further performed double immunostaining of the 66cl4-NS and 66cl4-Six2 KD tumors using a Lyve-1 antibody (which is a marker of lymphatic vessels) and MECA32 (a marker of blood vessels) and quantified the number of intra-tumoral lymphatic and blood vessels using slidebook software. A representative double immunofluorescence image is shown in Fig. 3D, and quantitation of the fluorescence showed no significant differences in the number of lymphatic or blood vessels between NS control and Six2 KD tumors in the 66cl4 model (Fig. 3E). Together, our results show that Six2 KD decreases distant metastasis without affecting primary tumor growth or tumor-associated lymphangiogenesis/angiogenesis, and that it is not functionally redundant with Six1 in this mouse model of metastasis.
Six2 expression increases metastasis in the 4TO7 mammary carcinoma model
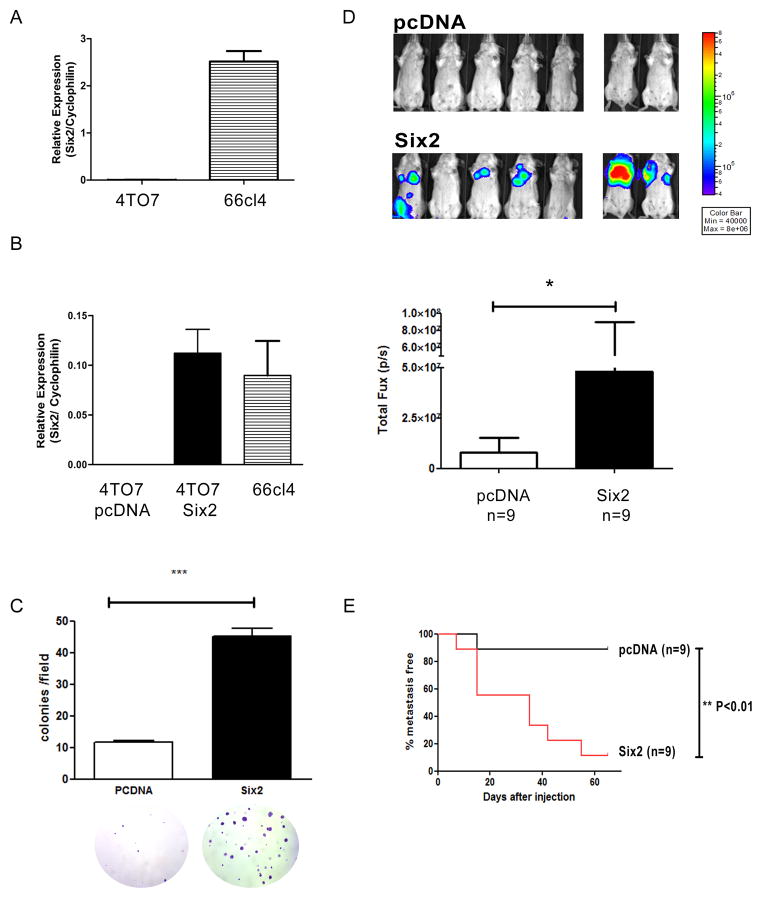
The aforementioned data suggested that unlike Six1, which participates in many aspects of tumor progression, Six2 may mediate tumor metastasis through means other than cell proliferation and lymphangiogenesis. To determine whether and how Six2 promotes metastasis, we utilized a second model of mouse mammary carcinoma, the 4TO7 model. 4TO7 and 66cl4 cells are syngeneic, but unlike 66cl4 cells, which are highly metastatic, 4TO7 cells are capable of micrometastasizing to the lungs after orthotopic injection, but are very inefficient at colonizing the lungs and forming macrometastases (26). Thus, 4TO7 cells are able to enter the bloodstream and exit into the secondary site, but they cannot efficiently grow at the secondary site. Since endogenous levels of Six2 are low in 4TO7 cells when compared to 66cl4 cells (Fig. 4A), we ectopically expressed Six2 in 4TO7 cells and determined if Six2 expression in these cells could enhance their ability to form metastatic lesions at the secondary site. To this end, we stably introduced pcDNA (as a control) or Six2 into 4TO7 cells and demonstrated that ectopic Six2 expression in 4TO7 cells led to similar levels of Six2 as observed in 66cl4 cells (Fig. 4B). Interestingly, we observed that ectopic Six2 expression in 4TO7 cells led to changes in cell morphology, with cells appearing smaller and more rounded (Supplementary Fig. 3A). We thus asked whether Six2 expression also changes cell behavior. Similar to our results in 66cl4 cells, Six2 expression in 4TO7 cells did not increase cell proliferation, measured using BrdU incorporation (Supplemental Fig. 3B). However, 4TO7 cells expressing Six2 displayed a significant increase in anchorage-independent cell growth (soft agar assay) when compared to control counterparts (Fig. 4C). Importantly, anchorage independence in vitro has been shown to correlate with both transformation capability and metastatic ability (29). To determine whether Six2 overexpression can promote metastasis in vivo, luciferase labeled 4TO7-pcDNA and 4TO7-Six2 cells were injected into female Balb/c mice via the tail vein, to enable us to examine the effect of Six2 on later stages of metastasis. Six2 expression in 4TO7 cells drastically enhanced the ability of the cells to form metastatic lesions in the lungs of mice as measured using IVIS imaging and confirmed by histologic examination (Fig. 4D and Supplemental Fig. 4). Kaplan-Meier analysis revealed that a significantly higher percentage of mice injected with Six2 expressing cells had metastatic lesions than control injected mice (Fig. 4E). Together, our data clearly demonstrate that expression of Six2 in mammary carcinoma cells with low metastatic potential significantly increases their ability to perform the later stages of met astasis.
