Abstract
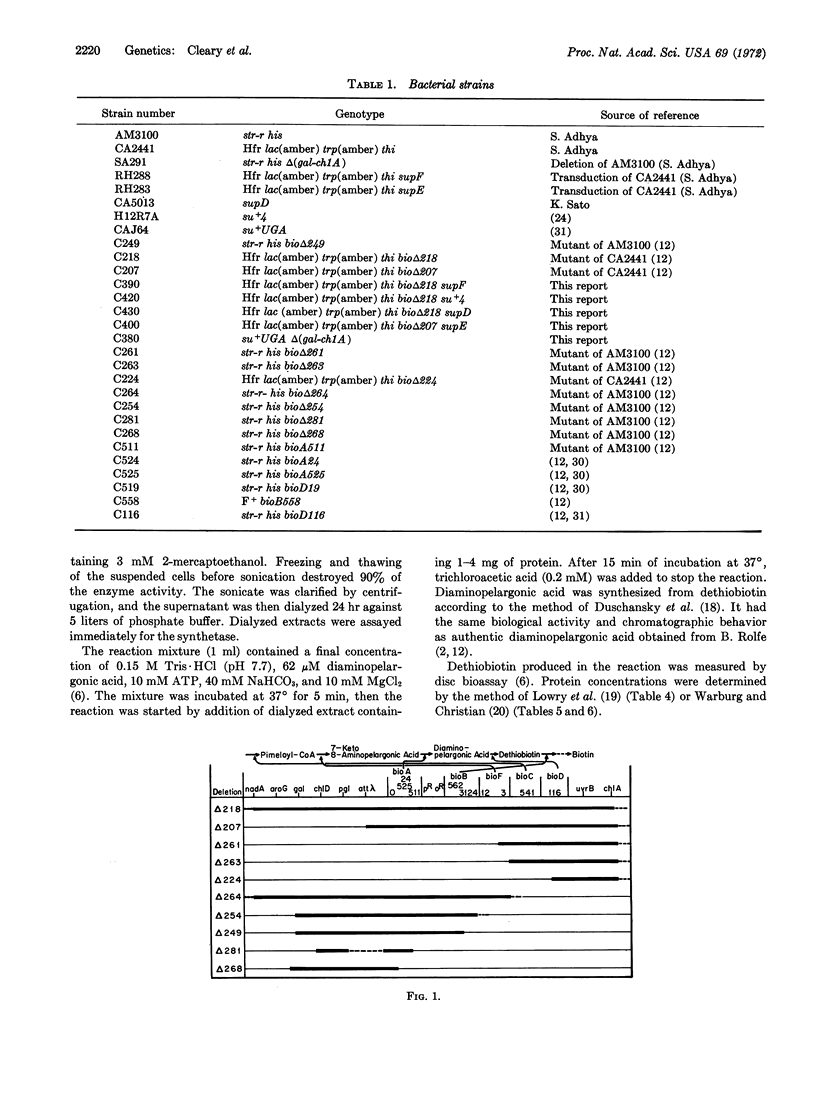
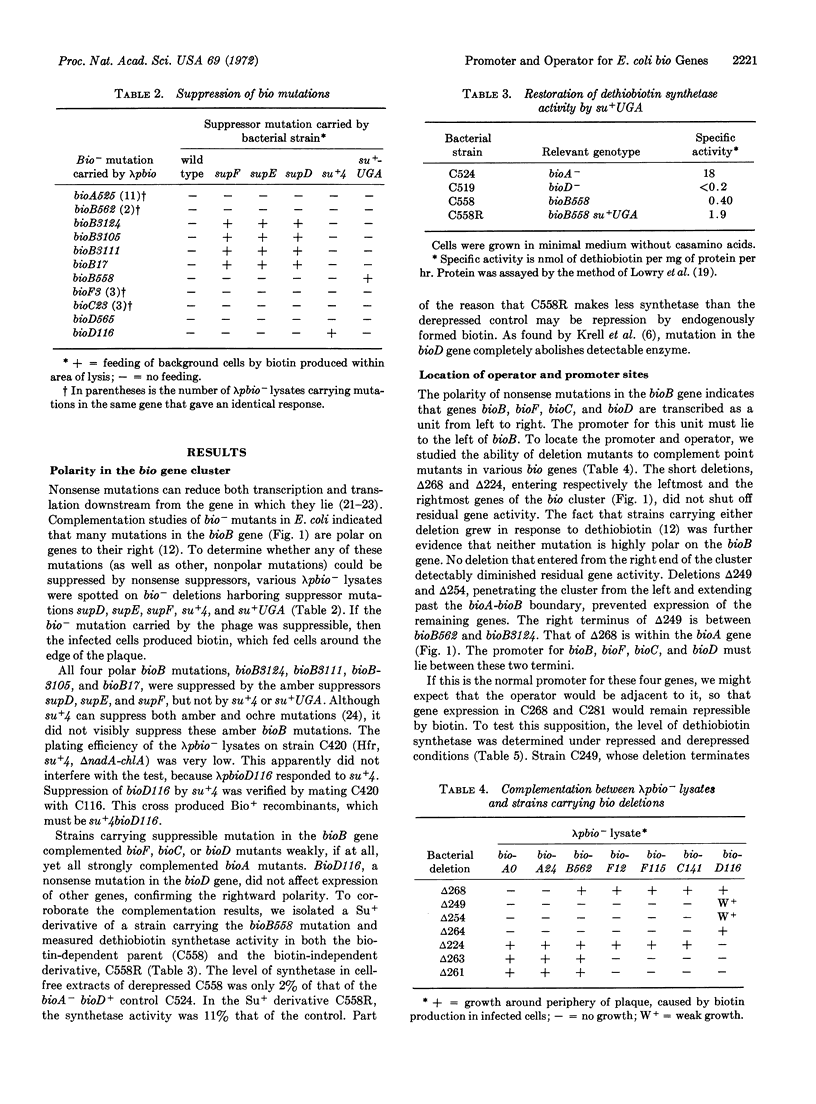
Biotin independence in E. coli requires five closely linked genes, bioA, bioB, bioF, bioC, and bioD. The residual gene activity of deletion mutants has been studied by complementation and enzyme assays. Deletion of the left end of the bioA gene does not impair expression of the remaining genes, but deletions from the left extending into bioB abolish all gene expression. Nonsense mutations in bioB reduce expression of bioC, bioF, and bioD. Therefore, the four genes, bioB, bioF, bioC, and bioD, are transcribed as a unit from left to right, from a promotor located between bioA and bioB.
