Abstract
Purpose of Review
Uptake of antiretroviral regimens with durable virologic suppression has been shown to reduce the risk of HIV transmission. Expanding ART programs at a population-level may serve as a vital strategy in the elimination of the AIDS epidemic.
Recent findings
The global expansion of ART programs has greatly improved access to life-saving therapies, and is likely to achieve the target of 15 million individuals on therapy set by UNAIDS. In addition to the incontrovertible gains in terms of life expectancy, growing evidence demonstrates that durable virologic suppression is associated with significant reductions in HIV transmission amongst heterosexual couples and men who have sex with men. Expansion in successful ART programs, best monitored by a program-level continuum of care cascade to monitor progress in diagnosis, retention in care and virologic suppression, is associated with reductions in HIV incidence at a population level.
Summary
Expanding and sustaining successful ART delivery at a global level is a key component in a comprehensive approach to combating the HIV epidemic over the next two decades.

Keywords: Antiretroviral therapy, Treatment as Prevention, Care Cascade, Millennium Development Goals
Introduction
The last decade has witnessed significant shifts in the approach to the management of HIV. Clinical trials have demonstrated the efficacy and tolerability of a substantial number of antiretroviral combinations, with once daily fixed-dose combinations being the norm. Concomitantly, global efforts have reduced the costs of these medications, allowing for unprecedented scale up of therapeutic programs in resource-limited settings. While initial antiretroviral therapy (ART) guidelines focused predominantly on CD4-cell count strata dependent initiation of treatment as a means to maximize the therapeutic index of therapy whereby both AIDS-related illness and potential drug toxicity were avoided(1), current guidelines are more nuanced, taking into account multiple other factors which might now influence ART initiation(2). One potential consideration for treatment initiation now well established in current international guidelines(2, 3) is a recognition of the secondary benefit of successful ART-related virologic suppression; namely decreased onward transmission. Treatment-related decreases in virologic transmission at a population level was first observed a decade ago (4, 5), and shortly thereafter formal mathematical models of expanded “Treatment as Prevention” (6, 7) paved the way for widespread consideration of the secondary population-level benefits derived from expanding treatment for individuals accessing therapy for the ever improving outcomes seen in HIV-related morbidity and mortality (8, 9).
The last year has seen continued progress in terms of improved access to ART at a global level. In 2013 approximately two million individuals started ART, the largest ever annual increase in initiations (10). An estimated 12.9 million individuals were receiving ART by the end of 2013, the majority (11.7 million individuals) in resource-limited settings.
These individuals represent 36% of the estimated 32.6 million HIV-infected individuals in these regions (10). UNAIDS estimates that the world is on track to meet the Millennium Development Goal-ART target of 15 million people on therapy by 2015(11). The expansion of ART programs in conjunction with additional ART prevention strategies in the form of prevention of mother to child transmission (PMTCT) has resulted in overwhelming individual level benefit. A systematic analysis of the UNAIDS Global Burden of Disease Study 2013 estimated the impact of these ART-based interventions on mortality, finding that overall 19.1 million life-years (confidence interval [CI] 16.6 – 21.5 million life-years) have been saved over this time period (12). Furthermore, further expansion of global treatment programs in order to implement current expanded WHO treatment guidelines (with at least 80% global coverage) would prevent an additional three million deaths and avert 3.5 million infections over the following decade in comparison to prior more conservative treatment recommendations (11). A cost-effectiveness analysis modelled on the epidemics in South Africa and India clearly supports the overwhelming benefits of early ART, with early ART found to be very cost-effective over a lifetime ($590 in South Africa and $1,800 per life-year saved) (13). Early ART was found also to prevent a greater number of cumulative transmissions compared to delayed ART. These data reflect the inextricable dual nature of successful ART expansion where treatment benefits the individual and secondarily limits transmission within the community. There is growing consensus that “Treatment as Prevention” has definitively moved from a topic of scientific enquiry to the domain of program design and implementation.
