Abstract
Galectin-3 is a family member of the carbohydrate binding proteins widely expressed by many cell types and exhibits multiple cellular functions. We demonstrate that melanocytes express galectin-3, which is predominantly localized to the cell body peripherally along the Golgi zone. Downregulation of galectin-3 in human melanocytes using shRNA technology resulted in reduction of both melanin synthesis and expression/activity of Tyrp-1. In the cell body, galectin-3 co-localizes with melanosome destined cargo, specifically tyrosinase and tyrosinase-related protein-1. We studied melanocytes cultured from patients with forms of Hermansky-Pudlak syndrome containing defects in trafficking steps governed by BLOC-2 (HPS5), BLOC-3 (HPS1) and adaptin-3 (HPS2). We found galectin-3 expression mimicked the defective expression of the tyrosinase cargo in dendrites of HPS-5 melanocytes, but was not altered in HPS1 or HPS2 melanocytes. In addition, galectin-3 co-localized predominantly with the HPS-5 component of BLOC-2 in normal human melanocytes. These data indicate that galectin-3 is a regulatory component in melanin synthesis affecting the expression of Tyrp-1.
Keywords: Melanization, Chaperones, Carbohydrate-Binding Protein, Cargo, Melanin, tyrosinase
INTRODUCTION
The melanocyte synthesizes a premelanosome organelle derived from the endosomal system within the cell (Marks & Seabra, 2001). Subsequently, several enzymes (specifically tyrosinase, Tyrp-1 and Tyrp-2) and regulatory proteins responsible for converting tyrosine to melanins are trafficked from the Golgi apparatus, through the endosomal system, and targeted for incorporation to the premelanosome (Raposo & Marks, 2007; Boissy, Huizing & Gahl, 2006). Predominate chaperones that facilitate this trafficking process are the family of protein complexes called BLOCs (Biogenesis of Lysosome-related Organelles Complex) (DiPietro, Falcón-Pérez, Tenza et al, 2006; Dell’Angelica, 2004). Many of the subunits of these BLOCs are products of genes that when mutated result in Hermansky-Pudlak Syndrome (HPS) (Wei, 2006; DiPietro & Dell’Angelica, 2005; Bonifacino, 2004). BLOC-2 is composed of at least the HPS-3, 5 and 6 proteins (DiPietro, Falcón-Pérez, & Dell’Angelica, 2004) and BLOC-3 is composed of at least the HPS-1 and 4 proteins (Kloer, Rojas, Ivan et al, 2010; Nazarian, Falcón-Pérez, & Dell’Angelica, 2003). When mutated, each of these HPS proteins compromise the integrity of their respective BLOCs and impairs efficient trafficking of the requisite enzyme to the melanosome in a distinctive fashion resulting in reduced melanin synthesis and cutaneous and ocular hypopigmentation (Boissy, Huizing & Gahl, 2006; Richmond, Huizing, Knapp et al, 2005). The molecular mechanisms utilized by these BLOCs to recruit and shuttle melanosome destined cargo remains unclear. However, two small GTPase, Rab32 and Rab38, have recently been shown to cooperate with BLOC-2 and/or BLOC-3 in trafficking of melanogenic enzymes (Marks, 2012; Bultema and DiPietro, 2013). Also unknown are additional protein and regulatory components that may be part of or participate with BLOCs.
Galectin-3 is a member of carbohydrate-binding proteins that interact primarily with β-galactoside residues of cell surface and extracellular matrix glycoprotein molecules (Dumic, Dabelic & Flögel, 2006; Wang, Gray, Haudek et al, 2004). By virtue of this property, galectin-3 has been implicated in cell-cell and cellsubstrate recognition. In addition, it has been proposed that galectin-3 may function as a chaperone involved in intracellular trafficking of cytosolic glycoproteins in various cell types (Delacour, Koch & Jacob, 2009; Vagin, Kraut, & Sachs, 2009; Delacour, Cramm-Behrens, Drobecq et al, 2006; Liu, Patterson & Wang, 2002). Expression of galectin-3 by the melanocytes has not been reported. However, brief mention of galectin-3 being a possible component of the melanosome was demonstrated in melanosomes purified using sucrose density gradient centrifugation and subsequently analyzed by protein digestion and mass spectrometry (Basrur, Yang, Kushimoto et al, 2003).
In this report, we demonstrate that galectin-3 is expressed by melanocytes, regulates melanogenesis, maintains expression of Tyrp-1, and co-localizes to chaperones upstream of the melanosome, particularly HPS-5, in the melanocyte cell body. These data implicate galectin-3 as a regulator of melanization by maintaining Tyrp-1.
RESULTS
Expression of galectin-3 by epidermal cells
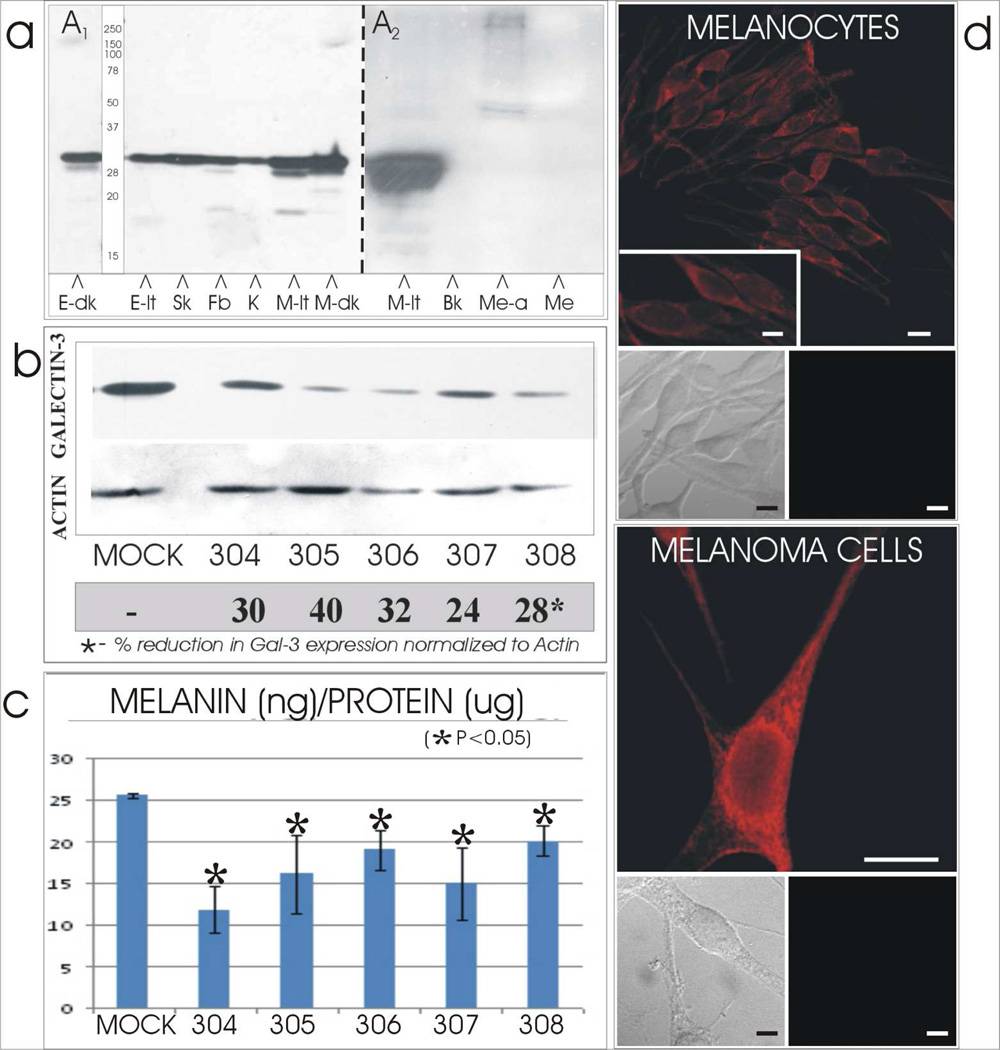
Galectin-3 was identified at the expected molecular weight of 30 kDa in cultured melanocytes from both light and dark skinned individuals (Figure 1A1). Galectin-3 was also identified in an established line of human melanoma cells, cultured human keratinocytes and fibroblasts, and in epidermal lysates from dark and light skin. The 30 kDa galectin-3 molecule expressed by cultured melanocytes was not found to be secreted into the media (Figure 1A2).
