Abstract
All-lymphoid progenitors (ALPs) yield few myeloid cells in vivo, but readily generate such cells in vitro. The basis for this difference remains unknown. We hypothesized that ALPs limit responsiveness to in vivo concentrations of myeloid-promoting cytokines by reducing expression of the corresponding receptors, potentially through post-transcriptional mechanisms. Consistent with such a mechanism, ALPs express higher levels of Csf1r transcripts than their upstream precursors, yet show limited cell surface protein expression of CSF1R. ALPs and other hematopoietic progenitors deficient in ADAM17, a metalloprotease that can cleave CSF1R, display elevated cell surface CSF1R expression. Adam17−/− ALPs, however, fail to yield myeloid cells upon transplantation into irradiated recipients. Moreover, Adam17−/− ALPs yield fewer macrophages in vitro than control ALPs at high concentrations of M-CSF. Mice with hematopoietic-specific deletion of Adam17 have grossly normal numbers of myeloid and lymphoid progenitors and mature cells in vivo. These data demonstrate that ADAM17 limits CSF1R protein expression on hematopoietic progenitors, but that compensatory mechanisms prevent elevated CSF1R levels from altering lymphoid progenitor potential.
Introduction
Hematopoietic stem cells traverse through a series of developmental intermediates, termed progenitors, en route to lineage commitment and maturation [1]. As differentiation progresses, these progenitors lose their ability to undergo self-renewing divisions. At specific developmental branchpoints, progenitors also lose their ability to generate specific subsets of mature blood lineages. At each of these branches, these progenitors are considered to be committed to the remaining blood lineages that they can still generate.
Complicating the definition and analysis of lineage commitment, in vivo assays can yield different results than in vitro experiments [2]. For example, common- or all-lymphoid progenitors (CLPs or ALPs) that yield primarily lymphocytes and dendritic cells in vivo can readily generate macrophages and neutrophils in vitro with high efficiencies [2–10]. These data demonstrate that CLPs and ALPs have not epigenetically silenced their myeloid programs [11], yet myeloid cells are infrequently generated from these progenitors under physiological conditions [4]. Thus, there is substantial disagreement on whether CLPs and ALPs should be considered lymphoid-committed.
As highlighted by this disagreement, the mechanisms by which lymphoid progenitors limit myeloid output in vivo remain incompletely understood. One possibility is that lymphoid progenitors home to distinct "niches" in vivo in which local concentrations of myeloid cytokines are low [12, 13]. Another non-mutually exclusive possibility is that ALPs reduce the expression of myeloid cytokine receptors such that they are unresponsive to the in vivo concentrations of such factors. Yet by providing excess amounts of myeloid lineage-promoting cytokines in vitro, lymphoid progenitors can still generate macrophages and neutrophils. Indeed, ectopic expression of certain cytokine receptors allows for robust myeloid cell production by lymphoid progenitors [14]. Endogenous cytokine receptor expression can be regulated both by transcriptional and post-transcriptional mechanisms. For example, the metalloprotease ADAM17 can cleave CSF1R, an essential and instructive cytokine receptor for M-CSF which mediates macrophage commitment and homeostasis [15–19].
ADAM17 belongs to a family of metalloproteases with broad target specificities and essential roles in many biological processes. ADAM17 is best known for its role in cell-intrinsic processing of TNFα to its secreted form, and is often referred to as TNFα-converting enzyme (TACE) [20, 21]. However, many studies have identified other ADAM17 targets in addition to TNFα, including CSF1R [18]. Although the role of ADAM17 in mature myeloid cells and responses to bacterial endotoxin challenge has been studied [22–26], to our knowledge there have been no reports describing its function during hematopoietic development. Given similar roles in other cell types, we hypothesized that ADAM17 limits CSF1R expression on lymphoid progenitors, thereby preventing macrophage and granulocyte production in vivo. Here we demonstrate that ALPs express Csf1r transcripts, and that ADAM17 does indeed limit cell surface expression of CSF1R on ALPs and other hematopoietic progenitors. Yet despite its role in limiting CSF1R on the surface of ALPs, ADAM17 is not required for preventing myeloid cell production by lymphoid progenitors in vivo.
Materials and Methods
Mice
Adam17fl/f [22], Vav1-iCre [27], C57BL/6, and B6.SJL mice were originally purchased from The Jackson Laboratory and subsequently housed and maintained in our animal care facility. The genotype of ADAM17 knockout mice in all experiments was ADAM17fl/fl Vav1-iCre+ while wild type mice were ADAM17fl/+ Vav1-iCre− or ADAM17fl/fl Vav1-iCre−. All studies were carried out according to the Institutional Animal Care and Use Committee at Washington University.