Antiretroviral therapy for the prevention of onward transmission
Recent evaluations of population-level mother to child transmission events in the United Kingdom and Ireland have demonstrated further decreases in the numbers of HIV-infected infants over the last decade, with rates dropping from 2.1% in 2000/1 to 0.7% in 2006 (for vaginal births) and 0.46% in 2010/11(14). Transmission remains clearly associated with the degree of maternal viremia, and the decrease in transmission events is associated with higher proportions of women accessing ART at conception as well as enhanced antenatal ART (14).
The prevention of onward transmission associated with ART has also been scrutinized with the application of the classic Bradford Hill criteria for causality (15). In addition to biologic plausibility considerations, the accumulated evidence to date for the effect of ART on transmission satisfies all other epidemiologic criteria, including consistency of association, with the results of the HPTN 052 randomized clinical trial evaluating the effect of early vs. deferred ART on transmission risk amongst serodiscordant couples demonstrating very similar results to those of prior cohort studies and meta-analyses of the effects of ART in similar populations (16–18). In the serodiscordant cohort nested within the Partners in Prevention study, use of ART was associated with a 92% reduction in transmission, from 2.24 (95% confidence interval [CI] 1.84 – 2.72)/100 person-years to 0.37 (95%CI 0.09 – 2.04)/100 person-years (17). Similarly a meta-analysis evaluated 5021 heterosexual couples and found an overall reduction in the rate of transmission for those receiving ART from 5.64 to 0.46 (95% CI 0.19 – 1.09)/100 person-years (again a 92% reduction in risk of transmission) (16). In the HPTN 052 trial involving 1763 couples (54% from Africa, 50% male HIV-infected partners), 28 linked transmission events were noted, only one occurring in the early ART group (hazard ratio 0.04; 95% CI 0.01 – 0.27), a 96% reduction in transmission risk (18). Satisfaction of the Bradford Hill criteria for causality help in the interpretation of other population-level ecologic data highlighting a consistent association between expansion of ART and decreased community HIV incidence (19, 20).
Evaluation of Treatment as Prevention in resource rich settings – recent findings
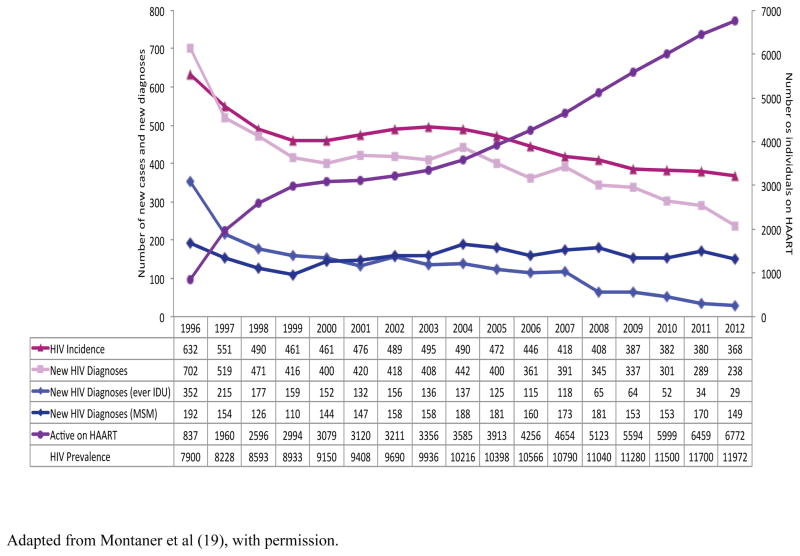
The Province of British Columbia, Canada, offers an ongoing opportunity to assess the population-level effects of sustained ART expansion, coupled with universal access to laboratory monitoring (19, 21). Antiretroviral therapy is provided free of charge to HIV-infected individuals within the province via a central Drug Treatment Program which maintains and monitors a population-based registry of individuals receiving ART, including monitoring of treatment adherence levels (determined through pharmacy refill data) and virologic resistance history. Evaluation of the British Columbia Treatment Program has provided early evidence of the population-level association between expanded access to ART therapy and concomitant reduction in new diagnoses (Table 1) (21). Ongoing evaluation of the Program has demonstrated continued expansion in ART uptake - between 1996 and 2012 the number of individuals receiving therapy increased substantially from 837 to approximately 6,672 individuals; a change in ART coverage from 11–57% of the estimated prevalent cases (19). During the time period 1999 – 2012 (the period for which the ultrasensitive HIV RNA assay was available) the proportion of individuals with HIV viral load <50 copies/mL increased from below 10% to 59% (p <0.0001). This dramatic expansion of successful ART uptake has been associated with matching decreases in the provincial HIV-related mortality rate (from 6.5/100,000 – 1.3/100,000 population) (an 80% decline, p< 0.0115). Significantly, overall new diagnoses of HIV and estimated incident cases of HIV have also undergone a corresponding decrease; dropping from 702 to 238 cases per year between 1996 and 2012 (a 66% reduction, p <0.0004, Figure 1), and from 632 – 368 cases per year (a 42% reduction, p<0.0003), respectively. The numbers of HIV diagnostic tests increased over this time period, and rates of syphilis, Neisseria gonorrhea and Chlamydia infection increased during this time period suggesting no change in ongoing sexual risk behaviours. In statistical analysis with HIV incidence as the outcome, and the explanatory variable being the percentage of individuals suppressed on ART (<500 copies/mL), for every 1% increase in the numbers of individuals with successful virologic suppression, the incident rate decreased by a corresponding 1% (estimated rate ratio, 0.9900; 95% CI 0.9887 – 0.9912).