Fig 4. Six2 expression promotes metastasis in the 4TO7 mammary carcinoma model.
(A) Six2 mRNA expression was measured using real-time RT-PCR in 4TO7 and 66cl4 cell lines. (B) pcDNA control or Six2 expressing vectors were transfected into 4TO7 cells and clones were pooled after hygromycin selection. Six2 over-expression in 4TO7 cells was measured by real-time PCR to compare endogenous levels of Six2 in 66cl4 cells to those obtained with ectopic expression of Six2 in 4TO7 cells. (C) Six2 expression increases anchorage independent cell growth. Quantification of colonies formed in soft agar by 4TO7-pcDNA or 4TO7-Six2 cells (upper) and representative pictures of colonies in 4TO7-pcDNA and 4TO7-Six2 cells (bottom). (D) Luciferase labeled 4TO7-pcDNA or Six2 cells were injected into Balb/c mice through the tail vein and in vivo metastasis was measured using IVIS imaging. Representative pictures of animals injected with 4TO7-pcDNA or 4TO7-Six2 cells (top). Quantification of whole body luciferase per animal in 4TO7-pcDNA (n=9) and 4TO7-Six2 (n=9) groups (bottom). (E) Kaplan-Meier plot shows % of metastasis free mice in 4TO7-pcDNA control and 4TO7-Six2 expressing groups. Statistical analysis was performed using the log-rank test.
Six2 induces metastasis via regulation of E-cadherin
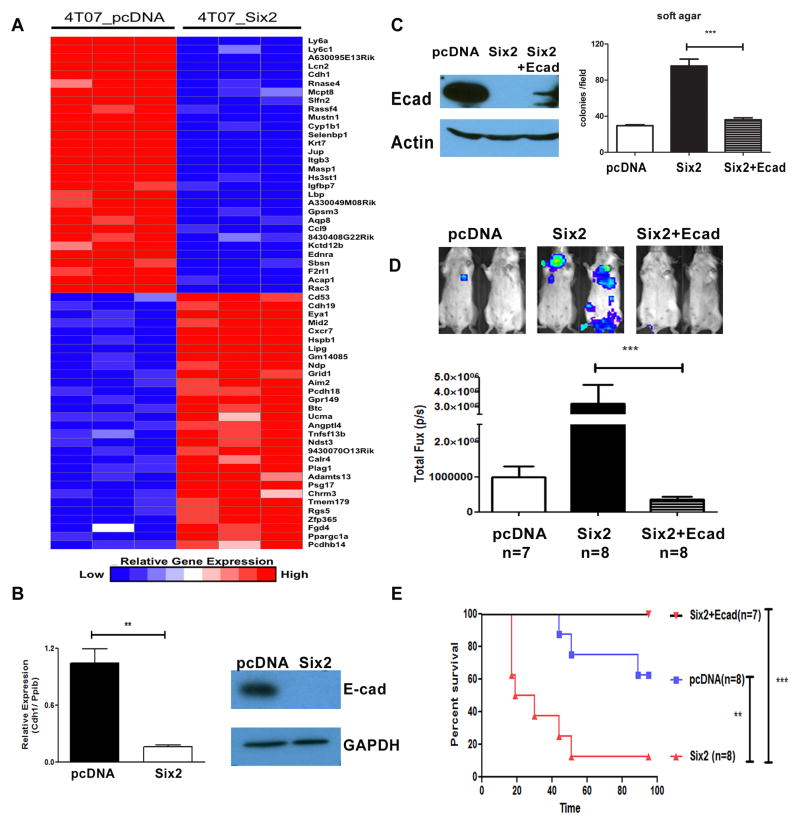
To determine the mechanism by which Six2 mediates metastasis, we performed microarray analysis on the 4TO7-pcDNA and Six2 expressing cells. Fig. 5A shows a heat map of the top 30 up- and down-regulated genes in the 4TO7-pcDNA cells as compared to the 4TO7-Six2 cells. Interestingly, the microarray analysis revealed that the E-cadherin gene (Cdh1) is significantly down regulated with Six2 overexpression, which we confirmed using both real-time RT-PCR and Western blot analysis (Fig. 5B). To investigate whether E-cadherin downregulation is required for Six2-induced metastasis, we restored E-cadherin to Six2 expressing cells (Six2+Ecad) at a level that did not exceed the level of E-cadherin protein in 4TO7-pcDNA control cells (Fig. 5C). In vitro, re-expression of E-cadherin in the 4TO7-Six2 cells decreases anchorage independent growth back to the levels observed in the control cells (Fig. 5C, right panel). Importantly, partial restoration of E-cadherin protein (Fig. 5C) was sufficient to inhibit Six2-mediated experimental metastasis (Fig. 5D), and significantly improve survival of the mice (Fig. 5E). Together, our results demonstrate that restoration of even low levels of the E-cadherin protein to Six2-expressing 4TO7 cells is sufficient to inhibit the ability of Six2 to mediate metastasis.