Microarray and quantitative real-time PCR
Csf1r expression levels were analyzed in LMPP and ALP from previously published microarrays [5]. For quantitative real-time PCR analysis, cells were double-sorted into TRIzol reagent (Invitrogen) using a BD FACSAria (BD Biosciences). SuperScript III First Strand Kit (Invitrogen) was used to generate cDNA using random hexamers per the manufacturer's instructions. SybrGreen PCR master mix (Applied Biosystems) was used for real-time PCR assays per the manufacturer's instructions. The ABI 7000 Sequence Detection System (Applied Biosystems) was used to quantify expression. Primer sequences were: Csf1r 5'-ACACGCACGGGCCACCATGAA-3': and 5'-GCATGGACCGTGAGGATGAGGC-3' and Gapdh, 5-GGCAAATTCAACGGCACAGT-3′ and 5-GATGGTGATGGGCTTCCC-3′.
M-CSF ELISA assay
Epiphyses were removed and dissected femurs from wild type mice were flushed with 1 ml PBS. Cells were pelleted, and M-CSF levels were quantified from bone marrow supernatant or blood serum using a mouse M-CSF ELISA kit as per manufacturer's instructions (Sigma). Bone marrow M-CSF concentrations were calculated by dividing the total amount of M-CSF in the bone marrow supernatant by the marrow volume of a mouse femur, estimated to be 9.4µl. This estimate is based upon the approximations that a mouse femur is a normal cylinder, the cross-sectional marrow radius r is 0.46mm [28], the length l is 15mm [28], and that in turn the volume can be calculated as πr2l.
Flow cytometry and cell sorting
Staining buffer consisted of 2% adult bovine serum (Hyclone)/PBS with 1mM EDTA. Dead cells were gated out using propidium iodide (Sigma-Aldrich). Cells were acquired and sorted on the FACSAria (BD Biosciences) or analyzed on a LSRII (BD Biosciences). Data were analyzed using FlowJo software (TreeStar). For a list of antibodies used in the experiments, see Supplemental Table 1
In vitro differentiation assay
500 ALPs were double-sorted into culture media that consisted of 10% Defined FBS (Hyclone) in DME-F12 + 10 mM Hepes (SAFC Biosciences) and supplemented with NEAA (Lonza), Sodium pyruvate (Lonza), penicillin/streptomycin (Sigma-Aldrich), Glutamax (Invitrogen), and 50µM 2-mercaptoethanol (Invitrogen). M-CSF (Peprotech) was added at the indicated concentration. Cells were cultured for 4 days before staining and flow cytometric analysis on a BD LSRII. To quantify total numbers of cells, software acquisition and recording was initiated before the sample was loaded and continued until the sample was completely consumed and no additional live events were observed. Viability was quantified by the percentage of cells incorporating propidium iodide.
In vivo differentiation assay
5000 ADAM17 wild type or knockout ALPs (CD45.2+) were double-sorted into PBS and then injected into each 800 cGy-irradiated B6.SJL (CD45.1+) recipient mice via retro-orbital injection. Ten days post-injection, bone marrow and spleens were harvested and mechanically dissociated in staining buffer. Cells were stained and analyzed as described in Results.
