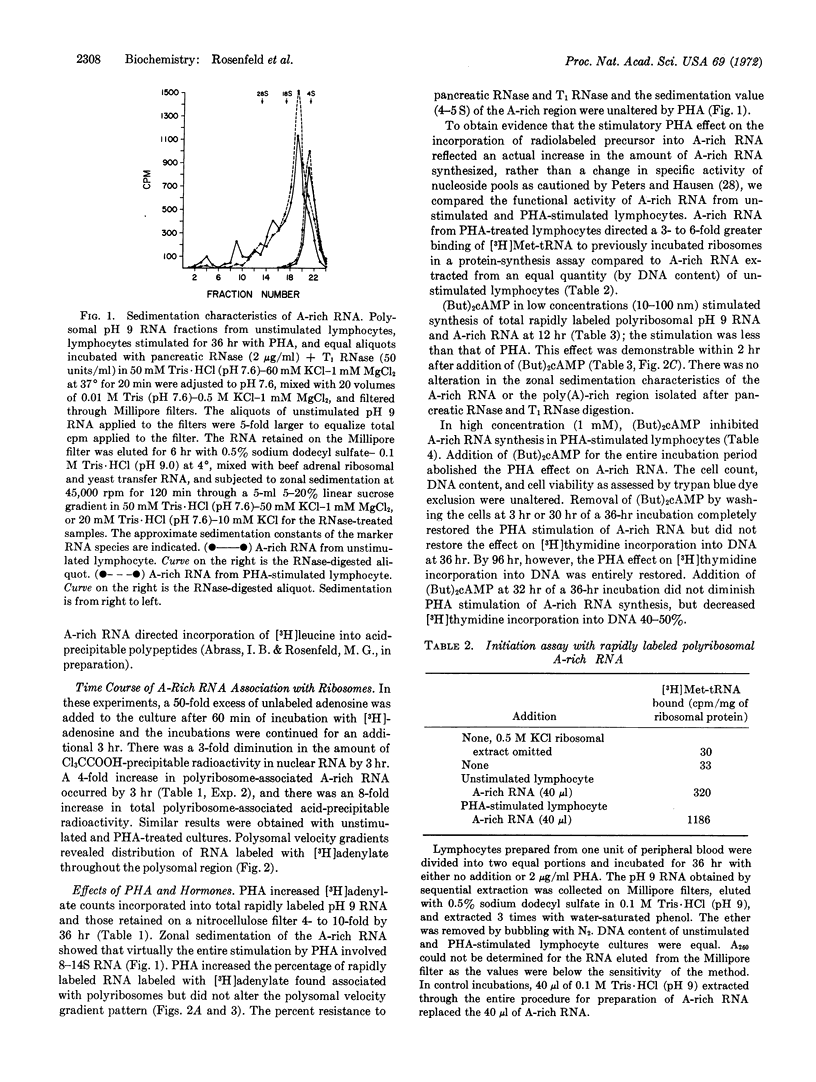
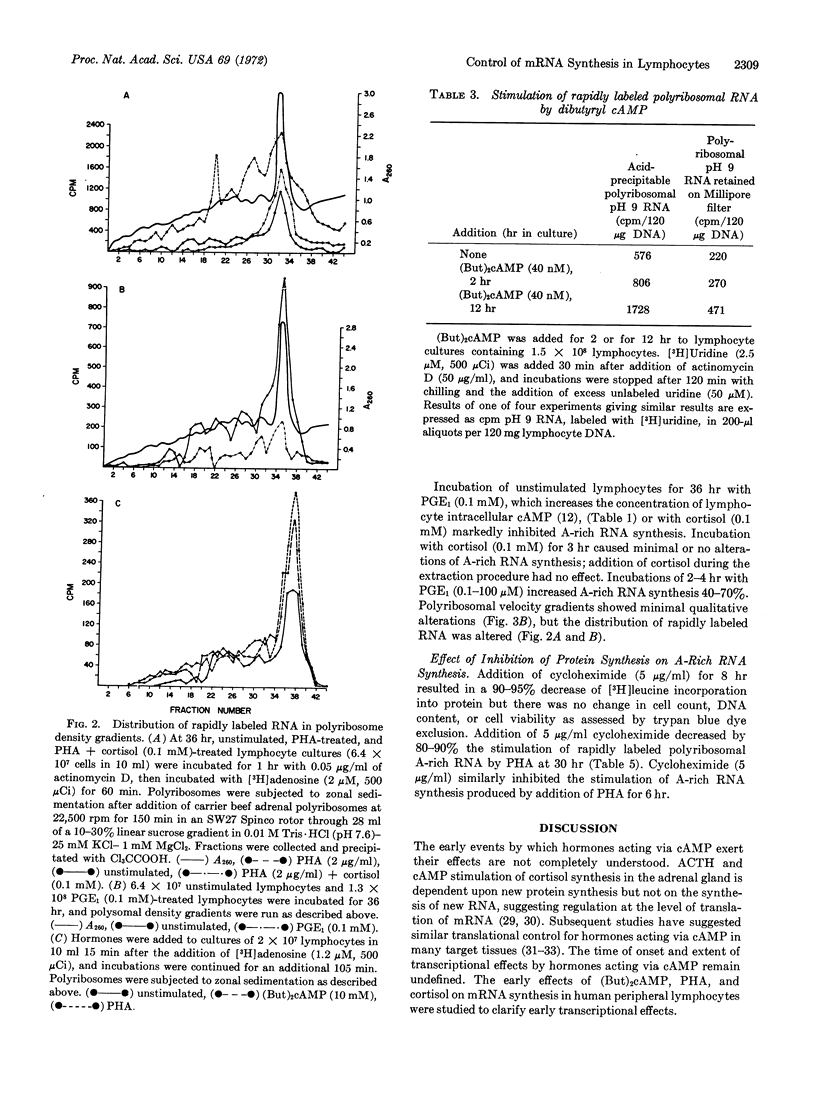
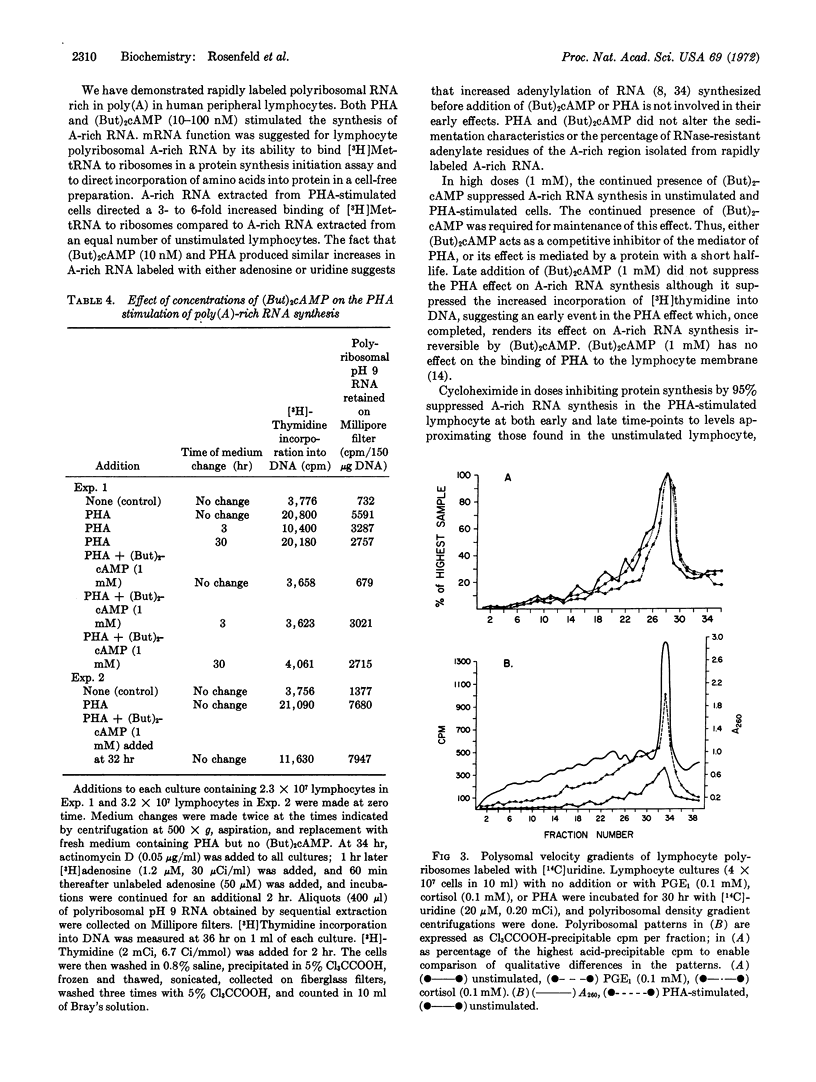
Abstract
Rapidly labeled polyribosomal RNA rich in poly(A) has been isolated from cultures of highly purified human peripheral blood lymphocytes. Messenger RNA function for this RNA is suggested by its ability to direct [3H]Met-tRNA binding to ribosomes and incorporation of amino acids into protein in a cell-free preparation. Phytohemagglutinin and low concentrations of dibutyryl cAMP (40 nM) increase poly(A)-rich RNA synthesis 40% within 2 hr, and 100-300% by 12 hr; the percent poly(A) content and the size of the poly(A)-rich portion remain constant. Higher concentrations of dibutyryl cAMP (1 nM), which prevent morphological transformation of lymphocytes by phytohemagglutinin, inhibit synthesis of poly(A)-rich RNA in phytohemagglutinin-treated lymphocytes without damaging cells. Cortisol (0.1 mM), which also prevents lymphocyte transformation, inhibits poly(A)-rich RNA synthesis by 80%. Cycloheximide (5 μg/ml), which decreases protein synthesis by 90%, decreases poly(A)-rich RNA synthesis 80% in cells stimulated by phytohemagglutinin. These studies demonstrate that, as part of the early molecular events of their action, phytohemagglutinin and cortisol regulate transcription of adenylate-rich RNA in human lymphocytes, and that similar transcriptional effects can be produced by dibutyryl cAMP.
