Abstract
T7 phage induces two negative control mechanisms of protein synthesis: (a) Host-gene expression is repressed by a “T7 repressor,” and (b) early T7 protein synthesis is inhibited by a late phage protein.
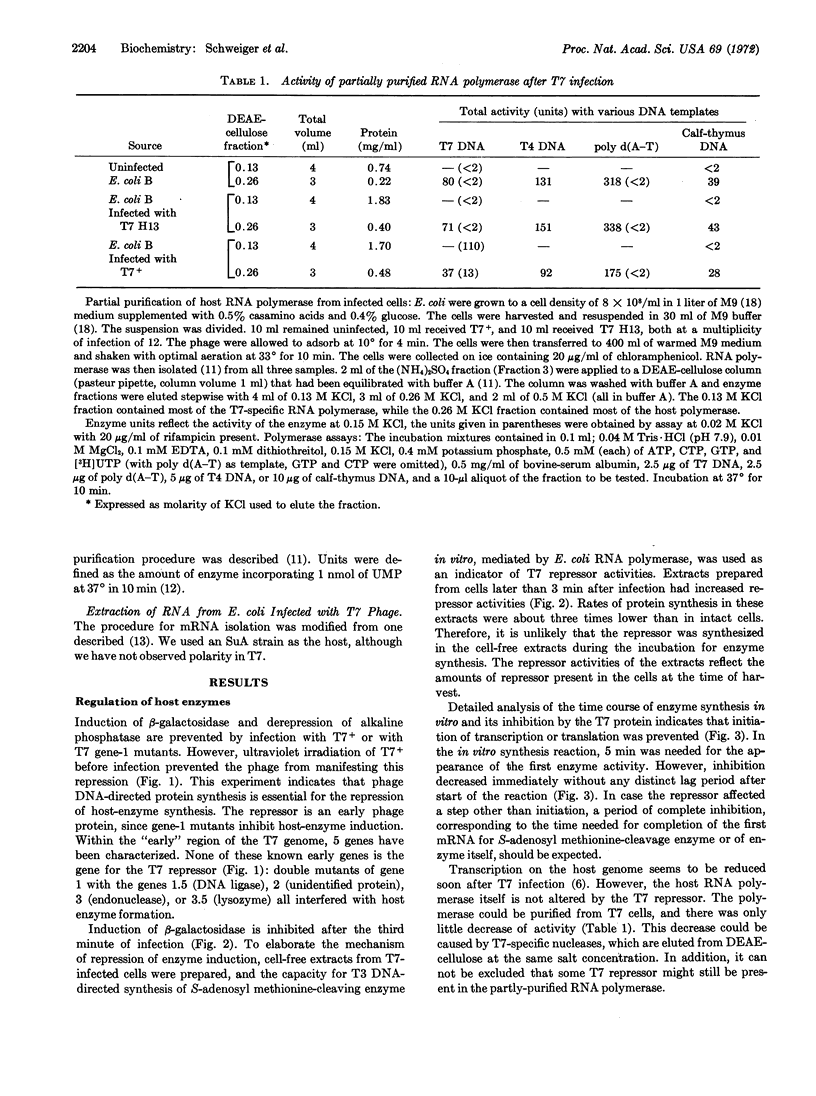
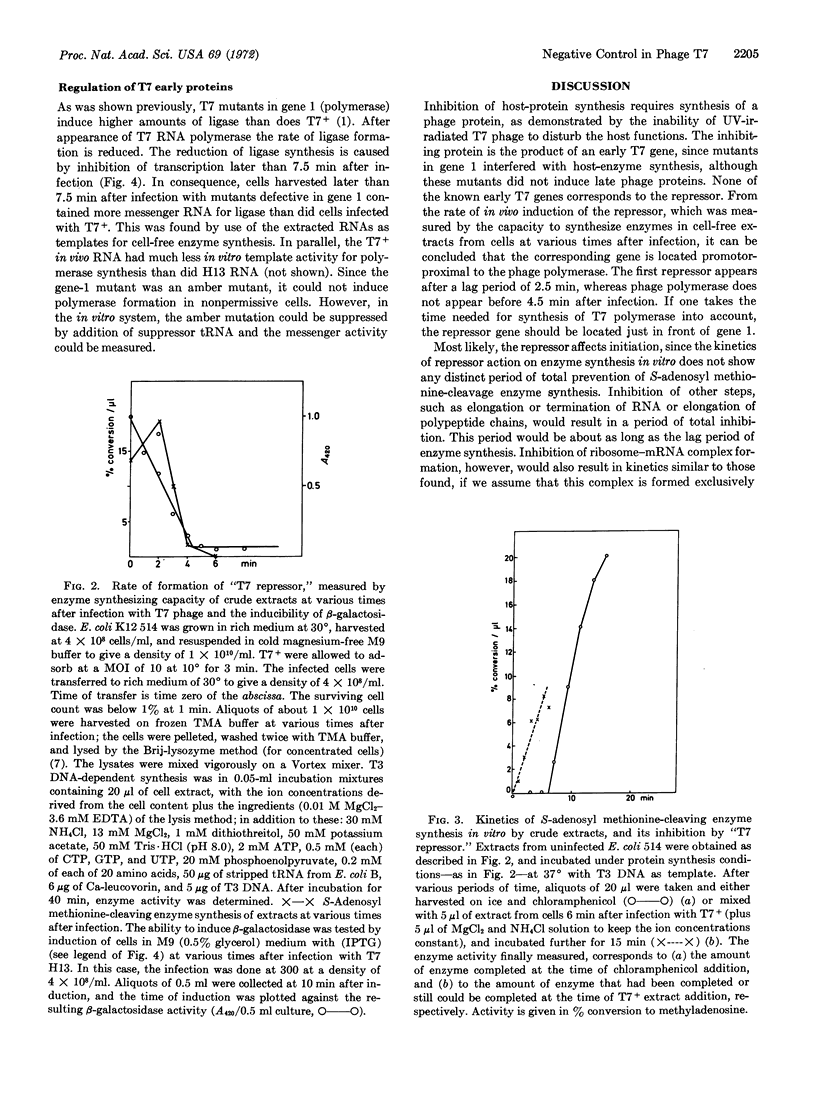
(a) The repressor for host enzyme synthesis is an early T7 protein. Its gene