Abstract
Repellents are important prophylactic tools for travelers and populations living in endemic areas of malaria, dengue, encephalitis, and other vector-borne diseases. DEET is a safe, broad spectrum repellent, which provides complete protection over a long period of time. Despite its low cost, more affordable alternatives are highly desirable, particularly for those in endemic areas where cost is an impediment. Alternative compounds like IR 3535 and picaridin have been developed using molecular modeling, but the lack of knowledge of the molecular target(s) for DEET has retarded progress towards low cost alternatives. It is known that DEET acts at a distance as an odorant as well as by direct contact, i.e., as a tastant, although DEET reception is primarily mediated by the olfactory system. There is unambiguous evidence that olfactory receptor neurons are involved, and that an odorant receptor co-receptor Orco is essential for DEET reception. In the southern house mosquito, Culex quinquefasciatus, DEET triggers repellence by direct activation of an odorant receptor, CquiOR136, which is also sensitive to a plant defense compound, methyl jasmonate.
Keywords: DEET, IR 3535, Picaridin, PMD, Orco, IR40a, CquiOR136, olfactory receptor neurons, odorant receptors
Introduction
Vector-borne diseases are major health problems for travelers and populations living in endemic regions. Among the most notorious vectors are mosquitoes that unwittingly transmit the protozoan parasites causing malaria and viruses that cause infections, such as dengue, yellow fever, chikungunya, and encephalitis. Therefore, mosquitoes are considered the deadliest animals on the planet, even more so than humans (The Blog of Bill Gates, http://www.gatesnotes.com/). Diseases transmitted by mosquitoes destroy more lives on a year basis than war, terrorism, gun violence, and other human maladies combined. Every year, malaria decimates countless lives – imagine a city of San Francisco perishing to malaria year after year. The number of people incapacitated by the disease every year is larger than the population of Brazil. The suffering and economic consequences in endemic areas are beyond imagination for those living in malaria-free countries. Therefore, people understandably detest mosquitoes as much as they, in general, dislike unnecessary wars, terrorism, and gun violence. Both natives and visitors to endemic areas want to keep these “infected needles” at bay. In the absence of vaccines for malaria, dengue, and encephalitis, one of the most ancient and effective prophylactic measures against mosquito-borne diseases is the use of insect repellents.
Brief history of repellent use
Repellents evolved, or more strictly speaking, were developed from plant-based smoke, extracts from plants (essential oils) into formulations with a single active ingredient or repellent substance. Smoke is still the most widely used means of repelling mosquitoes, typically by burning plants in rural tropics (Moore and Debboun 2007), but also with use of spiral-shaped incenses like katori senko – an archetypal icon of the humid Japanese summers. Citronella and eucalyptus essential oils are widely used not only in candles, but also as topical insect repellents. Low toxicity is of paramount importance in topical applications. Naturally occurring repellents may have very low toxicity, but “inactive ingredients” may render a natural mixture toxic or less desirable. Thus, there has been a shift from extract-based to active repellent substances, such as p-menthane-3,8-diol (PMD).
Repellents that repel and those that do not
Strictly speaking, a repellent is a chemical causing a responder to actively steer away from the stimulus source (Dethier et al. 1960; Miller et al. 2009). Therefore, not all “repellents” repel. To put it simply, a repellent is an odorant that must act in the vapor phase, but some “repellents” are either nonvolatile or have extremely low vapor pressure and, therefore, unable to convey a signal away from the source. On the other hand, other repellents have such high vapor pressure (low boiling point) that their repellency might be misleading as they only last for a short period of time. In practical terms, the end user wants to apply a repellent, which lasts for hours, not one that evaporates quickly – a criterion already known in the early days of product development (Gertler 1944). By contrast, an nonvolatile compound cannot be an odorant that acts at a distance and cause a mosquito to make movements oriented away from the source. It could, however, be a tastant, which disengages a mosquito from feeding. Whether a compound acts as a repellent sensu stricto (Dethier et al. 1960; Miller et al. 2009) or a contact disengagent (Miller et al. 2009) might not be relevant for the end user whose primary concern is to prevent an “infected needle” from making contact with his/her blood stream. However, these are important concepts for those attempting to seek mechanistic clarity. After all, how can one develop “better repellents,” if it is not known what properties one is trying to improve?
Our predecessors did not have at their disposal our current understanding of the molecular basis of insect olfaction, but they generated an impressive database of chemicals (ca. 20,000 compounds) with repellent activity that allowed them to find a repellent, DEET, that stood the test of time. DEET is inexpensive and has a remarkable protection time. A recent comprehensive re-assessment of DEET by the United States Environmental Protection Agency (EPA) concluded that insect repellents containing DEET do not present a health concern to the general population, including children (EPA, http://www2.epa.gov/insect-repellents/deet). Unfortunately, a huge proportion of those who can afford DEET do not use it, because of undesirable properties, such as its unpleasant odor (an embarrassment of the riches), whereas those who need it the most cannot afford daily use of DEET. Thus, our generation is challenged with the discovery of better, safer, and – more importantly - cheaper alternatives. With the current technology, it takes about 10 years and approximately $30 million to bring a new insect repellent to market (Gupta and Bhattacharjee 2007). There is a clear dichotomy between investment recovery and affordability of a newly developed product. I argue that we must understand how DEET works, discover what molecular target(s) it acts on, and mimic nature before an effective rationale design can be implemented to generate more affordable repellents for those who need them the most.
DEET’s inception
DEET is an acronym for N,N-diethyl-meta-toluamide, which has been renamed N,N-diethyl-3-methylbenzamide (Fig. 1), according to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature. Its predecessor, N,N-diethylbenzamide, albeit irritant to human skin and therefore barred from use, was designed to be relatively nonvolatile (boiling point 280°C) (McCabe et al. 1954). A high boiling point (and consequently lower vapor pressure) confers lower evaporation rate and, consequently, a long protection time – one of the essential properties of a repellent if it is to remain competitive in the market.