Table 1.
Population-level-and-observational cohorts evaluating expansion of treatment and risk of new HIV infection.
| Setting | Time Period | Evaluation | Outcomes | Reference |
|---|---|---|---|---|
| Taiwan | 1984 – 2002 | National HIV surveillance data. Transmission rate estimated by use of exponential model. |
Transmission rate 0.391 new cases/prevalent cases pre-ART Transmission rate 0.184 new cases/prevalent cases post-HAART. Overall decrease 53%. |
(4) |
| Vancouver, British Columbia, Canada | 1996– 2007 | Prospective cohorts of injection drug users. Median CVL*. Cox regression model to association with HIV incidence. |
CVL associated with time to HIV seroconversion (Hazard ratio 3.32 per log10 increase). After median viral load fell to <20,000 copies/mL, no statistical association with HIV incidence observed. |
(40) |
| San Francisco | 2004–2008 | HIV/AIDS public health surveillance for new diagnoses and calculated HIV incidence. Mean Community viral load. Poisson models for CVL and new HIV diagnoses. |
Significant decline in mean CVL 2004 –2008 (p=0.037). Reduction in CVL associated with decrease in new HIV diagnoses (p=0.003). |
(41) |
| British Columbia, Canada | 1996–2012 | ART† coverage from centralized registry. HIV public health surveillance for new diagnoses with estimated HIV incidence. General additive models with Poisson regression. |
709% increase in ART uptake. 42% decrease in HIV incidence. For every 1% expansion in individuals suppressed on ART, a corresponding 1% drop in HIV incidence was predicted. |
(19) |
| Hlabisa sub-district, KwaZulu-Natal, South Africa | 2004–2011 | Cohort of 16,667 HIV uninfected individuals. ART coverage and HIV prevalence within the surrounding community assessed, and rate of new seroconversions captured. |
As ART coverage within a community expands, risk of acquisition decreases: for communities with 30–40% ART penetration, risk of new infection dropped 38% compared to communities with <10% ART uptake. | (20) |
CVL = community viral load
ART = highly active antiretroviral therapy. Adapted from (42) with permission.
Figure 1. Changes in rates of HIV incidence and individuals accessing ART, British Columbia, Canada 1996–2012.
Adapted from Montaner et al (19), with permission.
