Abstract
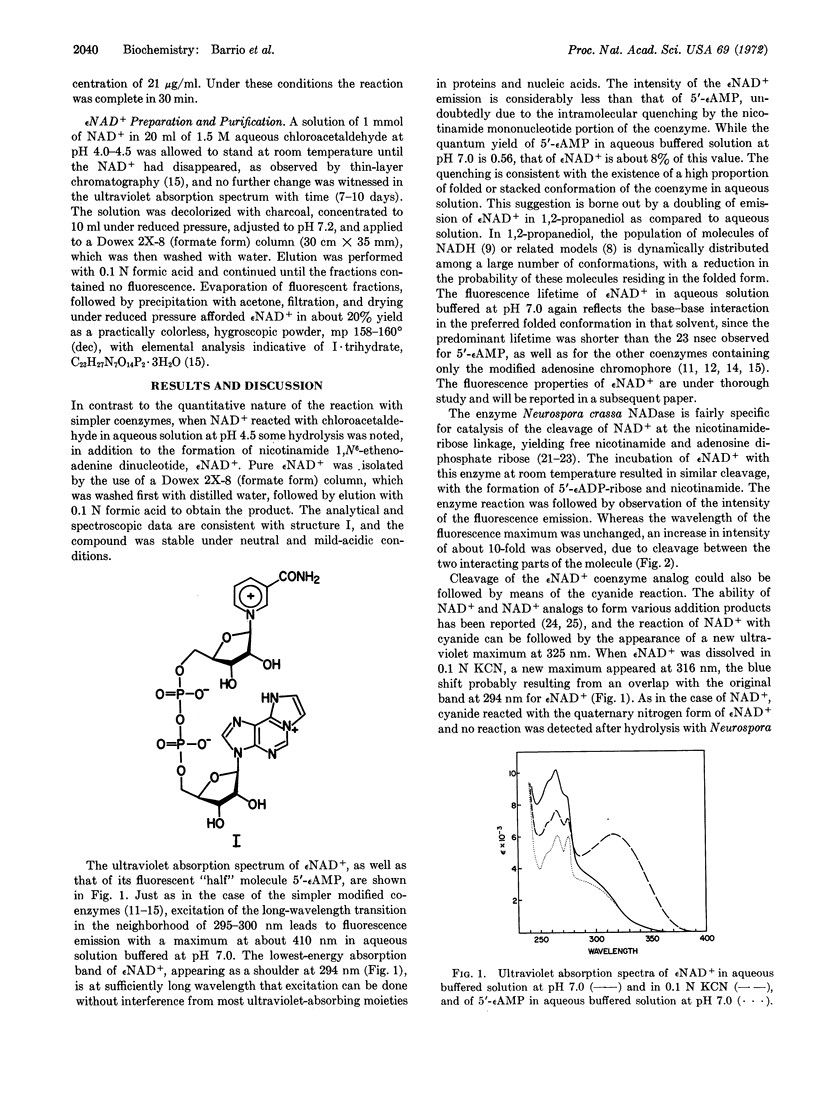
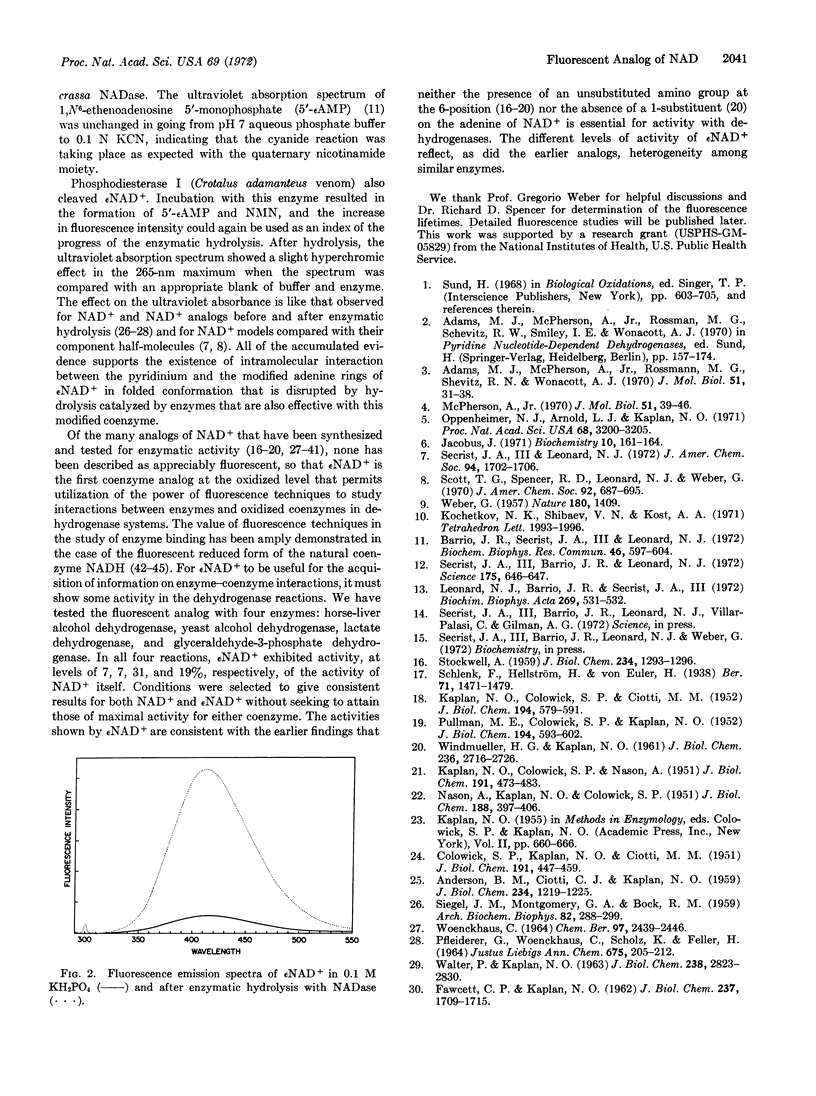
Nicotinamide 1,N6-ethenoadenine dinucleotide, a fluorescent analog of the coenzyme nicotinamide adenine dinucleotide, has been synthesized by the reaction of chloroacetaldehyde with the coenzyme. The technical fluorescence emission maximum of the analog is 410 nm, upon excitation at 300 nm. Its fluorescence yield is about 8% of that of the 1,N6-ethenoadenine 5′-phosphate, and its fluorescence lifteime is shorter. Upon hydrolysis of the modified coenzyme analog with Neurospora crassa NADase or phosphodiesterase I at room temperature, the intensity of fluorescence was increased 10-fold, corresponding to separation of the nicotinamide and ethenoadenine rings. The spectroscopic results with nicotinamide 1,N6-ethenoadenine dinucleotide are consistent with the concept of an intramolecular interaction between the modified adenine and pyridine moieties of the dinucleotide that is disrupted by enzymatic hydrolysis. The fluorescent analog showed reasonable activity as a substitute for NAD+ in four different dehydrogenase-catalyzed reactions.
Keywords: fluorescent coenzyme; quenching; enzyme activity; NAD+; 1,N6-ethenoadenosine derivative
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B. M., CIOTTI C. J., KAPLAN N. O. Chemical properties of 3-substituted pyridine analogues of diphosphopyridine nucleotide. J Biol Chem. 1959 May;234(5):1219–1225. [PubMed] [Google Scholar]
- Adams M. J., McPherson A., Jr, Rossmann M. G., Schevitz R. W., Wonacott A. J. The structure of the nicotinamide-adenine dinucleotide coenzyme when bound to lactate dehydrogenase. J Mol Biol. 1970 Jul 14;51(1):31–38. doi: 10.1016/0022-2836(70)90267-6. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. Fluorescent adenosine and cytidine derivatives. Biochem Biophys Res Commun. 1972 Jan 31;46(2):597–604. doi: 10.1016/s0006-291x(72)80181-5. [DOI] [PubMed] [Google Scholar]