Results
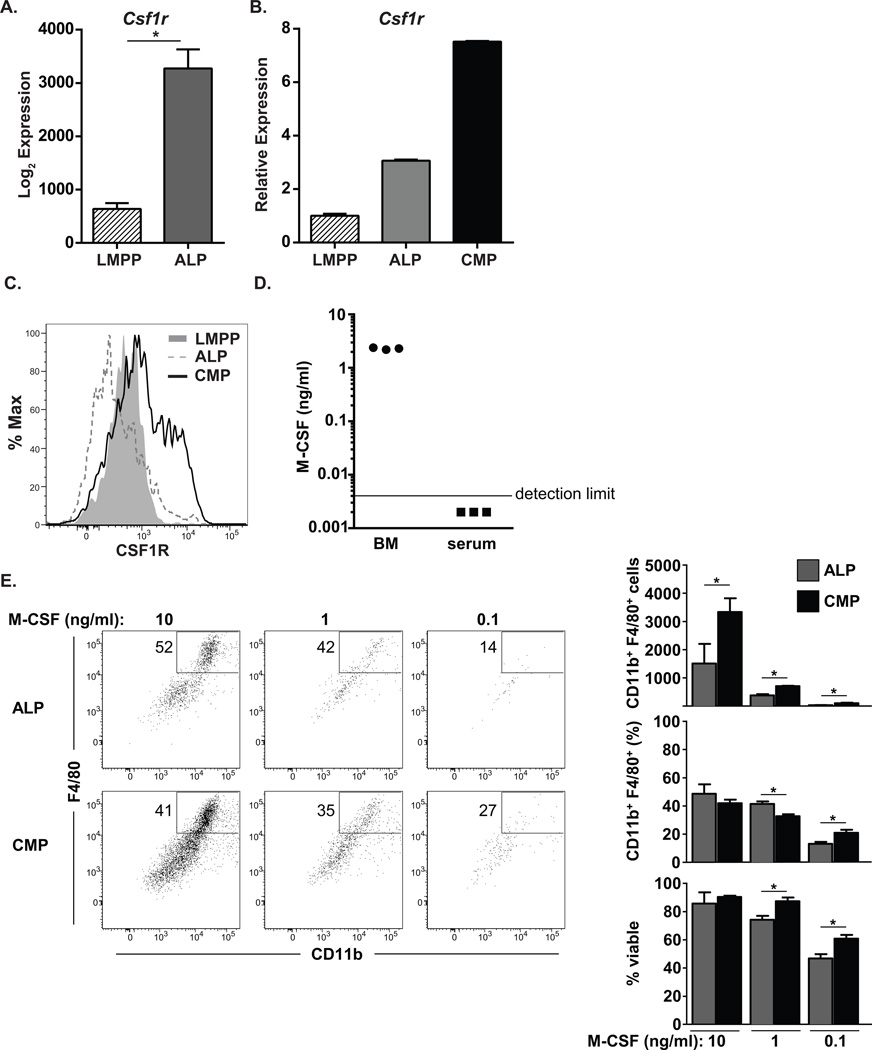
Cytokine signaling in hematopoietic progenitors can play an instructive role in directing fate decisions [14, 17, 19, 29]. Thus, we hypothesized that lymphoid progenitors display reduced expression of myeloid-promoting cytokine receptors relative to their uncommitted multipotent precursors. To test this hypothesis, we examined global gene expression profiles of ALPs, which generate only lymphocytes in vivo [5], and lymphoid-primed multipotent progenitors (LMPPs), which can generate macrophages in vivo through CSF1R-expressing progeny [5, 30–32]. Unexpectedly, the expression of CSF1R, which promotes monocyte and macrophage development [15], was increased in ALPs relative to LMPPs (Fig. 1A). This increase was also observed through quantitative RT-PCR analysis (Fig. 1B). Thus, ALPs express more Csf1r transcripts than do LMPPs, but less than do common myeloid progenitors (CMPs) (Fig. 1B).
Figure 1. ALPs express low levels of CSF1R and differentiate into monocytes in vitro with exogenous M-CSF administration.
(A) Microarray data obtained from Inlay et al. [5] showing log2 expression values for Csf1r. Signals from probeset 1419872_at are shown. Three biologically distinct samples were analyzed for each population. (B) Quantitative real-time PCR analysis of Csf1r for LMPPs, ALPs, and CMPs. Csf1r expression was normalized to Gapdh expression. Data are representative of 2 independent experiments. (C) Flow cytometry plots show the surface levels of CSF1R expressed on LMPPs, ALPs, and CMPs. These data are representative of 3 independent experiments. (D) ELISA analysis of bone marrow (BM) and serum M-CSF levels. Femurs were flushed with 1 ml of PBS, levels of M-CSF were quantified in the supernatant, and BM concentrations were estimated assuming a femur volume of 9.4µl (see Materials and Methods for details). Data are cumulative from two independent experiments. (E) Flow cytometric analysis of ALP output. 500 ALPs or CMPs were double-sorted and cultured in the presence of M-CSF for 4 days. Flow cytometric plots in the left panel depict representative data from ALP and CMP cultures. Values within the plots depict the percentage of F4/80+CD11b+ macrophages generated. Column graphs in the right panel show cumulative data for the absolute number or frequency of macrophages or the frequency of viable cells. Data are inclusive of 4 independent experiments and represent mean values ± SEM. * p ≤ 0.05, using students' 2-tailed unpaired t-test.