Expression of the bio genes is repressible by added biotin. Deletions removing the left end of bioA do not affect repressibility of bioD. Therefore the operator, as well as the promoter, lie to the right of bioA. One deletion that removes bioA, bioB, and bioF renders the bioD gene constitutive, presumably by fusion to an unknown operon. Therefore, the operator lies to the left of bioC.
Keywords: polar mutations, deletion mutations, nonsense suppressors, dethiobiotin synthetase
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Callahan R., Blume A. J., Balbinder E. Evidence for the order promoter-operator-first structural gene in the tryptophan operon of Salmonella. J Mol Biol. 1970 Aug;51(3):709–715. doi: 10.1016/0022-2836(70)90019-7. [DOI] [PubMed] [Google Scholar]
- Campbell A., Del Campillo-Campbell A., Chang R. A mutant of Escherichia coli that requires high concentrations of biotin. Proc Natl Acad Sci U S A. 1972 Mar;69(3):676–680. doi: 10.1073/pnas.69.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campillo-Campbell A., Kayajanian G., Campbell A., Adhya S. Biotin-requiring mutants of Escherichia coli K-12. J Bacteriol. 1967 Dec;94(6):2065–2066. doi: 10.1128/jb.94.6.2065-2066.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Krell K. Dethiobiotin synthesis from 7,8-diaminolargonic acid in cell-free extracts of a biotin auxotroph of Escherichia coli K-12. J Biol Chem. 1969 Oct 25;244(20):5503–5509. [PubMed] [Google Scholar]
- Eisenberg M. A., Maseda R. An early intermediate in the biosynthesis of biotin: Incorporation studies with [1,7-C(2)]pimelic acid. Biochem J. 1966 Dec;101(3):601–606. doi: 10.1042/bj1010601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Star C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J Bacteriol. 1968 Oct;96(4):1291–1297. doi: 10.1128/jb.96.4.1291-1297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Klopotowski T., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium. IV. A positive selection for polar histidine-requiring mutants from histidine operator constitutive mutants. J Mol Biol. 1967 Nov 28;30(1):81–95. doi: 10.1016/0022-2836(67)90245-8. [DOI] [PubMed] [Google Scholar]
- Gallucci E., Garen A. Suppressor genes for nonsense mutations. II. The su-4 and su-5 suppressor genes of Escherichia coli. J Mol Biol. 1966 Jan;15(1):193–200. doi: 10.1016/s0022-2836(66)80220-6. [DOI] [PubMed] [Google Scholar]
- Guha A. Divergent orientation of transcription from the biotin locus of Escherichia coli. J Mol Biol. 1971 Feb 28;56(1):53–62. doi: 10.1016/0022-2836(71)90083-0. [DOI] [PubMed] [Google Scholar]
- Ippen K., Miller J. H., Scaife J., Beckwith J. New controlling element in the Lac operon of E. coli. Nature. 1968 Mar 2;217(5131):825–827. doi: 10.1038/217825a0. [DOI] [PubMed] [Google Scholar]
- JACOB F., ULLMAN A., MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [PubMed] [Google Scholar]
- Krell K., Eisenberg M. A. The purification and properties of dethiobiotin synthetase. J Biol Chem. 1970 Dec 25;245(24):6558–6566. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATSUSHIRO A., KIDA S., ITO J., SATO K., IMAMOTO F. The regulatory mechanism of enzyme synthesis in the tryptophan biosynthetic pathway of Escherichia coli K-12. Biochem Biophys Res Commun. 1962 Oct 17;9:204–207. doi: 10.1016/0006-291x(62)90058-x. [DOI] [PubMed] [Google Scholar]
- Model P., Webster R. E., Zinder N. D. The UGA codon in vitro: chain termination and suppression. J Mol Biol. 1969 Jul 14;43(1):177–190. doi: 10.1016/0022-2836(69)90087-4. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- PAI C. H., LICHSTEIN H. C. THE BIOSYNTHESIS OF BIOTIN IN MICROORGANISMS. I. THE PHYSIOLOGY OF BIOTIN SYNTHESIS IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Apr 12;100:28–35. doi: 10.1016/0304-4165(65)90423-x. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Miller J. H., Scaife J. G., Beckwith J. R. A mechanism for repressor action. J Mol Biol. 1969 Jul 14;43(1):201–213. doi: 10.1016/0022-2836(69)90089-8. [DOI] [PubMed] [Google Scholar]
- SARABHAI A. S., STRETTON A. O., BRENNER S., BOLLE A. CO-LINEARITY OF THE GENE WITH THE POLYPEPTIDE CHAIN. Nature. 1964 Jan 4;201:13–17. doi: 10.1038/201013a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F., Fan D. P., Brenner S. A strong suppressor specific for UGA. Nature. 1967 Apr 29;214(5087):452–453. doi: 10.1038/214452a0. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Magasanik B. Nature and self-regulated synthesis of the repressor of the hut operons in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1493–1497. doi: 10.1073/pnas.68.7.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]