The Continuum of Care Cascade
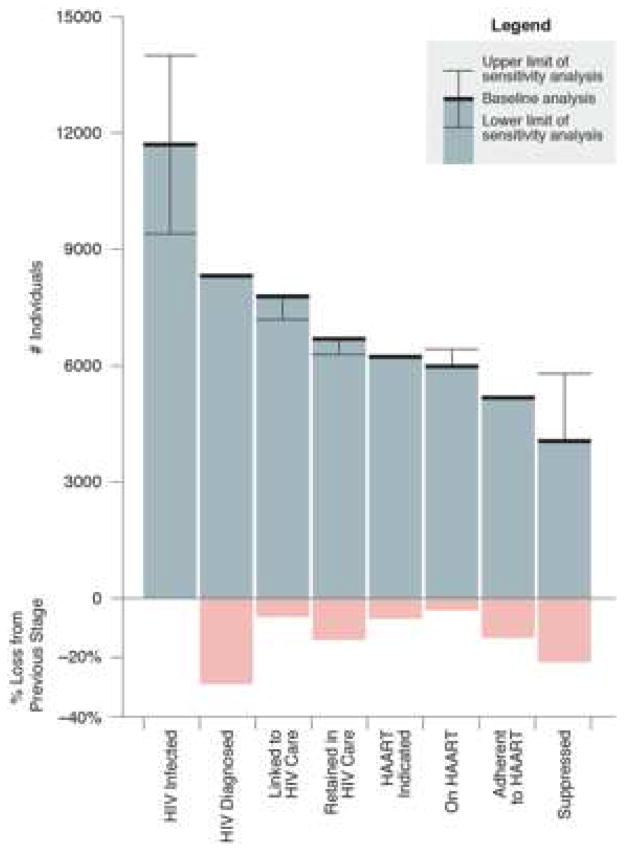
The British Columbia experience also highlights the application of a seminal, indeed crucial framework of evaluation for all HIV care programs – the HIV cascade of care. Initially formulated by Gardner et al. and applied to the United States as a whole, based on estimates of reported values for each step of the cascade for initial estimates of the entire HIV-infected population to those achieving virologic suppression on ART (22). Of the 1.2 million HIV-infected individuals in the U.S., approximately 20% were unaware of their diagnosis, and ultimately only 19% of individuals were successfully retained and suppressed on ART. These estimates are remarkably similar to those of the United States CDC using U.S. national surveillance instruments, where 28% of all HIV-infected individuals were found to be fully suppressed on ART (23). Each stage of the care cascade is amenable to scrutiny and this forms the basis of re-inforcing feedback loops for Treatment as Prevention Programs to assess deficiencies in areas such as diagnosis, linkage to care and adherence and target interventions accordingly for the population as a whole, or sub-populations of vulnerable individuals within the care cascade (such as people who inject drugs [PWID] or men who have sex with men [MSM]. The BC program has been subject to detailed application of the care cascade (Figure 2), and on a practical basis this is updated on a regular basis for specific regions within the Province and for specific populations (24).
Figure 2. The HIV cascade of care as a monitoring metric, British Columbia, Canada.
The HIV prevalence estimates are based on estimates from the Public Health Agency of Canada. From Nosyk et al (24) with permission.
MSM populations
One of the most challenging aspects of evaluating the transmission benefits of ART expansion has been amongst MSM populations (25). Unprotected receptive anal intercourse is associated with high per-act probability transmission risk when compared to vaginal intercourse (26, 27). Although the HPTN 052 study clearly demonstrated the effectiveness of ART at decreasing transmission amongst heterosexual couples, few MSM couples were included, limiting interpretation amongst this population (18). A recent observational study has provided reassurance that the effects of ART amongst MSM serodiscordant couples is likely similar to that observed in the HPTN 052 clinical trial. In the PARTNER study 767 serodiscordant couples (445 heterosexual and 282 MSM couples) where the HIV-infected partner had been stable on ART with HIV viral load of < 200 copies/mL, were followed to evaluate linked transmission events during periods of condomless sexual activity (28). Overall, 894 couple-years of followup (CYFU) were eligible for evaluation, with approximately 44,400 condomless sex acts included. No linked transmission events were observed amongst either the heterosexual or MSM participants during this period. While the observed transmission rate was zero, the estimated ten year within-couple transmission risk, derived from the upper boundary of the 95% confidence interval was 4% overall, and 10% for any anal sex (translating into a 1% risk per year) (28). Sub-analysis of the risks specifically amongst MSM were limited by the relatively short periods of observation for risk-type, however unprotected receptive anal sex with ejaculation was associated with a potential 10 year transmission risk of 32%, an estimate which may diminish with further observation and more data. Although these results confirm an individual-level benefit, recent evaluations of differing populations highlight the importance of establishing and implementing an HIV care cascade feedback loop if a population-level benefit amongst MSM is to be seen(19, 29–31). HIV incidence rates amongst MSM in England and Wales between 2001 and 2010 was estimated incorporating data from national surveillance sources, as well as CD4 cell count at diagnosis (29). Using a CD4-staged back calculation model, HIV incidence was estimated annually. In addition, engagement in care was assessed by reported ART uptake at last visit (however, virologic suppression amongst those accessing ART was not specifically recorded as an endpoint). Overall over the study period, testing rates increased over three-fold, with 65% of those diagnosed identified with a CD4 cell count > 350 cells/mm3 in late 2010 compared to only 48% of those diagnosed in 2001. In addition, the proportion of individuals accessing ART after diagnosis increased from 69% in 2001 to 80% in 2010. Despite these advances, the estimated overall HIV incidence rate was static across the time period at approximately 2300–2500 annual infections. In addition, the proportion of individuals estimated to be infected but undiagnosed declined slightly over time, with an estimated 22% of all HIV-infected MSM individuals in 2010 undiagnosed, and potentially responsible for the majority of forward transmissions in conjunction with overall increases in condomless sexual encounters (29).