Figure 1.
[A] Expression and secretion of galectin-3 by cutaneous cells and tissues. [A1] Galectin-3 of 30 kDa was identified by immunocytochemistry in lysates of dark (E-dk) and light (E-lt) neonatal foreskins and in cultured cells of skMel melanoma (Sk), dermal fibroblast (Fb), keratinocytes (K) and melanocytes derived from light (M-lt) and dark (M-dk) skin. [A2] Media collected from light skin derived melanocytes before (Me) or after (Me-a) acetone precipitation did not exhibit the 30 kDa galectin-3. However the latter did exhibit a 45–50 kDa triplet and a 250 kDa doublet. [Bk = blank lane]
[B & C] Silencing of galectin-3 results in reduced melanin synthesis. Cultured melanocytes derived from one light skin was transfected with control shRNA (Mock) or one of six galectin-3 shRNA (304-8). Amount of [B] galectin-3 protein and [C] melanin content were reduced in thegalectin-3 silenced melanocyte lines by 24–40% and 20–50%, respectively, compared to the Mock transfectants in all melanocyte lines.
[D] Cellular profile of galectin-3. Cultured normal human melanocytes and skMEL melanoma cells demonstrated a prominent perinuclear localization of galectin-3 and minimal dendritic localization. Doublet insets below the immunofluorescent micrographs represent phase (left) and corresponding fluorescent (right) images of cells stained with the galectin-3 primary antibody omitted and secondary used to demonstrate lack of non-specific staining. N=nucleus; C=cytoplasm. Bars = 10 microns; inserts = 5 microns.
Silencing of galectin-3 results in reduced melanin synthesis
Cultures of normal human melanocytes derived from a dark, medium and light skins were silenced for galectin-3 expression by transfection with several shRNAs as described in Materials and Methods. The dark line (1800 ng melanin/ug protein) and the medium line (800 ng melanin/ug protein) were silenced using the Santa Cruz shRNA (supplement Figure 1) and the light line (25 ng melanin/ug protein) was silenced using the SigmaTRCN clones (Figure 1B). Galectin-3 was dramatically reduced in all cell lines by 25–40%. Concurrently, melanin amount was significantly reduced in all melanocyte lines by 20–50% compared to the control shRNA transfectants (Figure 1C).
Cellular profile of galectin-3
In normal human melanocytes and melanoma cells, galectin-3 is expressed in a granular pattern that is prominent within the cell body and moderate along the dendrites, while being relatively devoid in the nucleus (Figure 1D). There is a distinctive lattice like and vesicular profile in the cell body that resembles the location of the endoplasmic reticulum and endosomes, suggesting that galectin-3 could participate in trafficking of glycoproteins in this significant area.
Tyrp-1 expression and activity is reduced in galectin-3 silenced NHM
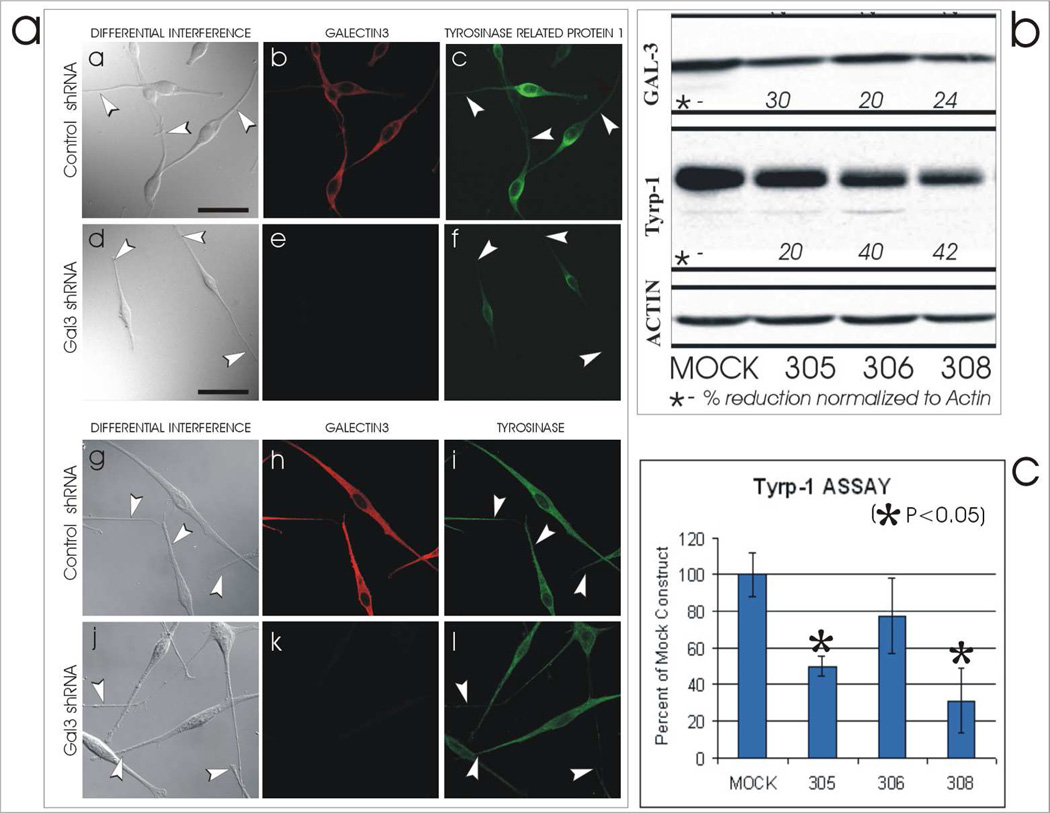
Cultures of normal human melanocytes derived from light skin were silenced for galectin-3 expression by shRNA transfection as described in Materials and Methods and presented in Figure 1. Control shRNA and Gal3 shRNA transfectants exhibited and lacked galectin-3 expression, respectively (Figure 2Ab & e). In addition, control shRNA and Gal3 shRNA transfectants demonstrate abundant and reduced expression of tyrosinase related protein-1 (Tyrp-1) throughout the cell, respectively (Figure 2Ac & f). In contrast, expression of tyrosinase was not altered in control shRNA and Gal3 shRNA transfectants (Figure 2Ag–l). Signal intensity per cell representing expression of Tyrp-1 in control shRNA and Gal3 shRNA transfectants was 17×104±15 and 1.9×104±0.4, respectively (P=0.021). For tyrosinase expression, signal intensity values were 116×104±19 and 157×104±20, respectively (P=0.111).