is none of the known early genes; it is located promotor-proximal to gene 1. The repressor function of this protein can be demonstrated by DNA-dependent enzyme synthesis in vitro.
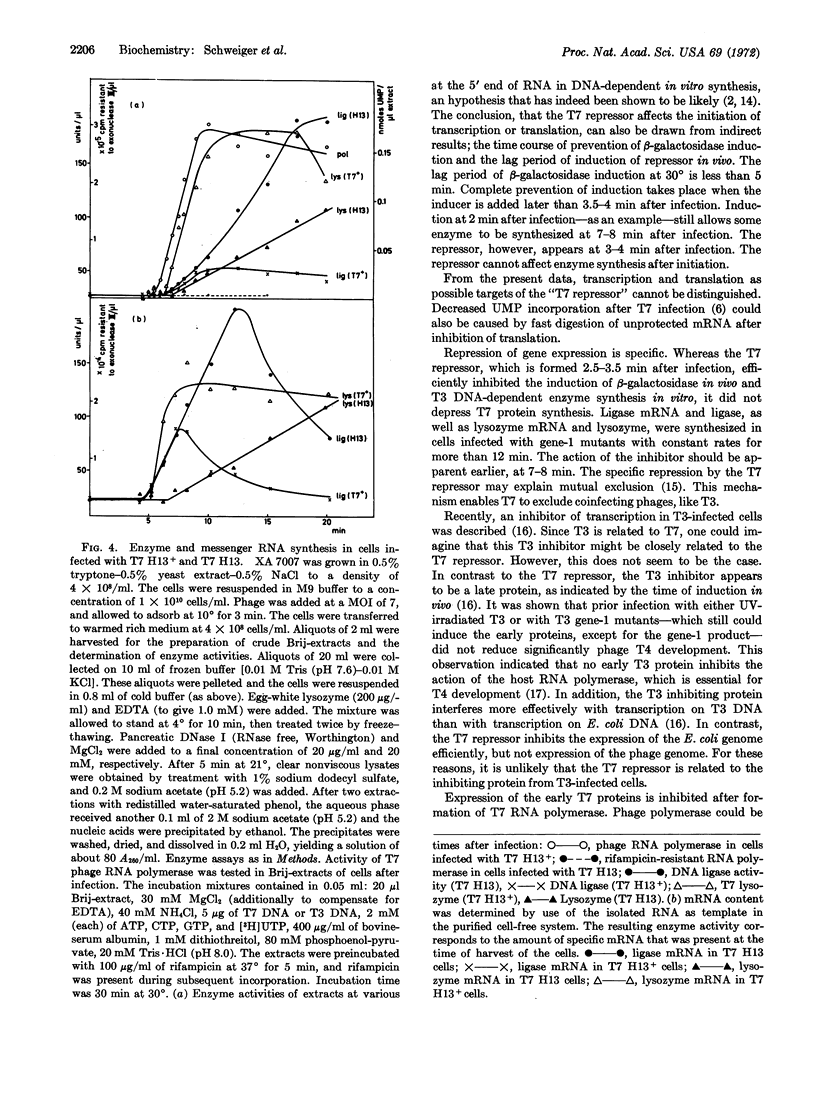
(b) Expression of early phage gene is depressed by a late phage protein or by T7 RNA polymerase. Control takes place on the level of transcription.