Protein levels of CSF1R can be regulated through several post-transcriptional mechanisms [33]. Therefore, increased transcript levels may not strictly correlate with increased cell surface protein levels or responsiveness to cytokines. To test cell surface expression of CSF1R in LMPPs and ALPs, we performed flow cytometric analysis. CSF1R surface expression was low on ALPs, similar to that seen in LMPPs and markedly less than that observed in CMPs (Fig. 1C, gating strategies shown in Supplemental Figure 1). Thus, ALPs use post-transcriptional mechanisms to limit CSF1R expression.
We hypothesized that the diminished levels of surface CSF1R on ALPs would render these cells insensitive to physiological concentrations of M-CSF. To quantify the endogenous levels of M-CSF, we performed ELISA analyses. These data demonstrated that the bone marrow M-CSF concentration is ~2 ng/ml (Fig. 1D). Interestingly, M-CSF was undetectable in the serum of these same animals (Fig. 1D). These data demonstrate that M-CSF levels are locally restricted. Local concentrations of M-CSF could potentially also vary greatly within distinct marrow regions from the average value of ~2 ng/ml. Thus, to determine if the low levels of cell surface CSF1R expression on ALPs would allow for macrophage development, we cultured purified ALPs in the presence of a broad range of M-CSF concentrations. ALPs readily generated macrophages at high concentrations (10ng/ml) of M-CSF, but this output fell sharply at lower doses (Fig. 1E). At all doses, CMPs generated more macrophages than did ALPs, potentially due to higher levels of surface CSF1R expression (Fig. 1E). The proportion of macrophages generated was comparable between ALPs and CMPs, with some small differences at the lower concentrations of M-CSF (Fig. 1E). Lowering the concentration of M-CSF led to a progressive decline in viability, although CMPs were less sensitive to death than were ALPs (Fig. 1E). These data demonstrate that ALPs express relatively little cell surface CSF1R despite transcription, and robustly respond to M-CSF only at high doses.
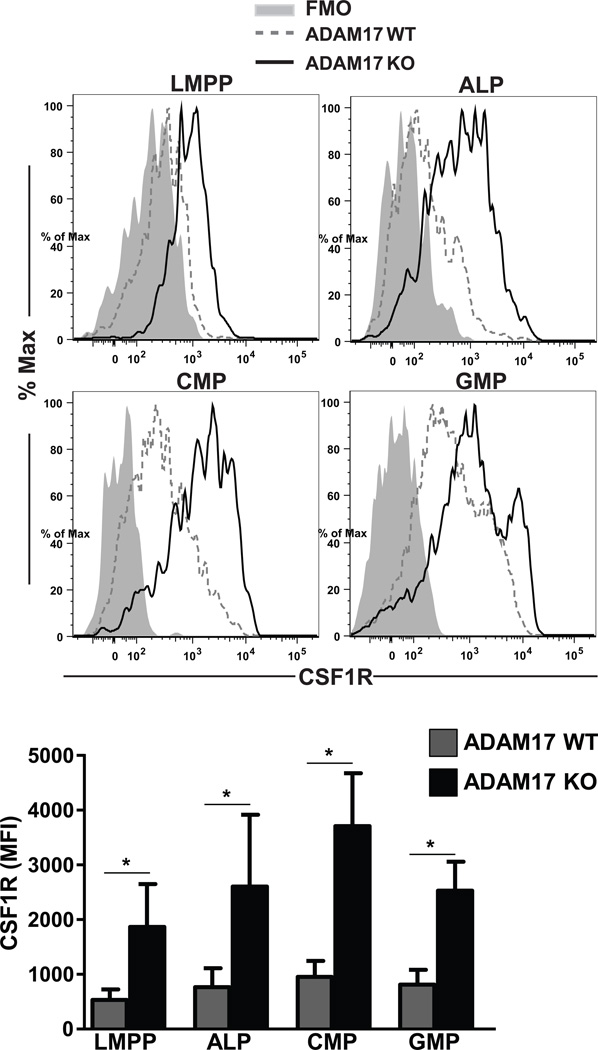
Previous studies have shown that the metalloprotease ADAM17 can cleave CSF1R protein in activated macrophages [18]. We thus hypothesized that a similar mechanism limits CSF1R expression in ALPs. To test this hypothesis, we generated Adam17fl/fl × Vav1-iCre mice, which selectively lack ADAM17 in the hematopoietic compartment [24]. ALPs and other progenitors were then assessed for cell surface CSF1R levels. Indeed, ALPs, and also granulocyte macrophage progenitors (GMPs), CMPs, and LMPPs all showed elevated expression of surface CSF1R (Fig. 2, gating strategies shown in Supplemental Figure 1). These data demonstrate that hematopoietic-intrinsic ADAM17 limits the expression of CSF1R in vivo.