Figure 2.
Galectin-3 influences Tyrp-1 expression but not that of tyrosinase. [A] Cultured melanocytes derived from light skins were transfected with control shRNA [Control shRNA] or galectin-3 shRNA [Gal3 shRNA] and co-labeled by immunofluorescence for galectin-3 and Tyrp-1 or tyrosinase. AmouhiRNA transfectants (a & c), was reduced in the Gal3 shRNA tranfectants (d & f). In contrast, tyrosinase expression was similar in both the shRNA transfectants (g & i) and the Gal3 shRNA transfectants (j & l). Bars = 40 microns.
[B] Cultured melanocytes transfected with control (Mock) or three galectin-3 (305,306, & 308) shRNAs demonstrating a reduction inexpression of galectin-3 (ranging between 20–30%) and Tyrp-1 (ranging between 20–42%) by silencing.
[C] Cell lines represented in [B] demonstrating a concurrent reduction in the catalytic activity of human Tyrp-1.
A fourth round of silencing on a light NHM line was performed using three of the SigmaTRCN clones and assessed for expression of Tyrp-1 and tyrosinase. Expression of galectin-3 was reduced by 20–30% (Figure 2B), Tyrp-1 by 20–42% (Figure 2B) and tyrosinase (not shown) was unchanged. Concurrently the tyrosine hydroxylase activity specific for human Tyrp-1 was significantly reduced in the galectin-3 silenced NHM (Figure 2C). These results demonstrate that expression and/or maintenance of Tyrp-1, as oppose to tyrosinase, was dependent on galectin-3.
Colocalization of galectin-3 with melanosome destined cargo, (i.e., tyrosinase and Tyrp-1)
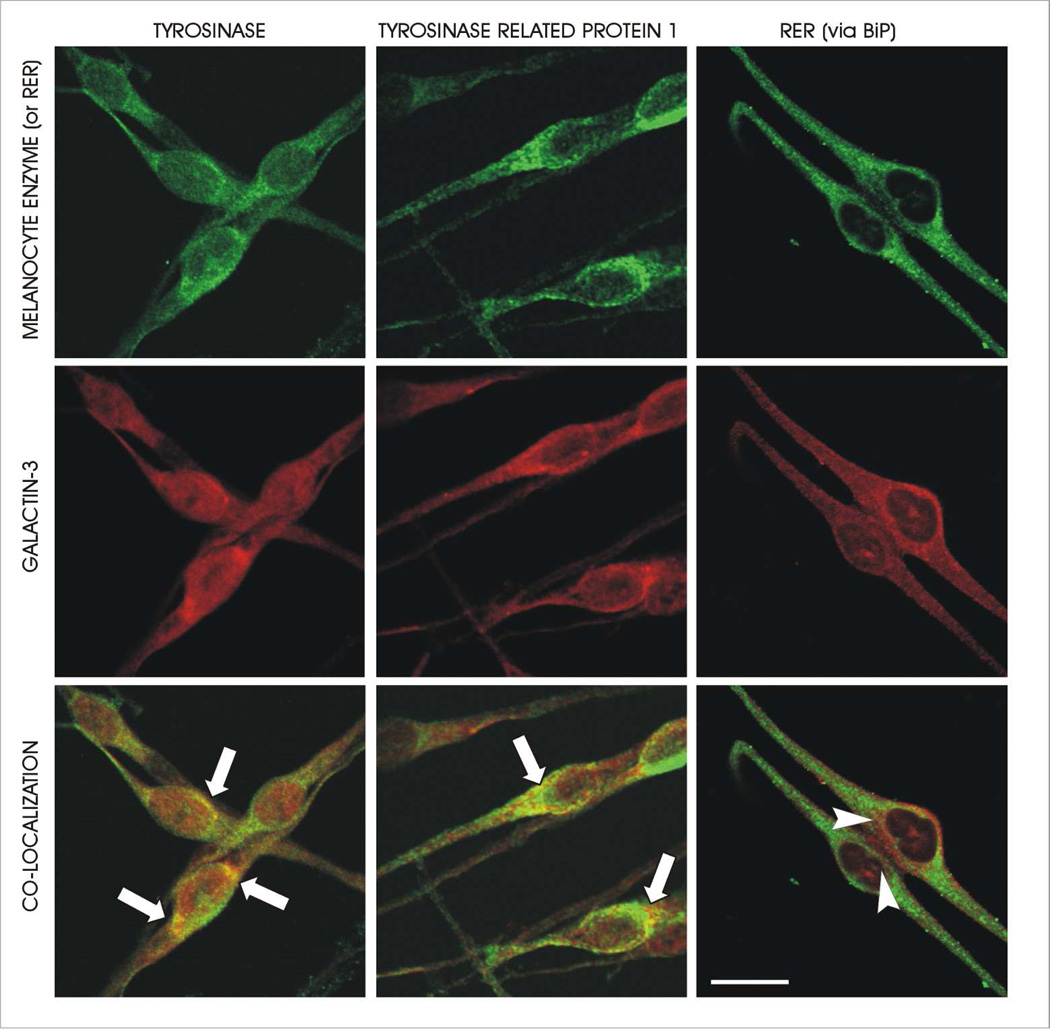
Galectin-3 was found to colocalize with both tyrosinase and tyrosinase related protein-1 (Tyrp-1) in normal human melanocytes (Figure 3). Both tyrosinase and tyrosinase related protein-1 had similar colocalization patterns with galectin-3. The Mander’s coefficient quantifying these colocalizations within the cell body is presented in Table 1. In contrast, colocalization of galectin-3 with the rough endoplasmic reticulum and the nucleus was relatively minimal. The colocalization of galectin-3 with Tyrp-1 and tyrosinase occurs lateral to the Golgi, where Golgi-derived cargo vesicles containing tyrosinase and Tyrp-1 fuse with either endosomes or stage II melanosomes. Galectin-3 also seemed to be somewhat restricted from the perinuclear Golgi zone. Additionally, colocalization between galectin-3 and both tyrosinase and Tyrp-1 does not appear to occur in the dendrites of the melanocytes. This pattern of colocalization between galectin-3 and the two melanocyte specific enzymes suggests that galectin-3 may be involved in the trafficking of these enzymes to melanosomes.
Figure 3.
Galectin-3 co-localizes with melanosome destined cargo. Galectin-3 co-localizes with both tyrosinase (left panel) and tyrosinase related protein-1 (middle panel). This co-localization is prominent in the peri-Golgi area (arrows) and does not occur in the dendrites. In contrast, the co-localization of galectin-3 with the RER (right panel) appeared minimal. G=Golgi. Bars = 30 microns.
TABLE 1.