Keywords: E. coli, S-adenosyl methionine cleavage, T3 phage, RNA polymerase
Full text
PDF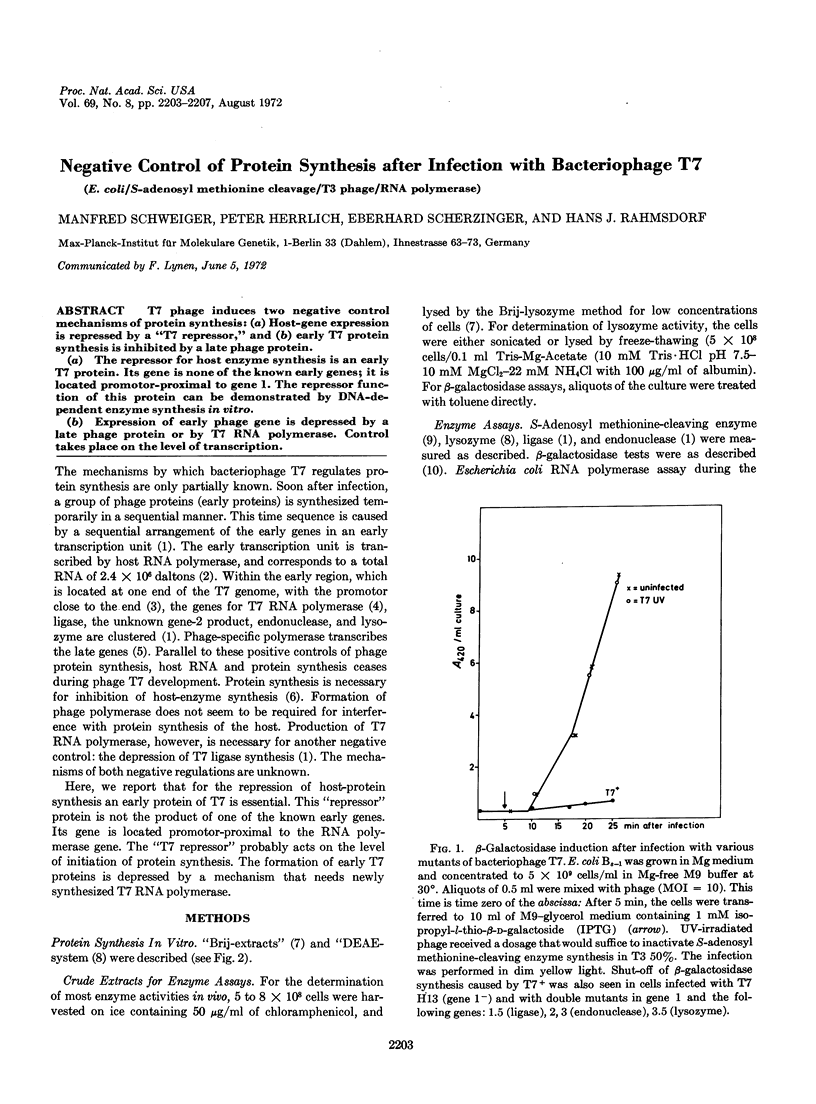

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunovskis I., Summers W. C. The process of infection with coliphage T7. V. Shutoff of host RNA synthesis by an early phage function. Virology. 1971 Jul;45(1):224–231. doi: 10.1016/0042-6822(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. T3 and T7 bacteriophage deoxyribonucleic acid-directed enzyme synthesis in vitro. J Virol. 1970 Dec;6(6):750–753. doi: 10.1128/jvi.6.6.750-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadik S. P., Dharmgrongartama B., Srinivasan P. R. An inhibitory protein of Escherichia coli RNA polymerase in bacteriophage T3-infected cells (core polymerase-sigma factor-host polymerase-phage polymerase-initiation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):162–166. doi: 10.1073/pnas.69.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Schweiger M., Herrlich P. Control of gene function in bacteriophage Tr. 3. Preventing the shutoff of early enzyme synthesis. J Virol. 1971 Nov;8(5):613–618. doi: 10.1128/jvi.8.5.613-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Herrlich P., Schweiger M., Schuster H. The early region of the DNA of bacteriophage T7. Eur J Biochem. 1972 Feb 15;25(2):341–348. doi: 10.1111/j.1432-1033.1972.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Herrlich P., Millette R. L. Gene expression in vitro from deoxyribonucleic acid of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6707–6712. [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Young E. T., 2nd, van Houwe G. Control of synthesis of glucosyl transferase and lysozyme messengers after T4 infection. J Mol Biol. 1970 Aug;51(3):605–619. doi: 10.1016/0022-2836(70)90011-2. [DOI] [PubMed] [Google Scholar]