Similar results were obtained from an analysis of HIV infectivity using the same national surveillance dataset of MSM in the United Kingdom over the period 2006–2010 (30). In this analysis, the proportion of MSM individuals deemed to be infectious, with a viral load > 1,500 copies/mL was evaluated, and infectivity of those undiagnosed was estimated by applying the viral load distribution amongst those diagnosed but untreated to the undiagnosed population estimates. The relative importance of undiagnosed individuals in potential forward transmission was again highlighted. In 2010 the proportion of undiagnosed individuals was estimated to be 26% (compared to 31% in 2006), however 85% were deemed to be infectious, compared to 79% of those diagnosed but not on ART, while only 3% of those on ART had a viral load over 1500 copies/mL and therefore would have been considered to be infectious (30). The overall proportion of infectious individuals (35% of all HIV-infected MSM) could be reduced to 29% by the expansion of ART initiation criteria to all individuals with a CD4 cell count < 500 cells/mm3 and to 21% by decreasing the proportion of those undiagnosed by half. The effects of ART on HIV incidence amongst MSM in the United Kingdom was more clearly delineated in an individual-based stochastic model developed to fit parameters for sexual risk behaviour, HIV transmission and progression, and the effects of ART in this population (31). Overall model outputs were consistent with observed surveillance data. HIV incidence was found to increase from 0.3/100 person-years in 1990–1997 to 0.45/100 person-years in 1998–2010, associated with increasing rates of condomless sex. The uptake of ART attenuated this risk; HIV incidence would have been 68% higher over the period 2006–10 if ART had not been introduced. Similarly, expansion of testing programs and immediate ART following diagnosis was modelled to be associated with a 62% lower incidence of HIV (31). In 2010, the majority of transmission events were associated with the undiagnosed proportion of the population with an association with acute HIV infection (31).
In the United States overall rates of new HIV diagnoses have decreased by 33% over the period 2002 – 2011, dropping from 24.1/100,000 to 16.1/100,000, however increased in the sub-group of MSM, particularly those aged 13–24 years where testing rates and uptake of ART were low (32). In contrast, in the British Columbia analysis, HIV diagnosis rates declined amongst MSM, dropping by 22% from 1.92 – 1.49 cases/100,000 (p. 0.0046) (19).
These data clearly highlight the need to develop population-specific HIV cascade of care metrics in order to maximize potential benefit of ART expansion. Increased testing frequency with associated early diagnosis and immediate linkage to ART is required as a key component of a Treatment as Prevention strategy, particularly amongst MSM.
Resource limited settings
Although ecologic data may not be able to determine causality rather than association, recent data from an observational cohort in South Africa has shown encouraging results from an ART expansion program (20). A cohort of 16,667 HIV uninfected individuals was followed in the Hlabisa sub-district, KwaZulu-Natal. Expansion of ART coverage and HIV prevalence within the surrounding community was assessed over the time period 2004– 2011, and rate of new seroconversions within the cohort was evaluated. As ART coverage within the community expanded, risk of an individual’s acquisition of HIV decreased: for communities with 30–40% ART penetration, risk of new infection dropped 38% compared to communities with <10% ART uptake, a benefit ratio of almost a 1% drop for every 1% of ART uptake in a community, a finding very similar to predicted in the British Columbia model(19).