Quantification of galectin-3 co-expression with tyrosinase and Tyrp-1
| CELL TYPE | CELL AREA | CO-LOCALIZED PROTEINS |
MANDER’S COEFFICIENT* |
|---|---|---|---|
| NHM | Cell body | Galectin-3 & Tyrp-1 | 0.945±0.04*1, |
| NHM | Cell body | Galectin-3 & tyrosinase | 0.977±0.01 |
| NHM | Cell body | Galectin-3 & RER | 0.732±0.02*2 |
| NHM | Cell body | Galectin-3 & nucleus | 0.277+0.01*2 |
| NHM | Entire cell | Galectin-3 & tyrosinase | 0.856±0.038 |
| HPS 1 (BLOC3) | Entire cell | “ | 0.647±0.116*3 |
| HPS 2 (adaptin-3) | Entire cell | “ | 0.693±0.062*3 |
| HPS 5 (BLOC2) | Entire cell | “ | 0.864±0.101 |
Mander’s Overlap coefficient ranges between 1 and zero with 1 being high-colocalisation and zero being low.
Average plus standard deviation.
P<0.05 compared to Galectin-3 & Tyrp-1 in Cell body of NHM
P<0.05 compared to Galectin-3 & tyrosinase in Entire cell of NHM
Colocalization of galectin-3 with melanosome destined proteins in BLOC2, BLOC3, and Adaptin-3 deficient HPS melanocytes
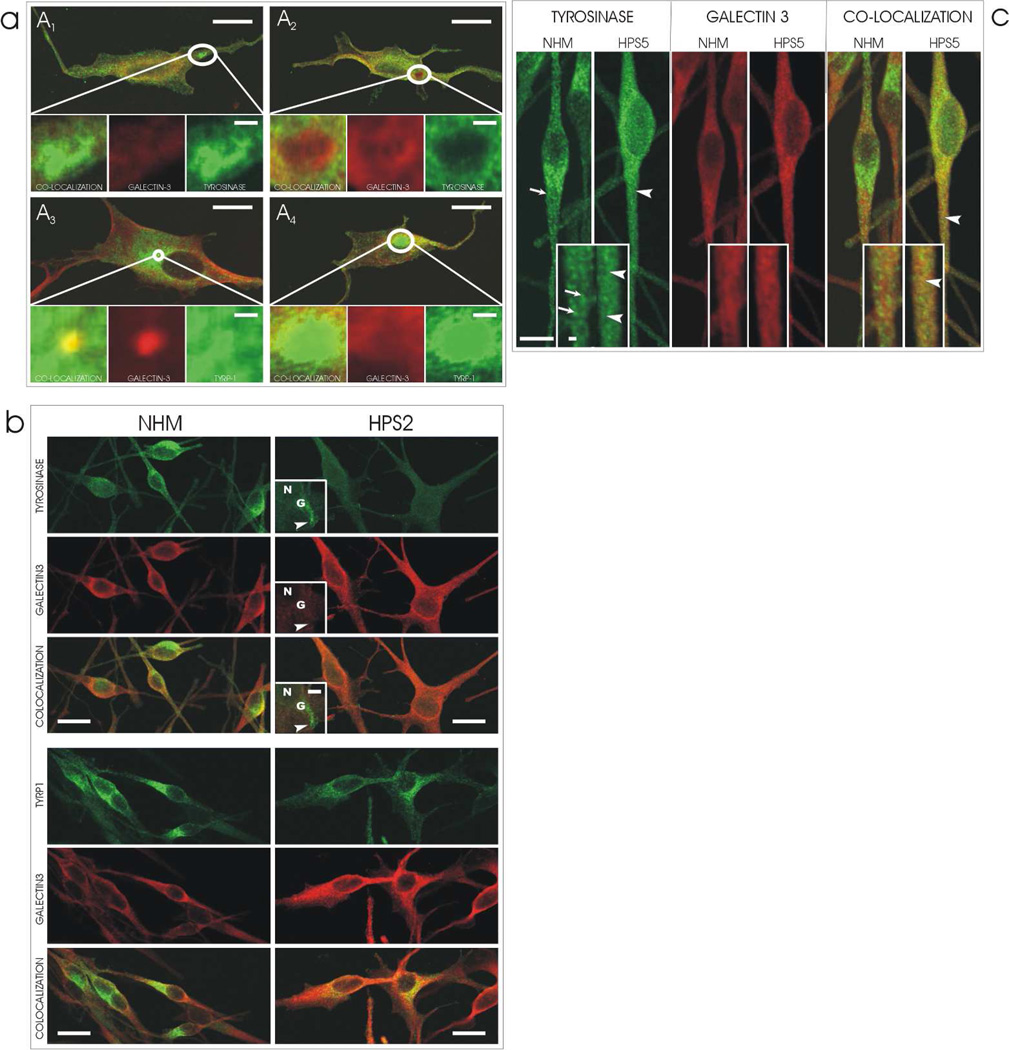
HPS-1 melanocytes contain genetic mutations that prevent the normal functioning of the BLOC-3 chaperone. These mutations cause the HPS-1 melanocytes to express macroautophagosomes to which tyrosinase and tyrosinase related protein-1 are targeted to because of their ineffective trafficking to melanosomes (Smith, Koshoffer, Morris et al, 2005). In HPS-1 melanocytes, galectin-3 demonstrated varied amounts of colocalization with melanocyte specific proteins in HPS-1 macroautophagosomes (Figure 4A). There was either dramatic, moderate, or no colocalization observed in various cells. This suggests that galectin-3 probably does not uniquely function in the BLOC-3 regulatory step in trafficking, but may be incorporated into macroautophagosomes by default. In support of this subjective evaluation, the Mander’s coefficient demonstrates that the colocalization of Galectin-3 with tyrosinase throughout the entire cell is lower in the HPS-1 cells as oppose to control NHM (Table 1).
Figure 4.
[A] Galectin-3 expression in HPS-1 melanocytes. In cultured human melanocytes from a patient with HPS-1, galectin-3 exhibits a variation in its localization to macroautophagosomes consisting of [A1] no, [A2 & A4] minimal, or [A3] prominent localization, respectively. In addition, the co-localization of galectin-3 with either tyrosinase or tyrosinase related protein-1 (TYRP-1) in the macroautophagosomes also varied consisting of [A1 & A2] no, [A4] minimal, or [A3] dramatic colocalization, respectively. Bars = 10 microns; inserts = 3 microns.
[B] Galectin-3 expression in HPS-2 melanocytes. In cultured human melanocytes from a patient with HPS-2, galectin-3 expression is restricted from the HPS-2 specific macroautophagosomes that accumulate tyrosinase (arrowhead). In contrast, co-localization of Galectin-3 with tyrosinase related protein-1 (TYRP1) is normal, i.e., prevalent around the perinuclear area as depicted in Figure 2. N=nucleus, G=Golgi zone. Bar = 10 microns; inserts = 5 microns.
[C] Galectin-3 expression in HPS-5 melanocytes. [Left doublet] In cultured human melanocytes from a patient with HPS-5, tyrosinase exhibits the characteristic fine (arrowheads) as oppose to granular (arrows) staining pattern present in normal human melanocytes (NHM), particularly noticeable in the dendrites. [Center doublet] Minimal expression of galectin-3 in the dendrites of both cultured NHM and HPS-5 melanocytes was intensified for observation by increasing the gain of the laser during image capture. [Right doubled] In normal human melanocytes there is minimal colozalization go galectin-3 and tyrosinase. In contrast, in HPS-5 melanocytes, there is co-localization between galectin-3 and tyrosinase in a fine pattern (arrowheads) containing of 50 nm vesicles as oppose to a granular pattern in NHM. Bars = 10 microns; insets = 2 microns.