Figure 2. ADAM17 regulates the surface expression of CSF1R on hematopoietic progenitor cells.
Flow cytometric analysis of CSF1R expression on wild type and ADAM17-deficient progenitors. Histograms are representative plots from three independent experiments. Bottom panels show an average of CSF1R mean fluorescence intensities (MFI) ± SEM for the same three experiments. * p ≤ 0.05, using students' 2-tailed unpaired t-test.
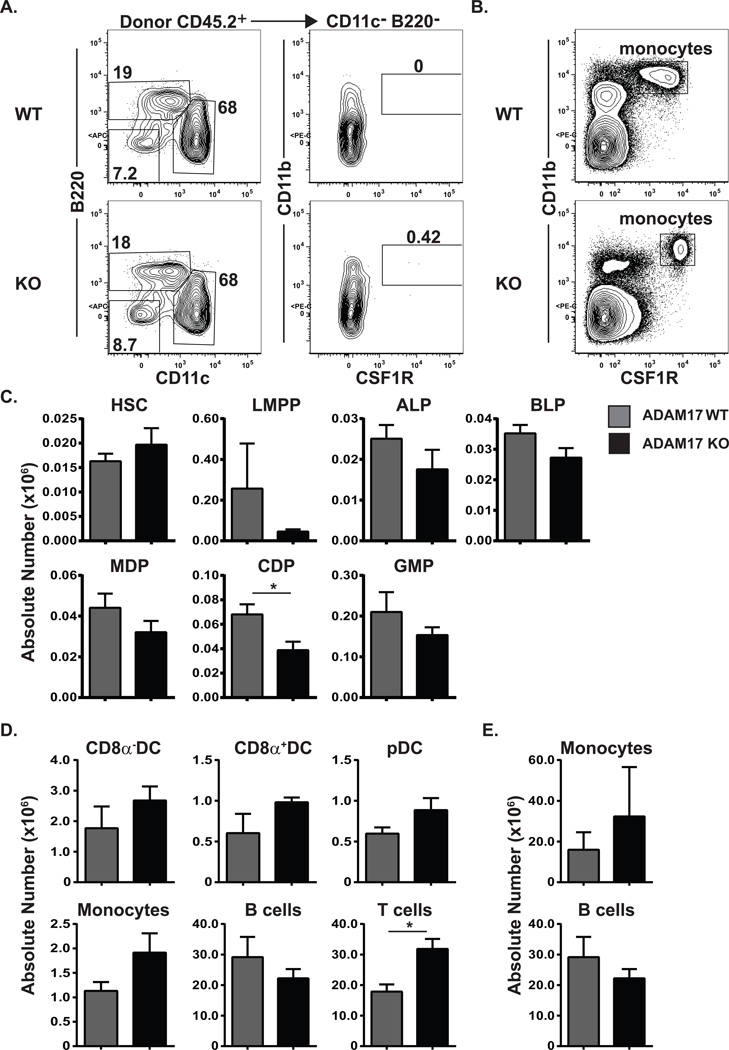
We next sought to determine the functional consequences of ADAM17-deficiency on ALP behavior in vivo. ALPs were purified from Adam17fl/fl × Vav1-Cre or control littermates and transferred into sublethally-irradiated recipients. For both wild type and ADAM17-deficient ALPs, mean splenic donor chimerism was identical at 1.9% (data not shown), and B cells and dendritic cells were readily generated from ALPs of both genotypes (Fig. 3A). However, we observed no evidence of monocyte production by ADAM17-deficient ALPs (Fig.3A). At this same timepoint, CMPs readily generate monocytes [31, 34]. We cannot exclude the possibility that ALPs generate mature cells such as monocytes with much different kinetics than do CMPs. However this possibility seems unlikely, as both of these progenitors yield mature dendritic cells with similar kinetics [34–36]. As monocytes are commonly identified using CSF1R expression as a marker, we were concerned that this strategy would not be faithful for ADAM17-deficient monocytes. To address this, we examined wild type and ADAM17-deficient splenocytes. Although ADAM17-deficient monocytes did indeed express elevated levels of CSF1R and required slightly different gates for quantification, they could still be readily identified (Fig. 3B). A similar proportion of wild type and ADAM17-deficient monocytes expressed Ly6C (data not shown), a marker of inflammatory monocytes, thus further validating the gating strategy. Therefore, in this adoptive transfer system, elevated levels of CSF1R are not sufficient to confer in vivo myeloid potential to ALPs.