Fig 5. Restoration of E-Cadherin in 4TO7-Six2 cells suppresses Six2-mediated metastasis and increases survival in animals.
(A) Gene expression using microarray analysis from 4TO7-pcDNA and 4TO7-Six2 was examined (each cell line microarray was performed in triplicate). Expression data is shown as top-regulated genes in 4TO7-pcDNA and 4TO7-Six2 cells. The color scale represents the expression level of a gene above (red) and below (blue) the mean expression level of that gene across all samples. (B) E-cadherin mRNA expression was determined by real-time PCR, normalized by Cyclophilin in 4TO7-pcDNA and 4TO7-Six2 cells (left). E-cadherin protein expression was measured by Western blotting. GAPDH was used as loading control (right). (C) E-cadherin expression in 4TO7-pcDNA, Six2, and Six2+Ecad was measured by Western blotting.β-actin was used as loading control (Left). Restoration of E-cadherin in 4TO7-Six2 expressing cells decreases anchorage independent cell growth (Right). Quantification of colony numbers formed by 4TO7-pcDNA, 4TO7-Six2, or 4TO7-Six2+Ecad cells. ***, P<0.001. (D) Luciferase labeled 4TO7-pcDNA, 4TO7-Six2, or 4TO7-Six2+Ecad cells were injected into Balb/c mice through the tail vein and in vivo metastasis was measured using IVIS imaging. Representative pictures from each group are shown (top) and quantification of luciferase signal in each animal injected with 4TO7-pcDNA (n=8), 4TO7-Six2 (n=8), or 4TO7-Six2+Ecad (n=7) is shown (bottom). (E) Kaplan-Meier plot shows overall survival of the injected mice. Statistical analysis was performed using the log-rank test.
Six2 regulates E-cadherin through transcriptional and epigenetic mechanisms
Six2 regulates the E-cadherin repressor Zeb2 to mediate metastasis
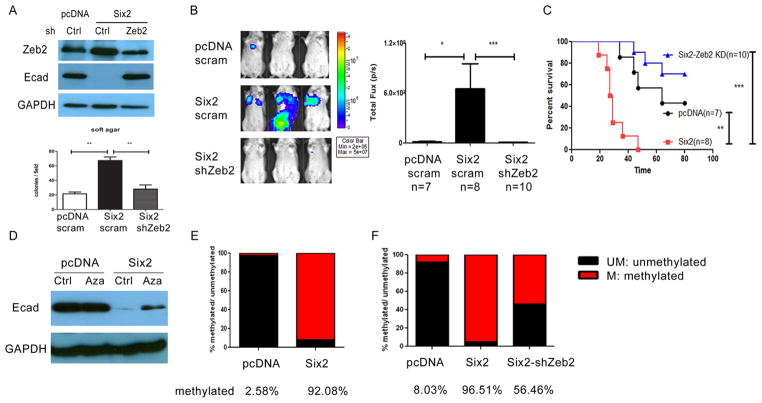
Increased expression of transcription factors (such as Twist, Snail and Zeb) has been detected in many cancers, and these transcription factors are well known to repress E-cadherin transcription directly. To determine the mechanism by which Six2 downregulates E-cadherin, we first examined whether Six2 can alter the expression of any of these known E-cadherin regulators. Of the aforementioned transcription factors, only Zeb2 showed altered expression in our microarray analysis comparing 4TO7-Six2 cells to 4TO7-pcDNA control cells, which we confirmed by real time PCR (Supplementary Fig. 5A–B). Zeb2 (zinc finger E-box binding homeobox 2) belongs to the Zeb family of transcription factors and is known to repress E-cadherin transcription by binding to its promoter and recruiting the transcriptional co-repressor, C-terminal binding protein (CtBP) (30). Downregulation of E-cadherin and upregulation of Zeb2 in response to Six2 overexpression was also observed in an additional cell line, human mammary epithelial cells (HMLE) (Supplementary Fig. 5C). To determine whether Six2 depends on Zeb2 to repress E-cadherin expression, we stably knocked down Zeb2 in the 4TO7-Six2 expressing cells and showed that loss of Zeb2 downstream of Six2 restores E-cadherin expression (Fig. 6A) and decreases anchorage-independent growth in vitro (Fig. 6A, lower panel). Similar to E-cadherin restoration in 4TO7-Six2 cells, Zeb2 KD in the 4TO7-Six2 cells significantly decreased metastasis and increased survival when the cells were injected into the tail vein of Balb/c mice (Fig. 6B). These data demonstrate that Zeb2 upregulation downstream of Six2 is important to repress E-cadherin and induce metastasis in the 4TO7 mammary carcinoma model.