HPS-2 melanocytes contain genetic mutations that affect the functioning of the adaptin-3 chaperone. These mutations cause HPS-2 melanocytes to exhibit normal expression of tyrosinase related protein-1 (Tyrp-1) and a drastically reduced expression of tyrosinase, which is mainly localized to multivesicular endosomes (Huizing, Sarangarajan, Strovel et al, 2001). In HPS-2 melanocytes, galectin-3 exhibited a normal colocalization pattern with Tyrp-1 in the peri-Golgi zone (Figure 4B). Galectin-3 did not colocalize with tyrosinase outside of the Golgi zone in multivesicular endosomes. This suggests that galectin-3 is probably not involved in the adaptin-3 step of cargo trafficking. In support of this subjective evaluation, the Mander’s coefficient demonstrates that the colocalization of galectin-3 with tyrosinase is lower in the HPS-2 cells as oppose to control NHM (Table 1).
HPS-5 melanocytes contain genetic mutations that affect the normal function of the BLOC-2 chaperone. These mutations cause HPS-5 melanocytes to retain tyrosinase and tyrosinase related protein-1 (Tyrp-1) in many cargo vesicles that become distributed through the dendrites and impart a fine granular pattern rather than the normal course granular fluorescence pattern in the dendrites (Helip-Wooley, Westbroek, Dorward et al, 2007; Boissy, Richmond, Huizing et al, 2005). In HPS-5 melanocytes, galectin-3 also demonstrated the abnormal fine granular fluorescent pattern resembling cargo vesicles and colocalization with tyrosinase in the dendrites (Figure 4C). In support of this subjective evaluation, the Mander’s coefficient demonstrates the colocalization of galectin-3 with tyrosinase did not differ between the HPS-5 and the control NHM, in contrast to the difference in HPS-1 and HPS-2 (Table 1). This suggests that galectin-3 may participate in BLOC-2 regulated trafficking step that is thought to function in the recognition, docking, and fusion of cargo vesicles with premelanosomes.
Colocalization of galectin-3 with BLOC-2 and BLOC-3
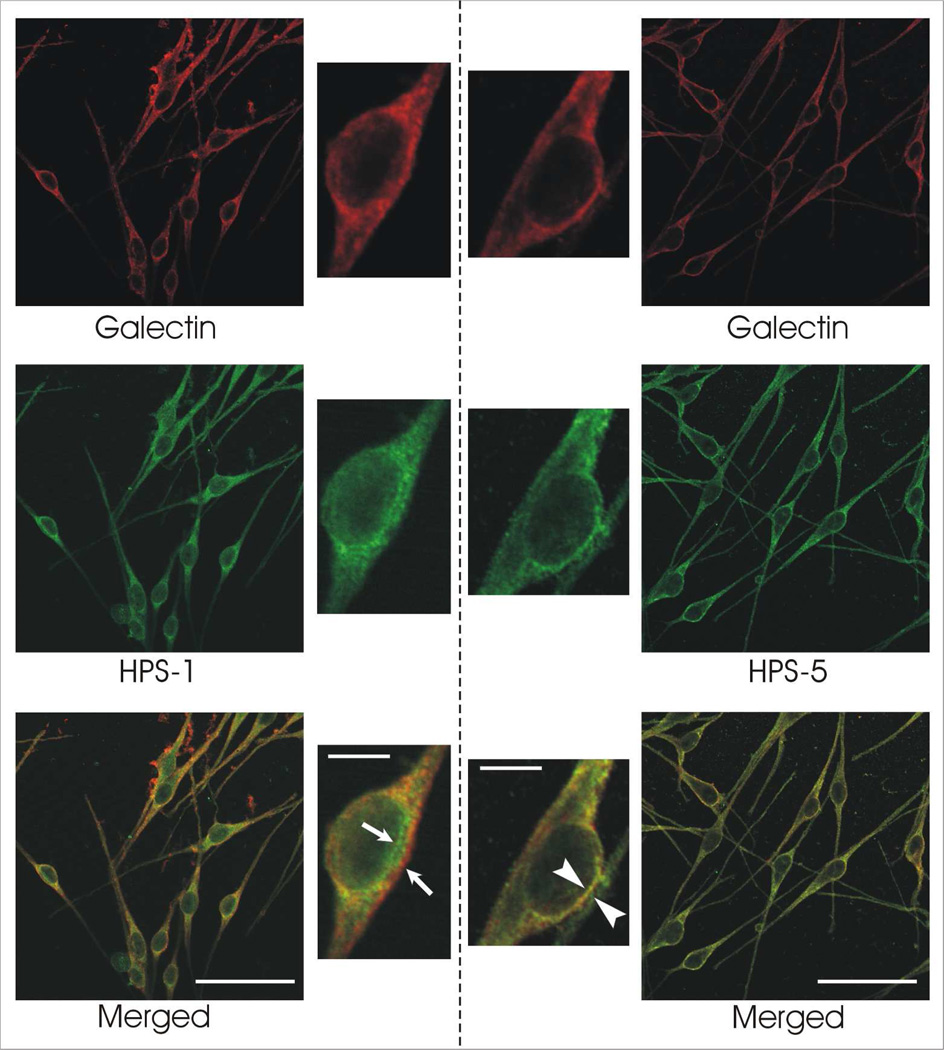
With immunocytochemistry, galectin-3 appears to colocalize with a greater extent, but not exclusively, to the BLOC-2 component HPS-5 compared to the BLOC-3 component HPS-1. This is seen by a greater overall area of yellow regions in the HPS-5 compared to the HPS-1 melanocytes (Figure 5). This was confirmed by the quantitative analysis of the images using MetaMorph (Table 2, supplementary material). In the latter study, the analysis of the colocalization was restricted to the Golgi and peri-Golgi areas of the melanocytes. The percentages determined by MetaMorph analysis were used in a t-test to confirm a significant difference in the colocalization between galectin-3 and HPS-1 versus HPS-5. MetaMorph analysis confirmed the increase in colocalization between galectin-3 and HPS-5 compared to the colocalization between galectin-3 and HPS-1 (Table 2, supplementary material). Since HPS-5 is a component of BLOC-2 and HPS-1 of BLOC-3, this suggests that galectin-3 may participate in BLOC-2 regulated trafficking, which functions in the recognition, docking and fusion of cargo vesicles with premelanosomes, rather than BLOC-3 regulated trafficking.
Figure 5.
Galectin-3 appears to co-localize more prominently with the BLOC2 component HPS-5 than the BLOC3 component HPS-1. Immunocytochemical staining of normal human melanocytes for galectin-3 with either HPS-1 [left panel] or HPS-5 [right panel] demonstrated relatively minimal co-localization in the former (arrows) and abundant co-localization in the latter (arrowheads). Bars = 50 microns; insets = 10 microns.
