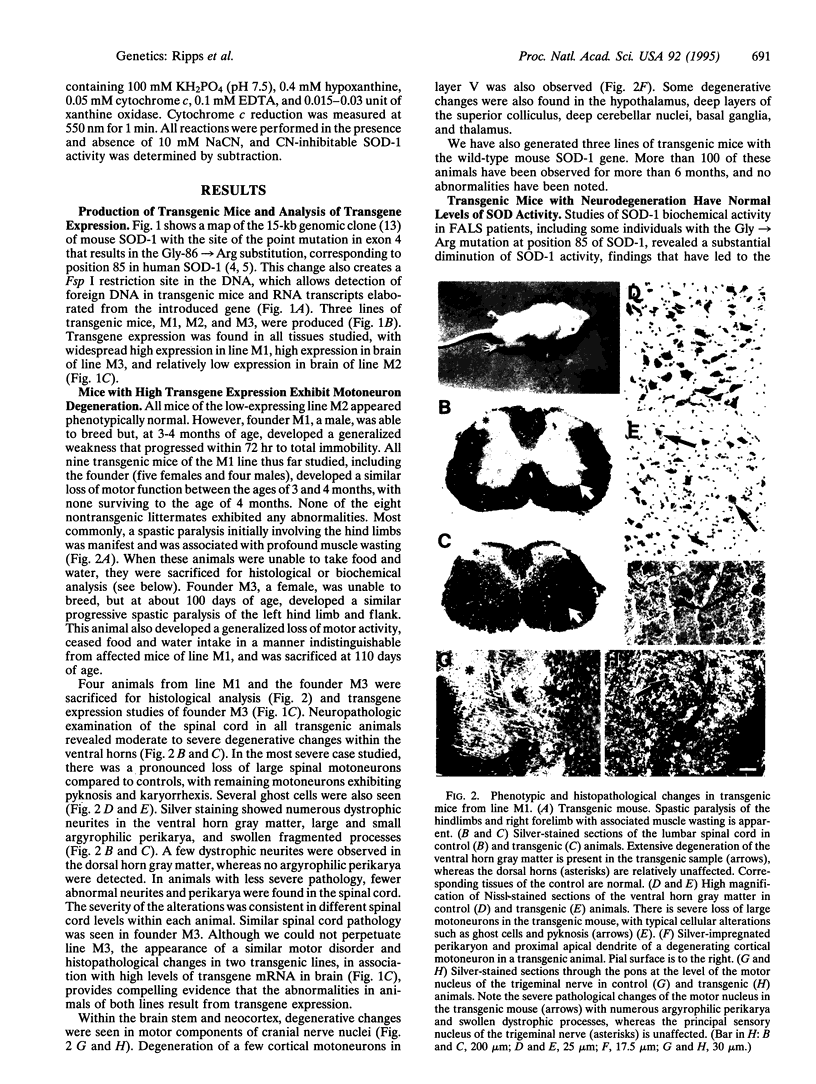
Abstract
Amyotrophic lateral sclerosis is a progressive neurodegenerative disorder primarily involving motoneurons. A subset of individuals with familial autosomal dominant forms of the disease have mutations of the copper/zinc superoxide dismutase (Cu/Zn SOD, SOD-1) gene, which encodes a ubiquitously expressed enzyme that plays a key role in oxygen free radical scavenging. This observation suggests that altered or reduced SOD-1 activity may play a role in the neurodegenerative process. To explore this possibility further, we have introduced a mutation into the mouse SOD-1 gene that corresponds to one of the changes found in the human gene in familial amyotrophic lateral sclerosis. Integration and expression of this mouse gene in transgenic mice was identified by the presence of a unique restriction enzyme site in the transgene coding sequence generated by introduction of the mutation. We report here that high expression of this altered gene in the central nervous systems of transgenic mice is associated with an age-related rapidly progressive decline of motor function accompanied by degenerative changes of motoneurons within the spinal cord, brain stem, and neocortex. These findings indicate a causative relationship between altered SOD activity and motoneuron degeneration. Moreover, biochemical studies indicate normal levels of total SOD activity in transgenic mouse tissues, results that indicate that the neurodegenerative disorder does not result from a diminution of activity and, as such, represents a dominant "gain of function" mutation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman A. D., Fackler J. C., Tuck-Muller C. M., Tarpey M. M., Freeman B. A., Rogers M. C. Partial monosomy 21, diminished activity of superoxide dismutase, and pulmonary oxygen toxicity. N Engl J Med. 1988 Jun 23;318(25):1666–1669. doi: 10.1056/NEJM198806233182506. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Benedetto M. T., Anzai Y., Gordon J. W. Isolation and analysis of the mouse genomic sequence encoding Cu(2+)-Zn2+ superoxide dismutase. Gene. 1991 Mar 15;99(2):191–195. doi: 10.1016/0378-1119(91)90126-v. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum A. I., Kukiełka E., Speisky H. Inhibition of rat liver microsomal lipid peroxidation by boldine. Biochem Pharmacol. 1992 Nov 3;44(9):1765–1772. doi: 10.1016/0006-2952(92)90070-y. [DOI] [PubMed] [Google Scholar]
- Côté F., Collard J. F., Julien J. P. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993 Apr 9;73(1):35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W. Production of transgenic mice. Methods Enzymol. 1993;225:747–771. doi: 10.1016/0076-6879(93)25048-7. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science. 1981 Dec 11;214(4526):1244–1246. doi: 10.1126/science.6272397. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W. Transgenic animals. Int Rev Cytol. 1989;115:171–229. doi: 10.1016/s0074-7696(08)60630-0. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Taketo M., Schroeder A. C., Mobraaten L. E., Gunning K. B., Hanten G., Fox R. R., Roderick T. H., Stewart C. L., Lilly F., Hansen C. T. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet P. G., Guntern R., Hof P. R., Golaz J., Delacourte A., Robakis N. K., Bouras C. A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer's disease. Acta Neuropathol. 1992;83(2):170–178. doi: 10.1007/BF00308476. [DOI] [PubMed] [Google Scholar]
- Wisniewski K., Dambska M., Jenkins E. C., Sklower S., Brown W. T. Monosomy 21 syndrome: further delineation including clinical, neuropathological, cytogenetic and biochemical studies. Clin Genet. 1983 Feb;23(2):102–110. doi: 10.1111/j.1399-0004.1983.tb01856.x. [DOI] [PubMed] [Google Scholar]