Fig 6. Six2 represses E-cadherin expression via multiple mechanisms.
(A) Zeb2 KD in 4TO7-Six2 cells reverses E-cadherin repression (Top). Expression of Zeb2 and E-cadherin in 4TO7-pcDNA, 4TO7-Six2 and 4TO7-Six2-Zeb2 KD cells was measured by Western blotting. shRNA targeting Zeb2 was delivered into 4TO7-Six2 cells and stable KD was selected using puromycin. Scramble shRNA was delivered into 4TO7-pcDNA and 4TO7-Six2 cells to serve as KD control. Zeb2 KD in 4TO7-Six2 expressing cells decreases anchorage independent cell growth (Lower). Quantification of colony numbers formed by 4TO7-pcDNA, 4TO7-Six2, 4TO7-Six2-Zeb2 KD cells in soft agar. **, P<0.01. (B) Luciferase labeled 4TO7-pcDNA, 4TO7-Six2 or 4TO7-Six2-Zeb2 KD cells were injected into Balb/c mice through the tail vein and in vivo metastasis was measured using IVIS imaging. Representative pictures (left) and quantification (right) of luciferase signal in animals injected with 4TO7-pcDNA (n=8), 4TO7-Six2 (n=8) or 4TO7-Six2-Zeb2 KD (n=7) cells are shown. (C) Kaplan-Meier plot shows overall survival of the injected mice. Statistical analysis was performed using the log-rank test. (D) Restored E-cadherin expression by treating 4TO7-Six2 cells with the DNA methylation inhibitor, 5Aza. 4TO7-pcDNA and Six2 cells were treated with DMSO (vehicle control) or 5Aza (10uM) for 48hrs and whole cell lysates were collected and Western blotting was performed for E-cadherin expression. (E) Six2 expression significantly increases E-cadherin promoter methylation. Genomic DNA from 4TO7-pcDNA and 4TO7-Six2 cells was collected and E-cadherin promoter CpG methylation status was detected using EpiTech Methyl II PCR primer assay (Qiagen) for the mouse Cdh1 promoter. (F) Zeb2 KD in 4TO7-Six2 cells decreases CpG methylation of the Cdh1 promoter. Relative amount of methylated and unmethylated E-cadherin promoter was detected using EpiTech Methyl II PCR primer assay for mouse Cdh1 in 4TO7-pcDNA, Six2, and Six2-Zeb2 KD cells. UM:un-methylated DNA. M: methylated DNA.
Since the Zeb2 promoter does not contain predicted Six binding sites, and since Six2-mediated regulation of the Zeb2 protein appeared to be somewhat greater than of the Zeb2 mRNA (Supplementary Fig. 5B and Fig 6A), we hypothesized that Six2 may regulate Zeb2 through a microRNA (miR) -mediated mechanism, especially as miR family members have been implicated in Zeb2 regulation (24). We found that miR-200 microRNAs that belong to the miR-8 family and are known to repress Zeb2 and exist in a feedback loop with Zeb2 (24), were decreased in the presence of Six2 (Supplementary Fig. 6). Because miR-200a and b were decreased by Six2 in both 4TO7 and HMLE cells, we reintroduced these miRs at similar levels (not shown) downstream of Six2 and examined their effect on Zeb2 levels. Our data suggest that Six2 increases Zeb2 primarily through repressing microRNA-200b in both mouse 4TO7 cells and human HMLE cells (Supplementary Fig. 6). Thus, Six2 increases Zeb2 expression at least in part via repressing miR-8 family members.