Figure 3. ADAM17 deficient ALPs do not produce monocytes in vivo.
(A) Flow cytometric analysis of ALP output in vivo. 5000 ALPs were double-sorted from ADAM17 wild type or knockout mice. Cells were injected into sublethally irradiated congenic recipients. Ten days post-injection, spleens were harvested and analyzed for donor-derived (CD45.2+CD45.1−) monocytes (B220−CD11c− CD11b+CSF1R+), B cells (B220+), and dendritic cells (CD11c+). (B) Flow cytometric analysis of CSF1R expression on splenic monocytes (pregated on B220− Ly6G− CD11c− cells) from ADAM17 wild type or knockout mice. (C–E) Flow cytometric analysis of hematopoietic stem and progenitor cells (C) or mature progeny (D, E) in the bone marrow (C, E) or spleens (D) of ADAM17 wild type or knockout mice. Bar graphs show the mean absolute number cells in each tissue ± SEM. Data are cumulative from three independent experiments. * p ≤ 0.05, using students' 2-tailed unpaired t-test.
Because irradiation can alter in vivo concentrations of cytokines and homing properties of progenitors [37–39], we next sought to determine if ADAM17-deficiency led to any changes in the numbers of progenitors or mature cells under steady-state conditions. Despite elevated surface levels of CSF1R, no significant defects were observed in the numbers of lymphoid or myeloid progenitors in ADAM17-deficient animals aside from a modest reduction in CDPs (Fig. 3C, gating strategies shown in Supplemental Figure 1). Similarly, no defects were observed in the numbers of mature dendritic cells, monocytes, or lymphocytes in the spleen (Fig. 3D) or bone marrow (Fig. 3E), except for a slight increase in splenic Adam17−/− T cell numbers (gating strategies shown in Supplemental Figure 2).
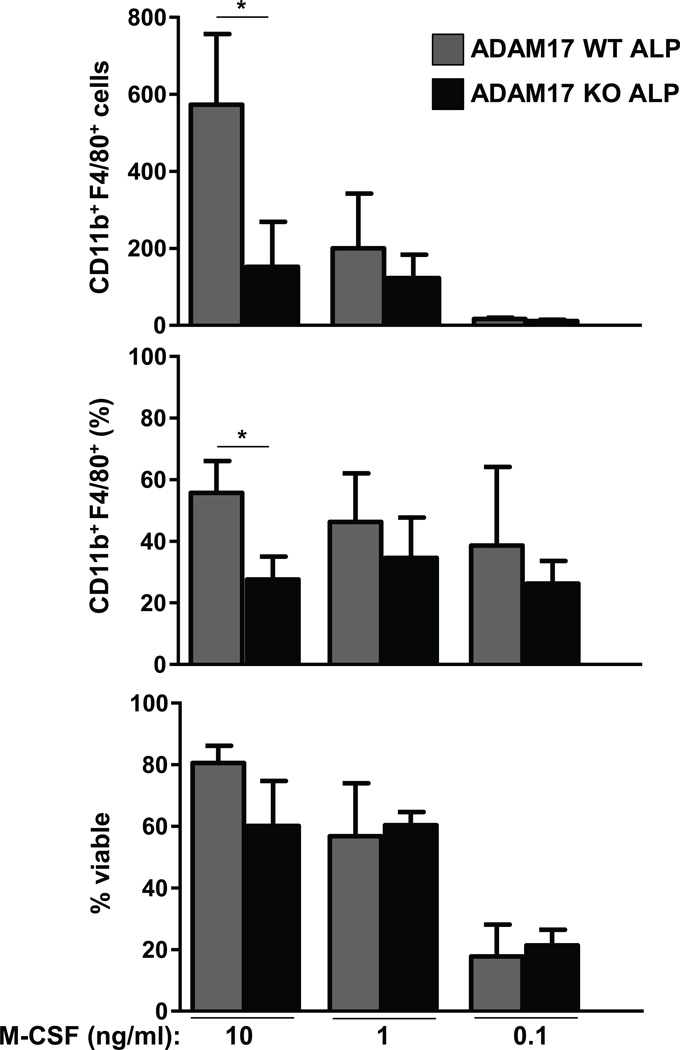
To explore the basis for the lack of dramatic in vivo effects of ADAM17-deficiency, we compared the in vitro responsiveness of wild type and Adam17−/− ALPs to M-CSF. Unexpectedly, ADAM17-deficient ALPs yielded fewer macrophages in vitro than did wild type ALPs at the highest doses of M-CSF (Fig. 4), despite expressing higher levels of CSF1R (Fig. 2). At lower M-CSF doses, the numbers and frequencies of macrophages generated were similar between wild type and ADAM17-deficient ALPs (Fig. 4). Overall viability was also similar between genotypes at all doses of M-CSF (Fig. 4, bottom panel). We cannot exclude the possibility that a distinct proteolytic target of ADAM17 somehow prevents monocyte and macrophage development. However, given that M-CSF is the only cytokine included in these in vitro cultures, our data suggest that excessive M-CSF signaling may either prevent the differentiation of macrophages from lymphoid progenitors or selectively kill those which have already formed. Thus, negative feedback signaling through CSF1R may prevent major changes from occurring in vivo in ADAM17-deficient animals. This may also help explain the modest reduction in ADAM17-deficient CDPs (Fig. 3C), which already express high levels of CSF1R even in ADAM17-sufficient animals [40, 41]. Together, these data demonstrate that although ADAM17 limits CSF1R expression in progenitors, it is not necessary for lymphoid lineage commitment or myeloid cell homeostasis under steady-state conditions.