- CIOTTI M. M., KAPLAN N. O., STOLZENBACH F. E. Reaction of pyridine nucleotide analogues with dehydrogenases. J Biol Chem. 1956 Aug;221(2):833–844. [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- FAWCETT C. P., KAPLAN N. O. Preparation and properties of some nicotinamide adenine dinucleotide analogues with pentose and purine modifications. J Biol Chem. 1962 May;237:1709–1715. [PubMed] [Google Scholar]
- FISHER H. F., McGREGOR L. L. On the nature of the fluorescence of enzyme-DPNH complexes. Biochim Biophys Acta. 1960 Mar 11;38:562–563. doi: 10.1016/0006-3002(60)91296-8. [DOI] [PubMed] [Google Scholar]
- Freda C. E., Stoppani A. O. Kinetics of bovine liver aldehyde dehydrogenase. Effect of coenzyme and aldehyde structure. Enzymologia. 1970 Apr 29;38(4):225–242. [PubMed] [Google Scholar]
- HONJO M., FURUKAWA Y., MORIYAMA H., TANAKA K. Synthesis of diphosphopyridine nucleotide analogs and their reaction with dehvdrogenases. Chem Pharm Bull (Tokyo) 1962 Jan;10:73–75. doi: 10.1248/cpb.10.73. [DOI] [PubMed] [Google Scholar]
- Hoffman D. J., Whistler R. L. Synthesis and properties of nucleotides containing 4-thio-D-ribofuranose. Biochemistry. 1970 May 26;9(11):2367–2372. doi: 10.1021/bi00813a022. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., CIOTTI M. M. Enzymatic deamination of adenosine derivatives. J Biol Chem. 1952 Feb;194(2):579–591. [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NASON A. Neurospora diphosphopyridine nucleotidase. J Biol Chem. 1951 Aug;191(2):473–483. [PubMed] [Google Scholar]
- Leonard N. J., Barrio J. R., Secrist J. A., 3rd A spray reagent for adenine-containing residues: detection by fluorescence. Biochim Biophys Acta. 1972 May 29;269(3):531–532. doi: 10.1016/0005-2787(72)90145-1. [DOI] [PubMed] [Google Scholar]
- NASON A., KAPLAN N. O., COLOWICK S. P. Changes in enzymatic constitution in zinc-deficient Neurospora. J Biol Chem. 1951 Jan;188(1):397–406. [PubMed] [Google Scholar]
- NISSELBAUM J. S., BODANSKY O. Purification and properties of human heart lactic dehydrogenase. J Biol Chem. 1961 Feb;236:323–327. [PubMed] [Google Scholar]
- Oppenheimer N. J., Arnold L. J., Kaplan N. O. A structure of pyridine nucleotides in solution. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3200–3205. doi: 10.1073/pnas.68.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULLMAN M. E., COLOWICK S. P., KAPLAN N. O. Comparison of diphosphopyridine nucleotide with its deaminated derivative in various enzyme systems. J Biol Chem. 1952 Feb;194(2):593–602. [PubMed] [Google Scholar]