Six2 represses E-cadherin via promoter methylation
Many studies demonstrate that epigenetic regulation (DNA methylation or histone modification) of E-cadherin is an important mechanism contributing to its downregulation in human cancers (20, 31). Because of the dramatic silencing of E-cadherin downstream of Six2 (Fig. 5B), we asked whether additional mechanisms may be at play in its ability to silence E-cadherin. To determine whether methylation of the E-cadherin promoter may play a role in Six2-mediated silencing of the gene, we treated 4TO7-Six2 cells with the methyltransferase inhibitor 5-Azacytidine (5Aza). Treatment of 4TO7-Six2 cells with 5Aza partially restored expression of E-cadherin; in contrast, no further increase in E-cadherin expression was observed when the 4TO7-pcDNA control cells were treated with 5Aza (Fig. 6D). Methylation analysis of the Cdh1 (E-cadherin) promoter revealed that cells expressing Six2 had a significant increase in Cdh1 CpG island methylation (Fig. 6E). We further found that breast cancer cell lines expressing high levels of SIX2 (MDA-MB-231, Sum159, and BT549) and low levels of E-cadherin, are those with high measured levels of CDH1 promoter methylation. In contrast, breast cancer cell lines (MCF7, T47D, HCC70) expressing low levels of SIX2 and high levels of E-cadherin had correspondingly low levels of CDH1 promoter methylation (Supplementary Fig. 7). Together, these results suggest that in addition to regulation by Zeb2, Six2 also represses E-cadherin expression via altering the methylation status of its promoter.
The Zeb transcription factor is known to repress Cdh1 (E-cadherin) via binding directly to E-boxes within the E-cadherin promoter (30). However, it has not been implicated in methylation of the E-cadherin promoter. Because Zeb2 is regulated downstream of Six2, we asked whether Zeb2 can in part mediate the Cdh1 promoter methylation seen downstream of Six2 expression. Intriguingly, stable Zeb2 KD in Six2 cells could partially restore Cdh1 promoter methylation status to that observed in control cells (Fig. 6F), suggesting that Zeb2 may participate in DNA methylation (directly or indirectly) downstream of Six2, and demonstrating a novel function for Zeb2 in epigenetic regulation of E-cadherin. Together, our studies demonstrate that Six2 represses E-cadherin through multiple mechanisms, including both transcriptional and epigenetic mechanisms.
SIX2 and CDH1 are inversely correlated in human breast cancers
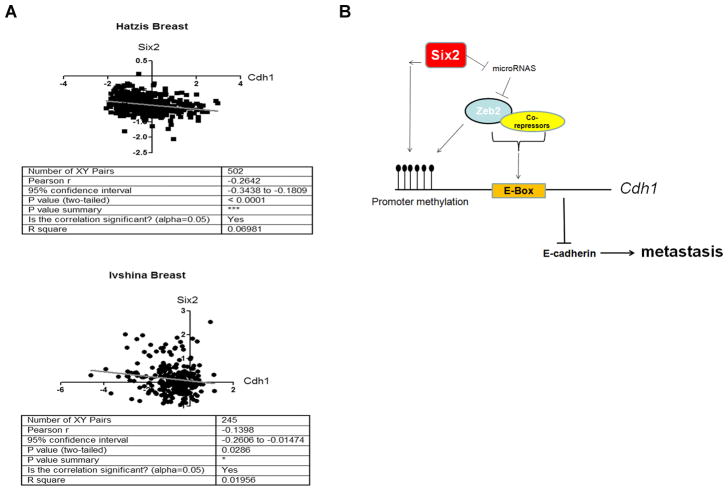
Based on our experimental finding that Six2 represses E-Cadherin to induce metastasis, we asked whether this relationship holds true in human breast cancer. We found that there is a significant inverse correlation between SIX2 and CDH1 in two different breast cancer datasets, encompassing 502 (Hatzis)(32) and 245 (Ivshina) (33) breast cancer samples respectively (Fig. 7A). Of note, in the Hatzis dataset, further analysis shows that there is an inverse correlation of SIX2 and CDH1 in both triple negative and non-triple negative breast cancers (supplementary Fig 8), implying that the regulation of E-cadherin by Six2 may not be restricted to specific subtypes of breast cancer. Together, these results suggest that regulation of E-cadherin by SIX2 is not confined to breast cancer cell lines, but may also occur in human breast tumors.
Fig 7. Six2 inversely correlates with E-cadherin expression in human breast cancers.
(A) SIX2 and CDH1 expression values were retrieved from an Oncomine microarray data set (as indicated in the figure) and were plotted by expression value. Statistical analysis was performed using the Pearson’s test.
(B) Model depicting the mechanism by which Six2 represses E-cadherin and promotes metastasis. Overexpression of Six2 in breast cancers leads to increased expression of Zeb2, at least in part through microRNA-mediated regulation. Increased expression of Zeb2 represses E-cadherin transcription by canonical E-box binding and also in part through DNA methylation. Six2 may also promote Cdh1 promoter methylation independent of Zeb2 expression. Decreased expression of E-cadherin by Six2 leads to increased metastasis and decreased survival.