DISCUSSION
Galectin-3 is a member of a family of carbohydrate-binding proteins that is widely expressed by cells and with multiple functions depending on the cell type (Wang, Gray, Haudek et al, 2004). Within the cell, galectin-3 can regulate cell growth, cell cycle, and apoptosis via its relation to known intracellular pathways associated with these processes (Liu, Patterson & Wang, 2002). An interesting and less explored cellular function of galectin-3 is the trafficking of glycoprotein cargo through the cell. In MDCK cells, galectin-3 contributes to delivering non-raft-dependent glycoproteins to the lumen of LPH-associated vesicles (LAVs) in a carbohydrate-dependant manner and depletion of galectin-3 results in missorting of the glycoprotein cargo (Delacour, Cramm-Behrens, Drobecq et al, 2006). In the trafficking of non-raft-independent glycoproteins, galectin-3 interacts directly with beta-galactoside moieties of the cargo resulting in cross-linked clusters to facilitate trafficking and targeting (Delacour, Greb, Koch et al, 2007).
It has previously been demonstrated that galectin-3 exists in the melanocytes (Basrur, Yang, Kushimoto et al 2003) as well as benign and malignant melanocytic lesions (Abdou, Hammam, Farargy et al, 2010; Braeuer et al, 2012). In the former report, galectin-3 was identified using melanosomes purified by sucrose density gradient centrifugation and subsequently analyzed by protein digestion and mass spectrometry (Basrur, Yang, Kushimoto et al 2003). In our present report, we confirmed that melanocytes express galectin-3 and exhibit a distinct localization pattern prevalent in the cell body peripheral to the Golgi zone. This positions galectin-3 to be a participant or chaperone in the trafficking of melanosome destined cargo from the Golgi to the premelanosome. Co-localization of galectin-3 with tyrosinase and Tyrp-1 at the periphery of the Golgi zone strengthens the hypothesis of this function.
BLOCs (Biogenesis of Lysosome-related Organelles Complex) have recently been identified as trafficking components in the biogenesis of lysosome related organelles including the melanosome (DiPietro, Falcón-Pérez, Tenza et al, 2006; Dell’Angelica, 2004). Mutations affecting the functions of BLOCs, as well as the chaperone adaptin-3, result in distinct forms of Hermansky-Pudlak Syndrome (HPS) (Boissy, Huizing & Gahl, 2006; Richmond, Huizing, Knapp et al, 2005). To further explore the role of galectin-3 in trafficking during the biogenesis of the melanosome, we analyzed the expression pattern in melanocytes cultured from patients with Hermansky-Pudlak Syndrome. As described above in the Methodology section, cultured melanocytes from each form of HPS exhibit distinct defects in the trafficking profile of melanosome destined cargo. Specifically, mutations affecting BLOC-3 (i.e., HPS-1 and 4) result in the accumulation of macroautophagosomes in the melanocytes where tyrosinase and Tyrp-1 are targeted (Smith, Koshoffer, Morris et al, 2005). Mutations affecting BLOC-2 (i.e., HPS-3, 5 and 6) result in the accumulation of 50 nm vesicles containing tyrosinase and Tyrp-1 cargo throughout the cell body and dendrites, with the inefficient incorporation of these enzymes into the melanosome (Helip-Wooley, Westbroek, Dorward et al, 2007; Boissy, Richmond, Huizing et al, 2005). Mutations affecting adaptin-3 (i.e., HPS-2) result in the accumulation of tyrosinase, but not Tyrp-1, in mulivescicular bodies of the perinuclear area (Huizing, Sarangarajan, Strovel et al, 2001). We rationalized that if galectin-3 participated in the trafficking step governed by either BLOC-3, BLOC-2 or adaptin3, an aberrant expression pattern for galactin-3 would result as well and mimic that of the associated cargo in melanocytes from the respective HPS form. The data demonstrated that only in BLOC-2 defective HPS-5 melanocytes does the co-localization of galectin-3 uniformly accompany that of the mistrafficked cargo. A recent report demonstrated that galectin-3 expression was increased in Hermansky-Pudlak syndrome pulmonary fibrosis (Cullinane, Yeager, Dorward et al. 2014).
To confirm that galectin-3 is predominantly associated with the BLOC-2 pathway, we performed quantitative analysis of the co-localization of galectin-3 with either BLOC-2 (via association with the HPS-5 component) versus BLOC-3 (via association with the HPS-1 component). This analysis supported the hypothesis that galectin-3 participates primarily, but not exclusively, in the BLOC-2 trafficking step. The inter-related roles of BLOCs and adaptins in shuttling cargo from the transGolgi cisterna, through various endosomal compartments and onto the premelanosome has yet to be clearly delineated. However, it is predicted that these chaperone modules work in tandem and/or concurrent pathway. It is not surprising that galectin-3 had some association with BLOC-3 in the universal mechanism of relaying cargo to the melanosome. Regardless we demonstrate in preliminary studies that downregulation of galectin-3 results in diminished melanin synthesis and a putative mechanism consists of the inefficient maintenance and/or targeting of as least Tyrp-1 but not tyrosinase to melanosome that are ultimately translocated down the dendrites.
Galectin-3 binds galactose and lactose residues on glycoproteins. Tyrosinase and Tyrp-1 are replete with these sugar residues to which galectin-3 could conceivably interact (García-Borrón & Solano, 2002). However, this is improbable since the sugar residues of Tyrp-1 and other cargo are sequestered in the lumen of the transport vesicles and melanosomes making them inaccessible to cytosolic galectin-3. Of interest is a report of an alternatively spliced form of galectin-3 that contains a single transmembrane-spanning region and a leucine zipper-like stalk domain that positions galectin-3 across the plasma membrane (Gorski et al, 2002). The doublet observed for galectin-3 on western analysis (Figure 1A1) may represent galectin-3 with and without the transmembrane domain.
Galectin-3 can also participate in protein-protein interactions (Liu, Patterson & Wang, 2002) and contains proline/glycine rich domain (PGAYPGXXX) known to be involved in protein binding (Pawson & Scott, 1997). Galectin-3 directly interacts with the carboxyl terminal 50 amino acids of Gemin4, a member of the SMN (survival of motor neuron) complex (Park, Voss, Grabski et al, 2001). Galectin-3 has been shown to bind Alix (Chen, Fermin, Vardhana et al, 2009), a component of ESCRT pathway required for the biogenesis of mulitvesicular bodies (Odorizzi, 2006). In addition, ESCRT is required for Tyrp-1 transport to the melanosomal membranes (Truschel et al, 2009), similar to BLOC-2. In conclusion, we demonstrate that galectin-3 in the melanocyte is a candidate chaperone molecule during melanosome biogenesis. Where and how galectin-3 molecularly interfaces with the cargo and known chaperones (i.e., ESCRT, BLOCs, adaptins, RABs, etc.) in this pathway needs to be explored.