- SCHWARCZ M. N., STOPPANI A. O. [Reaction of DPN-aldehyde dehydrogenase of yeast with analogues of diphosphopyridine-adenine dinucleotide (DPN)]. Rev Soc Argent Biol. 1960 Jun-Sep;36:319–333. [PubMed] [Google Scholar]
- SHIFRIN S., KAPLAN N. O., CIOTTI M. M. Fluorescence studies of coenzyme-binding to beef heart lactic dehydrogenase. J Biol Chem. 1959 Jun;234(6):1555–1562. [PubMed] [Google Scholar]
- SIEGEL J. M., MONTGOMERY G. A., BOCK R. M. Ultraviolet absorption spectra of DPN and analogs of DPN. Arch Biochem Biophys. 1959 Jun;82(2):288–299. doi: 10.1016/0003-9861(59)90124-9. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J. A fluorescent modification of adenosine triphosphate with activity in enzyme systems: 1,N 6 -ethenoadenosine triphosphate. Science. 1972 Feb 11;175(4022):646–647. doi: 10.1126/science.175.4022.646. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Leonard N. J. Synthetic spectroscopic models related to coenzymes and base pairs. An "abbreviated" nicotinamide adenine dinucleotide. J Am Chem Soc. 1972 Mar 8;94(5):1702–1706. doi: 10.1021/ja00760a046. [DOI] [PubMed] [Google Scholar]
- VAN EYES J., CIOTTI M. M., KAPLAN N. O. Yeast alcohol dehydrogenase. IV. Coenzyme binding sites. J Biol Chem. 1958 Mar;231(1):571–582. [PubMed] [Google Scholar]
- WALTER P., KAPLAN N. O. SUBSTITUTED NICOTINAMIDE ANALOGUES OF NICOTINAMIDE ADENINE DINUCLEOTIDE. J Biol Chem. 1963 Aug;238:2823–2830. [PubMed] [Google Scholar]
- WINDMUELLER H. G., KAPLAN N. O. The preparation and properties of N-hydroxyethyl derivatives of adenosine, adenosine triphosphate, and nicotinamide adenine dinucleotide. J Biol Chem. 1961 Oct;236:2716–2726. [PubMed] [Google Scholar]
- Woenckhaus C., Zumpe P. Uber die Bedeutung des Adeninringes im Coenzym Nicotinamid-adenin-dinucleotid. Eigenschaften des Coenzymmodells Nicotinamid-3-desazapurin-dinucleotid. Z Naturforsch B. 1968 Apr;23(4):484–490. [PubMed] [Google Scholar]