Keywords: mRNA function, dibutyryl cAMP, phytohemagglutinin, cycloheximide, cortisol
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- COOPER E. H., BARKHAN P., HALE A. J. Observations on the proliferation of human leucocytes cultured with phytohaemagglutinin. Br J Haematol. 1963 Jan;9:101–111. doi: 10.1111/j.1365-2141.1963.tb05446.x. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Redfield B., Weissbach H. Formylation of guinea pig liver methionyl-sRNA. Arch Biochem Biophys. 1967 Apr;120(1):119–123. doi: 10.1016/0003-9861(67)90605-4. [DOI] [PubMed] [Google Scholar]
- Cross M. E., Ord M. G. Changes in histone phosphorylation and associated early metabolic events in pig lymphocyte cultures transformed by phytohaemagglutinin or 6-N,2'-O-dibutyryladenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Aug;124(1):241–248. doi: 10.1042/bj1240241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMONDS M., ABRAMS R. Polynucleotide biosynthesis: formation of a sequence of adenylate units from adenosine triphosphate by an enzyme from thymus nuclei. J Biol Chem. 1960 Apr;235:1142–1149. [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garren L. D., Gill G. N., Masui H., Walton G. M. On the mechanism of action of ACTH. Recent Prog Horm Res. 1971;27:433–478. doi: 10.1016/b978-0-12-571127-2.50035-3. [DOI] [PubMed] [Google Scholar]
- Garren L. D. The mechanism of action of adrenocorticotropic hormone. Vitam Horm. 1968;26:119–145. doi: 10.1016/s0083-6729(08)60753-0. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Gross P. R. Translation-level control of amylase and protein synthesis by epinephrine. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1081–1088. doi: 10.1073/pnas.65.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwar S. S., Spears C., Weissbach H. Studies on the initiation of protein synthesis in animal tissues. Biochem Biophys Res Commun. 1970 Oct 9;41(1):78–84. doi: 10.1016/0006-291x(70)90471-7. [DOI] [PubMed] [Google Scholar]
- Labrie F., Béraud G., Gauthier M., Lemay A. Actinomycin-insensitive stimulation of protein synthesis in rat anterior pituitary in vitro by dibutyryl adenosine 3',5'-monophosphate. J Biol Chem. 1971 Mar 25;246(6):1902–1908. [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. A new fluorometric method for RNA and DNA determination. Anal Biochem. 1966 Oct;17(1):100–107. doi: 10.1016/0003-2697(66)90012-1. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Initiation of polysome formation in mouse sarcoma 180 ascites cells. Utilization of cytoplasmic messenger ribonucleic acid. Biochemistry. 1971 Mar 2;10(5):895–900. doi: 10.1021/bi00781a026. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- MACKINNEY A. A., Jr, STOHLMAN F., Jr, BRECHER G. The kinetics of cell proliferation in cultures of human peripheral blood. Blood. 1962 Mar;19:349–358. [PubMed] [Google Scholar]
- Means A. R., Abrass I. B., O'Malley B. W. Protein biosynthesis on chick oviduct polyribosomes. I. Changes during estrogen-mediated tissue differentiation. Biochemistry. 1971 Apr 27;10(9):1561–1570. doi: 10.1021/bi00785a009. [DOI] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. I. Stimulation of uridine uptake. Eur J Biochem. 1971 Apr 30;19(4):502–508. doi: 10.1111/j.1432-1033.1971.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Farber J., Baserga R. Gene activation in WI-38 fibroblasts stimulated to proliferate: requirement for protein synthesis. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1725–1729. doi: 10.1073/pnas.68.8.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Bretthauer R. K. Properties of a polyriboadenylate polymerase isolated from yeast ribosomes. Biochemistry. 1971 Apr 27;10(9):1576–1582. doi: 10.1021/bi00785a011. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N., Abrass I. B., Garren L. D. Phosphorylation of ribosome-associated protein by an adenosine 3':5'-cyclic monophosphate-dependent protein kinase: location of the microsomal receptor and protein kinase. Proc Natl Acad Sci U S A. 1971 May;68(5):880–884. doi: 10.1073/pnas.68.5.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Wicks W. D. Differential effects of glucocorticoids and adenosine 3',5'-monophosphate on hepatic enzyme synthesis. J Biol Chem. 1971 Jan 10;246(1):217–223. [PubMed] [Google Scholar]