Figure 4. ADAM17-deficient ALPs yield few macrophages at high M-CSF doses.
Flow cytometric analysis of wild type and Adam17−/− ALPs in vitro. 500 ALPs from wild type or ADAM17-deficient donors were cultured in the presence of varying concentrations of M-CSF for 4 days. CD11b+ F4/80+ macrophages and propidium iodide+ dead cells were quantified by flow cytometry. Three ADAM17-deficient animals and wild type siblings were analyzed. Bar graphs show mean values ± SEM. *p<0.05, using students 2-tailed t-test.
Discussion
Fate decisions during hematopoietic differentiation are regulated by complex interactions between cell-extrinsic and cell-intrinsic cues. At certain intermediates, progenitor cell differentiation in vivo is often more restricted than their epigenetic profiles or in vitro potentials might predict [2, 11]. In these cases, it is likely that extrinsic factors dictate cellular outcomes in vivo, either through instructive actions or by selectively permitting the survival or proliferation of specific downstream lineages. By tuning their sensitivities to these extrinsic factors, progenitors could regulate outcomes in vivo.
We hypothesized that one way in which lymphoid progenitors regulate their sensitivities to myeloid cytokines is by ADAM17-mediated cleavage of CSF1R. Indeed, CSF1R surface levels were significantly higher in Adam17−/− ALPs and other progenitors relative to their Adam17+/+ counterparts. Thus it seemed reasonable to expect that cells expressing elevated CSF1R levels would increase macrophage or monocyte production in vivo. Contrary to our hypothesis, however, we observed no differences in Adam17−/− ALP output in vivo compared to controls, and there were no major differences in mature cell subsets in the spleen or bone marrow. This may in part be attributable to negative feedback inhibition of CSF1R signaling, as Adam17−/− ALPs generated relatively few macrophages at the highest doses of M-CSF in vitro compared to Adam17+/+ ALPs.
The physiological importance of elevated CSF1R transcription in ALPs relative to LMPPs is unclear, as is the latent myeloid potential of these cells. Lymphoid progenitors can alter their lineage output when exposed to pathogen-associated molecular patterns, such as Toll-like receptor ligands [42]. By maintaining a reservoir of Csf1r transcripts, it is possible that ALPs can rapidly contribute to emergency myelopoesis upon systemic infection. Yet under steady-state conditions, this monocyte and macrophage potential is not utilized.
The full mechanisms by which lymphoid progenitors restrict myeloid output in vivo thus remain unresolved. Specific lymphoid niches and consequent restriction of access to M-CSF are possible explanations as to why ALPs generate so few myeloid cells in vivo [13, 43–45]. Our data are consistent with this mechanism, and justify further studies on specialized niches and cytokine gradients in vivo.
Supplementary Material
Highlights.
-
1)
Lymphoid progenitors yield macrophages in vitro robustly only at high doses of M-CSF.
-
2)
Lymphoid and other progenitors limit CSF1R through post-transcriptional mechanisms.
-
3)
Adam17−/− lymphoid and other progenitors have elevated CSF1R cell surface expression.
-
4)
Adam17−/− lymphoid progenitors yield few macrophages in vitro at high doses of M-CSF.
-
5)
ADAM17-deficiency does not alter lymphoid potential or myeloid homeostasis in vivo.