MATERIAL AND METHODS
Cell Culturing
Human melanocyte cultures were established from individual neonatal foreskins obtained from The University Hospital in Cincinnati or from The Christ Hospital in Cincinnati (IRB #03-1-23-1). Patient consent for experiments was not required because French laws consider human tissue left over from surgery as discarded material. HPS cell cultures were established from skin biopsies provided by W. Gahl (NIH, Bethesda, MD) from patients enrolled in a protocol approved by the National Human Research Institute Review Board with written informed consent. This study was conducted according to the declaration of Helsinki Principles. Tissues were transported to the laboratory in DMEM with 2% antibiotic/antimycotic. Using routine sterile techniques, the melanocytes were isolated from the tissues as follows. Tissues were incubated in 0.25% trypsin at 4°C overnight. The epidermis was separated from the dermis by vortexing in PBS. The tissue was pelleted at 1500 rpm for 5 minutes at 4°C and the PBS supernatant was aspirated off. The epidermis and cell suspension were resuspended in either melanocyte or keratinocyte media, and the dermis was resuspended in fibroblast growth media and plated in a T25 corning tissue culture flask. The melanocyte growth media consisted of MCDB-153 media (Sigma-Aldrich Co., St. Louis, MO) supplemented with 4% fetal bovine serum (Fisher Scientific, Pittsburgh, PA), 5 µg/mL Insulin, 0.6 ng/mL basic fibroblast growth factor (PeproTech Inc, Rocky Hill, NJ), 13 µg/mL bovine pituitary extract (Hammond Cell Tech, Windsor, CA), 8 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) and 1% antibiotic/antimycotic (Fisher Scientific, Pittsburgh, PA). The keratinocyte growth medium consisted of KC Epilife supplemented with HKGS (Cascade Biologics Inc., Portland, OR) and 1% antibiotic/antimycotic. The fibroblast growth medium consisted of DMEM medium (Sigma, St Louis, MO) supplemented with 5% fetal bovine serum, 2mM glutamine, 0.5mM sodium pyruvate and 1% penicillin-streptomycin (10,000 units/ml and 10,000 mg/ml, respectively), (Gibco, Carlsbad, CA). SKMEL-188 (a gift from R. Srinivasan, Sloan-Kettering Institute for Cancer Research, New York, NY) were maintained in the DMEM medium supplemented with 5% fetal bovine serum, 2 mM L-glutamine (Fisher Scientific, Pittsburgh, PA), 1 mM sodium pyruvate (Invitrogen, Carlsbad, CA) and 1% antibiotic/antimycotic. All the above reagents were purchased from Sigma-Aldrich Co. unless otherwise stated. Cultures were maintained in a humidified incubator with 95% air and 5% CO2 at 37°C. Subconfluent melanocytes and fibroblasts at passages 2–7 and keratinocytes at passages 1–2 were used for all experiments.
Western Blotting
Cells were lysed in RIPA extraction buffer and analyzed for their protein content. Each lane on a 10% acrylaminde gel was loaded with 30 ug of protein. After electrophoresis and transfer, the blot was blocked in 5% milk in TNET (TBS-tween) overnight, stained with amido black and destained, and immunoblotted with rabbit anti-Galectin-3 (1:2,000) (Novus Biologicals, Littleton, CO), mouse anti-Tyrp-1 (Mel 5, Covance, Princeton, NJ), or rabbit anti-actin HRP conjugated (Santa Cruz), for 2 hours. The blot was washed 3 times in TNET buffer, incubated in goat anti rabbit HRP in 5% milk in TNET (1:5,000 Santa Cruz) and washed twice with a final rinse in TBS. The blot was then developed using a Pierce ECL kit in a dark room.
To assess for secretion of galectin-3, media from normal human melanocyte cultures was extracted and saved while the cells were trypsinized and pelleted. 1 mL of media was put into a centrifuge tube along with 4 mL of −20°C acetone. The tube was vortexed and stored for 1 hour in a −20°C freezer. The tube was centrifuged for 10 minutes at 13,000 rpm at 4°C. The supernatant was removed without dislodging the protein pellet and the extra acetone was allowed to evaporate from the uncapped tube in a fume hood at room temperature for 30 minutes. 100 ul of RIPA+PI buffer was added to the pellet and it was vortexed to bring it to solution. This acetone precipitated sample was then used in a western blot as described above.
Silencing methodology
Norman human melanocytes were plated in 6 well plates at a concentration of 2×105 per well in 1 mL of melanocyte growth media. The cells were incubated overnight at 37°C, 5% CO2 to allow them to attach. The cells were then infected with 2.5 ug/mL polybrene and 1.25×105 IFU shRNA. Each plate contained a well that was not infected plus a well that contained either control shRNA (sh-), GFP shRNA, or one of six different galectin-3 shRNA. A dark line (dk) and one medium line (med) were infected with Santa Cruz Cat # sc-108080, sc-108084 and sc-155994-V in the GFP infected well. A light line was infected with one of 5 shRNA galectin 3 lentivirus plus a GFP and a mock shRNA clones obtained through Cincinnati Children’s Hospital Medical Center Heart Institute’s Lenti-shRNA Library Core. The viruses were generated using Sigma TRCN clones TRCN0000029304, TRCN0000029305, TRCN0000029306, TNCR0000029307 and TRCN0000029308 (Sigma, St. Louis, MO). For the dk and the med lines, the medium containing lentiviral particles was aspirated and replaced with fresh melanocyte growth media. For the light line, 40ul of virus was added and the cells were left for 5 days. A second round of infection was done with 40ul more plus 0.8ug/ml hexadimethrine bromide (Sigma H9268). After infection of all groups, puromycin (Sigma P8833) was added at 2ug/ml. The cells were treated with several rounds of puromycin selection. After sufficient puromycin selection, the cells were used for immunofluorescent, western blot analysis and melanin content.
Quantification of melanin content
Total melanin content was determined as follows. Cells were pelleted following trypsinization and lyzed (50mM Tris pH7.54, 2mM EDTA pH 7.87, 150mM sodium chloride, 1% Triton-X 100) in the presence of protease inhibitors (Complete, Roche, Indianapolis, IN). Cell extracts were spun at 14,000rpm for 5 min at 4°C. The supernatant was removed and protein content determined (Pierce, Rockford, IL). The pellet was washed twice in ethanol: ether (1:1) and dissolved in 0.2N sodium hydroxide at 60°C. A 75µl aliquot was measured for absorbance at 490nm in a plate reader (BioRad, Hercules, CA).
Tyrp-1 Assay
To assess the tyrosine hydroxylase activity of Tyrp-1 we used the method develop by Zhao et al (Zhao et al, 1994). Specifically, melanocyte lysates (10–25 µg protein) were incubated in 250 µl of reaction mixture (0.1 µCi/ml tyrosine plus 0.08mM unlabelled tyrosine) for 19 hours at 37°C. Subsequently, tritium H2O produced was assessed with a Packard 1900 CA scintillation analyzer.
Immunofluorescent methodology
Cells were plated on chamber slides at 2×104 cells per well and fixed after 24 hours with 3% paraformaldehyde. Cells were washed with PBS and blocked with a 5% BSA solution. Primary antibodies at optimized concentrations in a 1% BSA/PBS solution were added to the slide and left at room temperature for 1 hour. Cells were washed and Alexa Fluor secondary antibodies were added at 1:250 in a 1% BSA/PBS solution at room temperature for 45 minutes in the dark. Slide was mounted and let dry overnight before viewing on a Zeiss LSM510 META confocal microscope. The primary antibodies used consisted of goat polyclonal antiserum to galectin-3 provided by F-T Liu (University of California at Davis, Sacramento, CA); rabbit polyclonal antiserum to tyrosinase provided by R. King & W. Oetting (hPEP1; University of Minnesota, MN); mouse monoclonal antiserum to Tyrp-1 (Mel-5; Signet Laboratories, Dedham, MA); rabbit polyclonal antiserum to BiP (Santa Cruz); rabbit polyclonal serum to the HPS1 and HPS5 proteins provided by B. Gahl (NIH, Bethesda, MD); rabbit polyclonal antiserum to adaptin 3 provided by M. S. Robinson (β3A; Cambridge Institute for Medical Research, Cambridge, Great Britain).