Discussion
The Six2 homeoprotein has been extensively studied during embryonic development. Loss of Six2 results in postnatal lethality due to premature differentiation of mesenchymal cells in the kidney, resulting in precocious nephrogenesis (7). Given the fact that Six2 is important for kidney development, but becomes silenced in the adult kidney, it is not surprising that Six2 expression has been implicated in the pathogenesis of renal carcinoma (5, 34). However, the mechanism by which Six2 contributes to renal carcinoma is unclear. In addition, a role for Six2 in other cancers has not been examined. Interestingly, Six2 upregulation was observed in a bone-metastatic signature derived using in vivo selection of MDA-MB-231 breast cancer cells (35), suggesting that Six2 may play a role in breast cancer associated metastasis. In this study, we demonstrate for the first time, that Six2 is a novel regulator of breast cancer metastasis, through a mechanism that differs at least in part from that of its highly related family member, Six1. We show that Six2 KD decreases distant metastasis without influencing primary tumor growth or lymphangiogenesis, two phenotypes that are significantly affected by loss of Six1. Studies examining the developmental roles of Six1 and Six2 in mice have demonstrated that although Six1 and Six2 are broadly expressed during embryogenesis, loss of Six1 results in more severe phenotypes than loss of Six2, the latter resulting in phenotypes mainly in the developing kidney. In this study, we show that Six2 plays a role in later stages of metastasis, while not influencing some earlier metastatic properties, such as proliferation and lymphangiogenesis. However, we cannot rule out the possibility that Six2 may influence additional steps in the metastatic cascade that we have not directly examined.
Our data suggest that Six2 mediates breast cancer metastasis in a manner that differs from Six1, at least in part. However, we cannot exclude the possibility that Six1 and Six2 still contribute to tumor progression via overlapping mechanisms. Interestingly, SIX1 was recently shown to regulate E-cadherin levels via regulating a microRNA-200 family-ZEB1 axis, and it has also been shown to directly transactivate the ZEB1 promoter (36, 37). However, high endogenous Six1 expression in the 4TO7 mammary carcinoma cell line does not interfere with E-cadherin expression, nor does it correlate with high E-cadherin promoter methylation. In contrast, introduction of Six2 into this same cell line, which does not significantly express Six2 endogenously, leads to significant E-cadherin promoter methylation and downregulation. Together, these data implicate the related SIX family member, SIX1, in E-cadherin regulation, but not via the same mechanism as SIX2, particularly with respect to promoter methylation. Nonetheless, these data do suggest that the two proteins impinge on the same pathways, albeit at least in part through different mechanisms. Interestingly, we were unable to establish Six1/Six2 double KD cells, suggesting that loss of both proteins may result in loss of viability. These data suggest that the two proteins cooperate to mediate metastasis, and that the total level of these two proteins together may impart aggressive phenotypes to tumor cells.
Mesenchymal cells expressing Six2 in the kidney are known to maintain the nephron progenitor population through promoting self-renewal properties and also through suppressing signals required for epithelial differentiation (8). Although Six2 plays a critical role in the kidney, few downstream targets of the transcription factor are known in this context. We have identified a target of Six2 in breast cancer, that may potentially also be a developmental target of Six2. Microarray analysis revealed that E-cadherin, which is widely implicated in many different kinds of cancer, including breast cancer, is downregulated with Six2 overexpression. In Six2-null kidney explants, expanded expression of E-cadherin has been observed (7). When taken together with the fact that Six2 loss results in premature epithelialization of the metanephric mesenchyme (7), these results support our finding that Six2 plays a role in repressing epithelial molecule (in particular E-cadherin) expression. Based on our results, we propose a working model (Fig. 7B) in which overexpression of Six2 in breast cancer cells promotes metastasis at least in part by repression of E-cadherin expression. Six2 inhibits E-cadherin through induction of the transcriptional repressor Zeb2, which represses E-cadherin by binding to E-boxes in the Cdh1 promoter. In addition, Six2 can also suppress Cdh1 transcription by enhancing promoter methylation; thus repressing E-cadherin both through transcriptional and epigenetic mechanisms. Interestingly, Zeb2 stable KD in Six2 overexpressing 4TO7 cells leads to a decrease in Cdh1 promoter methylation, demonstrating that Cdh1 promoter methylation downstream of Six2 is partially dependent on Zeb2. While Zeb2 is known to repress E-cadherin transcription by binding to its promoter and recruiting the transcriptional co-repressor CtBP, which in turn mediates histone deacetylation and histone methylation (30, 38, 39), Zeb2 has not previously been implicated in Cdh1 promoter methylation. However, it should be noted that histone modifications and DNA methylation are frequently coupled; thus, the reduction of Zeb-CtBP mediated histone modifications (H3K9) may lead to a decrease in promoter methylation. Together, our studies identify a novel mode of regulation of E-cadherin by Six2 that involves transcriptional repression by Zeb2 as well as DNA methylation. It should also be noted that while downregulation of E-cadherin is important for Six2-mediated metastasis, our microarray analysis revealed that Six2 also up-regulates additional genes which have been implicated in cancer progression, specifically for lung metastasis, such as MMPs and Angptl4 (40). Therefore, Six2 likely regulates other pathways that contribute to its ability to mediate metastasis.