Moving forward
Currently a number of clinical trials seek to clarify the benefits of early initiation of ART at an individual level (the START trial – Strategic Timing of Antiretroviral Treatment comparing outcomes for ART initiation at >500 cells/mm3 compared to < 350 cells/mm3) as well as that of community level benefit in terms of transmission effect of early ART (33, 34). While the results of these trials will be important, current guidelines have already incorporated the presently available data to guide clinical decision-making. In this context, the case for early initiation of ART has been supported by cohort studies (35, 36) and the recognition that the period of time in which ART may safely be deferred is of relatively short duration compared to the decades spent on ART regardless of CD4 count threshold selected (37). Treatment for treatment’s sake is clearly beneficial and worthy of immediate implementation, regardless of setting. The challenges remain significant, and serve as the focus of system-wide operations research and intervention. Prior to the implementation of the HIV continuum of care cascade as a metric to monitor progress at a regional or country-wide level, purely practical considerations remain. These concern the ongoing need to monitor and ensure the availability of a wide array of factors such as testing kits, availability of antiretroviral agents, laboratory supplies, and decisions regarding healthcare staff and staffing roles for follow-up of patients engaged in ART programs. More distally, global level financing is crucial for the implementation of treatment programs. Although the costs associated with ART expansion may seem daunting, cost-effectiveness analyses continue to confirm the cost-savings associated with this approach. The overall economic benefits of returning individuals to the workforce must not be overlooked (38). Strategies to minimize losses along the care cascade continue to be evaluated and recommended using an evidence-based approach where possible (39), and in this context the secondary benefits of successful ART uptake can be maximized to reduce transmission events. Moving forward beyond 2015, new aggressive targets are required to meet the goal of “ending the epidemic by 2030”, defined as ‘the rapid reduction of AIDS-related deaths as well as new HIV infections, stigma and discrimination experienced by people living with HIV and vulnerable and key populations, by 90 percent of 2010 levels’. In this context, there is increasing momentum behind the the ambitious but achievable UNAIDS proposed post 2015 MDG-ART target of 90-90-90, which specifically proposes that by 2020, 90% of those infected be aware of their infection, 90% of those infected access ART, and 90% of those on ART be virologically suppressed. All together the strategy would lead to 73% of virological suppression among people living with HIV globally, and this would dramatically decrease morbidity, mortality and HIV transmission to levels consistent with “ending the epidemic by 2030” as defined above.
Conclusion
Ongoing evaluation of ART expansion at a global level confirms an overwhelming individual-level benefit in terms of morbidity and mortality. There can be no doubt that continued sustained and successful expansion of ART programs is necessary. The secondary reduction in transmission events accrued by ART expansion is clear at an individual level, with compelling evidence of benefit in communities and the general population. Expanded treatment programs require innovative implementation of the HIV continuum of care with close monitoring and feedback to maximize ART success and associated prevention benefits. Full implementation of the UNAIDS proposed post 2015 MDG-ART target of 90-90-90 provides a unique evidence-based opportunity to dramatically decrease morbidity, mortality and HIV transmission, to levels consistent with “ending the epidemic by 2030”.
Key Points.
Virologic suppression associated with antiretroviral therapy is associated with decreased risk of transmission amongst individuals, including men who have sex with men.
Expanded ART programs with associated durable virologic suppression is associated with decreasing HIV incidence at a population level in studies in developed world and resource-limited settings.