Metamorph methodology
For quantitating expression of tyrosinase and Tyrp-1 in control and galectin-3 silenced NHM, captured photographs were cropped to include the entire cell (n=25 per group) and fluorescence intensity per cell quantited using MetaMorph and express as signal intensity per cell. For quantitating expression of galectin-3 with HPS-1 or HPS-5, Captured photographs were cropped to include only individual cell’s nuclei and surrounding cell body (n=25). Dendrites were not included in the cropped images. In the former group, the fluorescence intensity per cell was quantitated using the quantitative software MetaMorph and expressed as signal intensity in the former group. In the latter group, the color channels were separated, each of which represented a different primary antibody: either galectin-3, HPS-1, or HPS-5. Each color channel from each photograph was set on a threshold value, and then the colocalization of each color was measured. A t-test was done on the colocalization values comparing galectin-3 to HPS-1 and galectin-3 to HPS-5 to test for a significant difference between the primary antibodies.
Analysis of Mander’s Coefficient
To quantitate the Mander’s coefficient for colocalization of (a) galectin-3 with Tyrp-1 or tyrosinase in cell bodies (20 cells per group) or (b) galectin-3 with tyrosinase in HPS cells (25 cells per group) the following was performed. Digital images were obtained using the Zeiss LSM510 META confocal microscope. Using the quantitative software Image J, images were analyzed with the Intensity Correlation Analysis plugin that generated Mander’s coefficients. Mander’s overlap coefficient ranges between 1 and zero with 1 being high-colocalization and zero being low. Appropriate thresholds were set for each image to eliminate noise.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by a National Instituted of Health grant R21 AR056059 (REB). The authors thank the recommendations of the reviewers.
Abbreviations
- BLOC
biogenesis of lysosome-related organelle complex
- HPS
Hermansky-Pudlak Syndrome
- Tyrp-1
tyrosinase related protein-1
- RER
rough endoplasmic reticulum
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Abdou AG, Hammam MA, Farargy SE, et al. Diagnostic and prognostic role of galectin 3 expression in cutaneous melanoma. Am J Dermatopathol. 2010;32:809–814. doi: 10.1097/DAD.0b013e3181e02f29. [DOI] [PubMed] [Google Scholar]
- Basrur V, Yang F, Kushimoto T, et al. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res. 2003;2:69–79. doi: 10.1021/pr025562r. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Richmond B, Huizing M, et al. Melanocyte specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak Syndrome-Type 3. Am J Path. 2005;166:231–240. doi: 10.1016/S0002-9440(10)62247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy RE, Huizing M, Gahl WA. Chapter 7: Biogenesis of melanosomes. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System. Edition 2. Oxford, UK: Blackwell Publishing; 2006. pp. 155–170. [Google Scholar]
- Bonifacino JS. Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann N Y Acad Sci. 2004;1038:103–114. doi: 10.1196/annals.1315.018. [DOI] [PubMed] [Google Scholar]
- Braeuer RR, Shoshan E, Kamiya T, et al. The sweet and bitter sides of galectins in melanoma progression. Pigment Cell Melanoma Res. 2012;25:592–601. doi: 10.1111/j.1755-148X.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- Bultema JJ, DiPietro SM. Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles. Small GTPases. 2013;4:16–21. doi: 10.4161/sgtp.22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Fermin A, Vardhana S, et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:14496–14501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane AR, Yeager C, Dorward H, et al. Dysregulation of galectin-3. Implications for hermansky-pudlak syndrome pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50:605–613. doi: 10.1165/rcmb.2013-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour D, Cramm-Behrens CI, Drobecq H, et al. Requirement for galectin-3 in apical protein sorting. Curr Biol. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Delacour D, Greb C, Koch A, et al. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic. 2007;8:379–388. doi: 10.1111/j.1600-0854.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Delacour D, Koch A, Jacob R. The role of galectins in protein trafficking. Traffic. 2009;10:1405–1413. doi: 10.1111/j.1600-0854.2009.00960.x. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Falcón-Pérez JM, Dell'Angelica EC. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic. 2004;5:276–283. doi: 10.1111/j.1600-0854.2004.0171.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Dell'Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Falcón-Pérez JM, Tenza D, et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- García-Borrón JC, Solano F. Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002;15:162–173. doi: 10.1034/j.1600-0749.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Gorski JP, Liu F-T, Artigues A, et al. New alternatively spliced form of galectin-3, a member of the β-galactoside-binding animal lectin family, containina a predicted transmembrane-spanning domain and a leucine xipper motif. JBC. 2002;277:18840–18848. doi: 10.1074/jbc.M109578200. [DOI] [PubMed] [Google Scholar]
- Helip-Wooley A, Westbroek W, Dorward H, et al. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome-5. J Invest Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M, Sarangarajan R, Strovel E, et al. AP-3-dependent vesicles carry tyrosinase, but not TRP-1 in cultured human melanocytes. Mol Biol Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloer DP, Rojas R, Ivan V, et al. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Marks MS. Organelle biogenesis: En BLOC exchange for RAB32 and RAB38. Curr Biol. 2012;22:R963–R965. doi: 10.1016/j.cub.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Falcón-Pérez JM, Dell'Angelica EC. Biogenesis of lysosome-related organelles complex 3 (BLOC-3): a complex containing the Hermansky-Pudlak syndrome (HPS) proteins HPS1 and HPS4. Proc Natl Acad Sci U S A. 2003;100:8770–8775. doi: 10.1073/pnas.1532040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G. The multiple personalities of Alix. J Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- Park JW, Voss PG, Grabski S, et al. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29:3595–3602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Raposo G, Marks MS. Melanosomes--dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond B, Huizing M, Knapp J, et al. Melanocytes from Hermansky-Pudlak Syndrome types 1–3 express distinct defects in cargo trafficking. J Invest Dermatol. 2005;124:420–427. doi: 10.1111/j.0022-202X.2004.23585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Koshoffer A, Morris RE, et al. Membranous Complexes Characteristic of Melanocytes Derived from Patients with Hermansky-Pudlak Syndrome Type 1 Are Macroautophagosomal Entities of the Lysosomal Compartment. Pigment Cell Res. 2005;18:417–426. doi: 10.1111/j.1600-0749.2005.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truschel ST, Simoes S, Setty SR, et al. ESCRT-1 function is required for Tyrp-1 transport from early endosomes to the melanosome limiting membrane. Traffic. 2009;10:1318–1336. doi: 10.1111/j.1600-0854.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296:F459–F469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Gray RM, Haudek KC, et al. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhao Y, Nordlund JJ, et al. Human TYP-1 has tyrosine hydroxylase but not DOPA oxidase activity. Pigment Cell Res. 2004;7:131–140. doi: 10.1111/j.1600-0749.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.