Loss of the epithelial marker E-cadherin accompanied by increased expression of mesenchymal molecules is considered a hallmark of the epithelial to mesenchymal transition (EMT). Cancer cells that undergo EMT are thought to gain migratory and invasive capabilities, which can facilitate the spreading of tumor cells and contribute to metastasis. In this study, we demonstrate that Six2 represses E-cadherin expression and that this downregulation is important for Six2-mediated metastasis. This finding is in contrast to what we have previously observed with SIX1 overexpression in MCF7 cells, which does not lead to downregulation of E-cadherin, but rather leads to relocalization of E-cadherin away from the cell membrane and into the cytoplasm (3). Therefore, although Six1 and Six2 share highly conserved domains for DNA and cofactor binding, Six1 and Six2 may regulate tumor progression and metastasis by slightly different means. Of note, the additional C-terminal activation domain of Six2 (Six1 does not contain a C-terminal activation domain) has been implicated in activation of Gdnf and of its own promoter in vitro (41), suggesting that Six2 may not require the co-activator Eya to activate transcription and that this difference in activation activity may in part explain different functions of Six2 and Six1. Alternatively, interaction with different cofactors many influence DNA binding of the two proteins. Analysis of gene regulation by Six1 and Six2 using ChIP-seq as well as RNA-seq may shed light onto both similarities and differences in the gene programs that the two transcription factors regulate.
It has recently been reported that high levels of SIX1 predict adverse outcomes particularly in the luminal B subtype of breast cancer (28), which have a high proliferative index. Analysis of 1881 breast cancer samples in multiple breast cancer datasets demonstrated that in contrast to SIX1, SIX2 expression correlates with poor prognosis specifically in luminal A subtypes of breast cancer. Based on these observations, it is interesting to speculate that SIX1 may thus be more involved in subtypes of breast cancer that are highly proliferative, and SIX2 may not be important in promoting tumor cell proliferation. Luminal A breast cancers make up 40% of all breast cancers, and while patients with luminal A breast cancers have a better prognosis, high SIX2 expression may identify those patients who are more likely to succumb to metastatic disease. It should be noted that examination of the Yu-multi-cancer database led to the discovery that in addition to breast cancer, SIX2 expression is increased in lung carcinoma and esophageal cancer compared to corresponding normal tissue (Supplemental Fig. 1B); however, whether Six2 influences the progression of other cancers remains to be determined.
Epigenetic therapies are emerging as new means to reactivate tumor-suppressor genes and there are some promising results in preclinical studies (42). In breast cancer cell lines, it has been demonstrated that demethylating agents can sensitize cells to chemotherapy or irradiation (43, 44). In addition, azacytidine is currently in clinical trials for advanced breast cancers in combination with other therapies (National Cancer Institute, NCI). In this study we demonstrated, using an immune-competent mouse model, that restoration of E-cadherin downstream of Six2 can abrogate Six2-induced metastasis and increase survival. The fact that E-cadherin expression can be reactivated by 5Aza treatment in the Six2 expressing cells raises the interesting possibility that inhibitors of DNA methylation may be more efficacious in breast cancer patients with SIX2 overexpression.
Supplementary Material
Acknowledgments
The authors acknowledge the University of Colorado Comprehensive Cancer Center (P30CA046934), which supports numerous core facilities used to carry out this work, including the Functional Genomics, Genomics, Animal Imaging, Flow Cytometry, and Tissue Biobanking and Processing Shared Resources. This work was funded by grants from the NCI (R01CA157790 and R01CA095277) to H.L. Ford. C.-A. Wang was funded by a pre-doctoral fellowship from the Department of Defense Breast Cancer Research Program (W81ZWH-10-1-0162) and a postdoctoral fellowship from the Cancer League of Colorado, Inc. David Drasin was funded from a National Research Service Award from the NCI (1 F31 CA165617).
Footnotes
Conflict of interest statement: H.L. Ford reports receiving a commercial research STTR grant from NIH 1R41CA180347 and a BDEG (Bioscience Discovery Evaluation grant). No potential conflicts of interest were disclosed by the other authors.