Given the incontrovertible primary benefits of ART, and compelling evidence of decreased transmission associated with a robust HIV cascade of care designed to improve diagnosis and retention in care, efforts must shift to operationalize program expansion at a global level in order to meet UNAIDS Development Goal targets for the next decade as the means of combating the HIV epidemic.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is dedicated to the memory of our colleague, friend and collaborator, Professor Joep Lange, who was a globally inspirational leader in the field of HIV/AIDS. The photo reproduced above was taken during the opening day of the 2014 International AIDS Conference in Melbourne, Australia. The slide was shown by the UN Undersecretary General and Executive Director of UNAIDS, Dr. Michel Sidibé, in homage of Professor Lange, as he unveiled the proposed UNAIDS post 2015 Millennium Development Goals Antiretroviral Therapy Targets. In the slide, he used a famous quote from Professor Lange urging us to learn from the success of other industries as we attempt to make antiretroviral therapy available to every person in need in any corner of the world. Dr. Sidibé’s presentation was interrupted at this point by a community manifestation, asking for virological suppression to become the new global goal standard for Antiretroviral Therapy monitoring. Indeed, this is what the proposed UNAIDS post 2015 MDG 90-90-90 Antiretroviral Therapy Target calls for. Professor Lange would have been proud to see the consensus emerging on this issue as he was a champion major contributor to the development of the proposed 90-90-90 target, which specifically proposes that by 2020, 90% of all people living with HIV will know their HIV status, 90% of all people with diagnosed HIV infection will receive sustained antiretroviral therapy, and 90% of all people receiving antiretroviral therapy will have durable viral suppression. Achieving this target will lead to 73% of all people living with HIV to be virologically suppressed, globally. This would be expected to meet the goal of “ending the epidemic by 2030”, defined as ‘the rapid reduction of AIDS-related deaths as well as new HIV infections, stigma and discrimination experienced by people living with HIV and vulnerable and key populations, by 90 percent of 2010 levels’.
Conflict of Interest
Mark Hull receives support from the U.S. National Institute on Drug Abuse (grant number R01DA031043-01). He has served on speakers bureau or advisory boards of Merck, Janssen, Vertex, Bristol-Myers-Squibb, ViiV Healthcare, Pfizer.
Julio S. G. Montaner is supported, with grants paid to his institution, by the British Columbia Ministry of Health. He has also received financial support from the US National Institutes of Health, International AIDS Society, United Nations AIDS Program, World Health Organization, France Recherche Nord & Sud SIDA HIV Hépatites (ANRS), International Association of Providers of AIDS Care (IAPAC), UNICEF, MAC AIDS Fund and Open Society Foundation. He has received grants from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
References
Papers of particular interest, published recently, have been highlighted as:
** Of major importance
* Of great interest
- 1.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. Jama. 2006 Aug 16;296(7):827–43. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 2.Gunthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. Jama. 2014 Jul 23–30;312(4):410–25. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a public health Approach. Jun 26, 2013. [PubMed] [Google Scholar]
- 4.Fang CT, Hsu HM, Twu SJ, Chen MY, Chang YY, Hwang JS, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. The Journal of infectious diseases. 2004 Sep 1;190(5):879–85. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- 5.Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006 Aug 5;368(9534):531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 6.Lima VD, Johnston K, Hogg RS, Levy AR, Harrigan PR, Anema A, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. The Journal of infectious diseases. 2008 Jul 1;198(1):59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 7.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS (London, England) 2012 Jan 28;26(3):335–43. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 9*.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the Gap: Increases in Life Expectancy among Treated HIV-Positive Individuals in the United States and Canada. PloS one. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. Updated evaluation of life-expectancy of individuals accessing ART in the NA-ACCORD cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Global Update on the Health Sector. HIV. 2014 Jul;2014 [Google Scholar]
- 11.UNAIDS. [Accessed July 22, 2015];Treatment. 2015 Available from www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2484_treatment-2015_en.pdf.