Acknowledgements
We thank E. Lantelme and D. Brinja for assistance with flow cytometry and L. Hursey for animal colony maintenance.
Support:
D.B. is a New York Stem Cell Foundation Robertson Investigator. This work was supported by National Institutes of Health grants T32CA009547 (A.M.B.) and K01DK078318 (D.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Declaration:
The authors have no competing financial interests.
References
- 1.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. The American journal of pathology. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrlich LI, Serwold T, Weissman IL. In vitro assays misrepresent in vivo lineage potentials of murine lymphoid progenitors. Blood. 2010 doi: 10.1182/blood-2010-05-287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rumfelt LL, Zhou Y, Rowley BM, Shinton SA, Hardy RR. Lineage specification and plasticity in CD19- early B cell precursors. The Journal of experimental medicine. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlenner SM, Madan V, Busch K, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Inlay MA, Bhattacharya D, Sahoo D, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes & development. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008 doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 8.Hao QL, Zhu J, Price MA, Payne KJ, Barsky LW, Crooks GM. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 2001;97:3683–3690. doi: 10.1182/blood.v97.12.3683. [DOI] [PubMed] [Google Scholar]
- 9.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 10.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nature immunology. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Ehrlich LI, Seita J, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Lai AY, Watanabe A, O'Brien T, Kondo M. Pertussis toxin-sensitive G proteins regulate lymphoid lineage specification in multipotent hematopoietic progenitors. Blood. 2009;113:5757–5764. doi: 10.1182/blood-2009-01-201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo M, Scherer DC, Miyamoto T, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 15.Dai XM, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Sarrazin S, Mossadegh-Keller N, Fukao T, et al. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell. 2009;138:300–313. doi: 10.1016/j.cell.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 17.Mossadegh-Keller N, Sarrazin S, Kandalla PK, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovida E, Paccagnini A, Del Rosso M, Peschon J, Dello Sbarba P. TNF-alpha-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J Immunol. 2001;166:1583–1589. doi: 10.4049/jimmunol.166.3.1583. [DOI] [PubMed] [Google Scholar]
- 19.Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 20.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 21.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi K, Kimura T, Miyamoto T, et al. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007;179:2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wu J, Newton R, Bahaie NS, Long C, Walcheck B. ADAM17 cleaves CD16b (FcgammaRIIIb) in human neutrophils. Biochimica et biophysica acta. 2013;1833:680–685. doi: 10.1016/j.bbamcr.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long C, Wang Y, Herrera AH, Horiuchi K, Walcheck B. In vivo role of leukocyte ADAM17 in the inflammatory and host responses during E. coli-mediated peritonitis. Journal of leukocyte biology. 2010;87:1097–1101. doi: 10.1189/jlb.1109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long C, Hosseinkhani MR, Wang Y, Sriramarao P, Walcheck B. ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. Journal of leukocyte biology. 2012;92:667–672. doi: 10.1189/jlb.0312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arndt PG, Strahan B, Wang Y, Long C, Horiuchi K, Walcheck B. Leukocyte ADAM17 regulates acute pulmonary inflammation. PloS one. 2011;6:e19938. doi: 10.1371/journal.pone.0019938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 28.Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Molecular and cellular biology. 2010;30:711–721. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi K, Kasai H, Watanabe A, Lai AY, Kondo M. IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression. J Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Fogg DK, Sibon C, Miled C, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 32.Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell stem cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horiuchi K, Miyamoto T, Takaishi H, et al. Cell surface colony-stimulating factor 1 can be cleaved by TNF-alpha converting enzyme or endocytosed in a clathrin-dependent manner. J Immunol. 2007;179:6715–6724. doi: 10.4049/jimmunol.179.10.6715. [DOI] [PubMed] [Google Scholar]
- 34.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa F, Niiro H, Iino T, et al. The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007;110:3591–3660. doi: 10.1182/blood-2007-02-071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 37.Mazo IB, Quackenbush EJ, Lowe JB, von Andrian UH. Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow. Blood. 2002;99:4182–4191. doi: 10.1182/blood.v99.11.4182. [DOI] [PubMed] [Google Scholar]
- 38.Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. The Journal of clinical investigation. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xun C, Brown SA, Jennings CD, Henslee-Downey PJ, Thompson JS. Acute graft-versus-host-like disease induced by transplantation of human activated natural killer cells into SCID mice. Transplantation. 1993;56:409–417. doi: 10.1097/00007890-199308000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nature immunology. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 41.Naik SH, Sathe P, Park HY, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nature immunology. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 42.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.