References
- 1.Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Adv Cancer Res. 2008;101:93–126. doi: 10.1016/S0065-230X(08)00405-3. [DOI] [PubMed] [Google Scholar]
- 2.Coletta RD, Christensen K, Reichenberger KJ, et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci U S A. 2004;101:6478–83. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micalizzi DS, Christensen KL, Jedlicka P, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009;119:2678–90. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CA, Jedlicka P, Patrick AN, et al. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J Clin Invest. 2012;122:1895–906. doi: 10.1172/JCI59858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy AJ, Pierce J, de Caestecker C, et al. SIX2 and CITED1, markers of nephronic progenitor self-renewal, remain active in primitive elements of Wilms’ tumor. J Pediatr Surg. 2012;47:1239–49. doi: 10.1016/j.jpedsurg.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senanayake U, Koller K, Pichler M, et al. The pluripotent renal stem cell regulator SIX2 is activated in renal neoplasms and influences cellular proliferation and migration. Hum Pathol. 2012;44:336–45. doi: 10.1016/j.humpath.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Self M, Lagutin OV, Bowling B, et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–28. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, Valerius MT, Mugford JW, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–81. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 10.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 11.van Horssen R, Hollestelle A, Rens JA, Eggermont AM, Schutte M, Ten Hagen TL. E-cadherin promotor methylation and mutation are inversely related to motility capacity of breast cancer cells. Breast Cancer Res Treat. 2012;136:365–77. doi: 10.1007/s10549-012-2261-8. [DOI] [PubMed] [Google Scholar]
- 12.St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–71. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day ML, Zhao X, Vallorosi CJ, et al. E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. J Biol Chem. 1999;274:9656–64. doi: 10.1074/jbc.274.14.9656. [DOI] [PubMed] [Google Scholar]
- 14.Derksen PW, Liu X, Saridin F, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–49. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22:2423–35. doi: 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105:394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- 17.Umbas R, Isaacs WB, Bringuier PP, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–33. [PubMed] [Google Scholar]
- 18.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Tillo E, Liu Y, de Barrios O, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–56. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graff JR, Herman JG, Lapidus RG, et al. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 1995;55:5195–9. [PubMed] [Google Scholar]
- 21.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2009;126:2575–83. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–56. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Zhao J, Zhang PY, et al. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit. 2012;18:BR299–308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: at the crossroads of signal transduction in cancer. Int J Cancer. 2012;132:745–54. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto T, Nigam SK. Cell-cell dissociation upon epithelial cell scattering requires a step mediated by the proteasome. J Biol Chem. 1999;274:24579–84. doi: 10.1074/jbc.274.35.24579. [DOI] [PubMed] [Google Scholar]
- 26.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 27.Ringner M, Fredlund E, Hakkinen J, Borg A, Staaf J. GOBO: gene expression-based outcome for breast cancer online. PLoS One. 2011;6:e17911. doi: 10.1371/journal.pone.0017911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwanaga R, Wang CA, Micalizzi DS, et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res. 2012;14:R100. doi: 10.1186/bcr3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori S, Chang JT, Andrechek ER, et al. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28:2796–805. doi: 10.1038/onc.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Tillo E, Siles L, de Barrios O, et al. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am J Cancer Res. 2011;1:897–912. [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Q, Yu J, Dhanasekaran SM, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–84. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzis C, Pusztai L, Valero V, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305:1873–81. doi: 10.1001/jama.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivshina AV, George J, Senko O, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 34.Senanayake U, Koller K, Pichler M, et al. The pluripotent renal stem cell regulator SIX2 is activated in renal neoplasms and influences cellular proliferation and migration. Hum Pathol. 44:336–45. doi: 10.1016/j.humpath.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 36.Ono H, Imoto I, Kozaki K, et al. SIX1 promotes epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation. Oncogene. 2012;31:4923–34. doi: 10.1038/onc.2011.646. [DOI] [PubMed] [Google Scholar]
- 37.Cieply B, Farris J, Denvir J, Ford HL, Frisch SM. Epithelial-mesenchymal transition and tumor suppression are controlled by a reciprocal feedback loop between ZEB1 and Grainyhead-like-2. Cancer Res. 2013;73:6299–309. doi: 10.1158/0008-5472.CAN-12-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinnadurai G. Transcriptional regulation by C-terminal binding proteins. Int J Biochem Cell Biol. 2007;39:1593–607. doi: 10.1016/j.biocel.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–87. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 41.Brodbeck S, Besenbeck B, Englert C. The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev. 2004;121:1211–22. doi: 10.1016/j.mod.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 43.Mirza S, Sharma G, Pandya P, Ralhan R. Demethylating agent 5-aza-2-deoxycytidine enhances susceptibility of breast cancer cells to anticancer agents. Mol Cell Biochem. 2010;342:101–9. doi: 10.1007/s11010-010-0473-y. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Zhang Y, Li R, et al. 5-aza-2′-Deoxycytidine enhances the radiosensitivity of breast cancer cells. Cancer Biother Radiopharm. 2012;28:34–44. doi: 10.1089/cbr.2012.1170. [DOI] [PubMed] [Google Scholar]
- 45.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.