- 12*.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Jul 21; doi: 10.1016/S0140-6736(14)60844-8. An important update evaluating current indicators of the HIV epidemic at a global level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. The New England journal of medicine. 2013 Oct 31;369(18):1715–25. doi: 10.1056/NEJMsa1214720. Important analysis demonstrating cost-effectiveness of ART expansion in South Africa and India. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS (London, England) 2014 Apr 24;28(7):1049–57. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 15.Nosyk B, Audoin B, Beyrer C, Cahn P, Granich R, Havlir D, et al. Examining the evidence on the causal effect of HAART on transmission of HIV using the Bradford Hill criteria. AIDS (London, England) 2013 Apr 24;27(7):1159–65. doi: 10.1097/QAD.0b013e32835f1d68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS (London, England) 2009 Jul 17;23(11):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 17.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Montaner JS, Lima VD, Harrigan PR, Lourenco L, Yip B, Nosyk B, et al. Expansion of HAART Coverage Is Associated with Sustained Decreases in HIV/AIDS Morbidity, Mortality and HIV Transmission: The “HIV Treatment as Prevention” Experience in a Canadian Setting. PloS one. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. Updated ecologic analysis of the association of expanded ART programs and decreasing HIV incidence in British Columbia, Canada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science (New York, NY. 2013 Feb 22;339(6122):966–71. doi: 10.1126/science.1228160. Similar analysis of the effects of ART expansion on HIV acquisition in a resource-limited setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011 Mar 15;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vital signs: HIV prevention through care and treatment--United States. Mmwr. 2011 Dec 2;60(47):1618–23. [PubMed] [Google Scholar]
- 24.Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. The Lancet infectious diseases. 2014 Jan;14(1):40–9. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muessig KE, Smith MK, Powers KA, Lo YR, Burns DN, Grulich AE, et al. Does ART prevent HIV transmission among MSM? AIDS (London, England) 2012 Nov 28;26(18):2267–73. doi: 10.1097/QAD.0b013e328355713d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. International journal of epidemiology. 2010 Aug;39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin F, Jansson J, Law M, Prestage GP, Zablotska I, Imrie JC, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS (London, England) 2010 Mar 27;24(6):907–13. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodger A, Bruun T, Cambiano V, et al. HIV transmission risk through condomless sex if HIV+ partner on suppressive ART: PARTNER study. Program and abstracts of the 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, Massachusetts. p. Abstract 153LB. [Google Scholar]
- 29.Birrell PJ, Gill ON, Delpech VC, Brown AE, Desai S, Chadborn TR, et al. HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study. The Lancet infectious diseases. 2013 Apr;13(4):313–8. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AE, Gill ON, Delpech VC. HIV treatment as prevention among men who have sex with men in the UK: is transmission controlled by universal access to HIV treatment and care? HIV medicine. 2013 Oct;14(9):563–70. doi: 10.1111/hiv.12066. [DOI] [PubMed] [Google Scholar]
- 31*.Phillips AN, Cambiano V, Nakagawa F, Brown AE, Lampe F, Rodger A, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PloS one. 2013;8(2):e55312. doi: 10.1371/journal.pone.0055312. Thought-provoking model of HIV incidence in the MSM population in England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AS, Hall HI, Hu X, Lansky A, Holtgrave DR, Mermin J. Trends in diagnoses of HIV infection in the United States, 2002–2011. Jama. 2014 Jul 23–30;312(4):432–4. doi: 10.1001/jama.2014.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. National Institutes of Health and the University of Minnesota Clinical and Translational Science Institute. [Accessed July 22, 2014.];The START study (Strategic Timing of Antiretroviral Treatment) 2012 http://clinicaltrials.gov/ct2/show/study/NCT00867048#locn.
- 34.Iwuji CC, Orne-Gliemann J, Tanser F, Boyer S, Lessells RJ, Lert F, et al. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14:230. doi: 10.1186/1745-6215-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. The New England journal of medicine. 2009 Apr 30;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, Kirk O, et al. The incidence of AIDS-defining illnesses at a current CD4 count >/= 200 cells/muL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013 Oct;57(7):1038–47. doi: 10.1093/cid/cit423. [DOI] [PubMed] [Google Scholar]
- 37.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiebaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 Cells/mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011 Oct;53(8):817–25. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 38*.Thirumurthy H, Chamie G, Jain V, Kabami J, Kwarisiima D, Clark TD, et al. Improved employment and education outcomes in households of HIV-infected adults with high CD4 cell counts: evidence from a community health campaign in Uganda. AIDS (London, England) 2013 Feb 20;27(4):627–34. doi: 10.1097/QAD.0b013e32835c54d8. Compelling argument for the secondary economic benefits of successful ART programs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Annals of internal medicine. 2012 Jun 5;156(11):817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ (Clinical research ed) 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS one. 5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hull MW, Montaner J. Antiretroviral therapy: a key component of a comprehensive HIV prevention strategy. Curr HIV/AIDS Rep. 2011 Jun;8(2):85–93. doi: 10.1007/s11904-011-0076-6. [DOI] [PubMed] [Google Scholar]