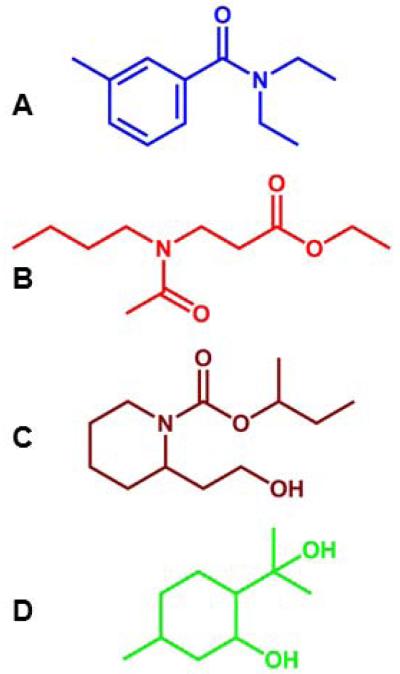
Figure 1.
Structures of the major four insect repellents currently in the market. (A) DEET, (B) IR3535, (C) Picaridin, and (D) PMD. Note the lack of an amide carbonyl moiety and nitrogen atom in PMD, the only natural product in the group, compared to the synthetic counterparts.
Major natural and synthetic repellents in the current market
There is a trend nowadays to believe that if a chemical is a natural product then it is non-toxic and safe, the corollary being that synthetic compounds are toxic and not safe. Both are wrong when taken literally. Strychnine is a natural product and yet its estimated LD50 to humans is 1-2 mg/Kg (stay away!). Even worse, the calculated LD50 for ricin is 20 ng/Kg! That is to say that ricin would kill half of the population exposed to a dose 100,000X lower than that of strychnine. Both are natural products. Indeed, the top ten most toxic compounds are natural products. There is no absolute safety in toxicology. Although the least toxic chemical compound, water is toxic (LD50 90 ml/Kg). DEET is deemed safe for human use. Its acute dermal LD50 in rabbits is 4,280 mg/Kg thus falling into the low toxicity category (>2,000-5,000 mg/Kg) (Jackson et al. 2008). PMD is a natural insect repellent. In studies using laboratory animals, PMD showed no adverse effects except for eye irritation (EPA Fact Sheet 011550, http://www.epa.gov/opp00001/chem_search/reg_actions/registration/fs_PC-011550_01-Apr-00.pdf). PMD appeals to natural product aficionados, but it never debunked DEET, in part, because of the short (2h) complete protection time (National Pesticide Information Center, http://pi.ace.orst.edu/repellents/search.s;jsessionid=D0CE56EBE422B33033CB23A36D1 F7D67?sortBy=ingredient#selectTop). Out of the four top tier insect repellents that remain competitive in the worldwide market, two have been designed more recently by using molecular modeling. In the 70s, Merck developed the insect repellent 3535 thus named IR 3535 or Merck 3535 (IUPAC name: ethyl 3-[acetyl(butyl)amino]propanoate, a derivative of β-alanine (Klier and Kuhlow 1976). In the sunset of last century, Bayer developed KBR 3023 (IUPAC name: 1-piperidinecarboxylic acid 2-(2-hydroxyethyl)-1-methylpropylester), which is also known as picaridin, icaridin, and Bayrepel™ (Boeckh et al. 1996). The four major insect repellents in today’s market are of low toxicity, with DEET and picaridin being the top performing products. Although the literature is dichotomous with some studies, depending on the vector being tested and many other factors, suggesting that DEET is a better repellent while others documenting a better performance of picaridin, DEET remains as the gold standard of insect repellents, but undoubtedly picaridin is a close alternative (Moore and Debboun 2007).
DEET is more than a repellent
DEET is a multitasking compound. First and foremost, it is a repellent sensu stricto (Dethier et al. 1960; Miller et al. 2009), an odorant that causes mosquitoes to steer away from the stimulus source. It acts at a distance, but not too far away from the source given its relatively low volatility (bp 297°C, ChemSpider, http://www.chemspider.com/Chemical-Structure.4133.html). However, there is growing evidence suggesting that DEET is also a tastant, i.e., a compound that acts by direct contact with the insect’s gustatory system (Lee et al. 2010). According to the terminology suggested by Grieco et al. (Grieco et al. 2007), DEET in that context would be a “contact irritant,” whereas as an odorant it would be a “spatial repellent.” It is worth mentioning that Miller et al. (Miller et al. 2009) convincingly argued that unnecessary confusion could be avoided if behaviorally unambiguous terms like spatial repellent and contact irritant were replaced by “non-contact disengagent” and “contact disengagent,” respectively, to indicate, regardless of the mechanism, a reduction of interaction between a responder and the source of the stimulus. The term “special repellency” is widely used (ca. 80,000 hits in Google at the time of this writing), so it is the synonym “aerial repellent” (a product applied somewhere between a host and the vector), but a more rigorous and mechanistically accurate terminology laid out by Miller and colleagues (Miller et al. 2009) is recommended. Although we all believe that we have seen mosquitoes making movement directed away from sources (hosts or attractants) “spiked” with DEET, demonstration that disengagement is indeed repellency is yet to be forthcoming. DEET also acts as a contact disengagent. There is even evidence that DEET, most likely detected by the gustatory system, leads to reduced oviposition (Tikar et al. 2014). Since the specific mechanisms are unknown, DEET is here acting as a contact disengagent. These concepts are relevant when designing bioassays and unraveling the molecular basis of DEET detection.
DEET modes of action may not be universal
DEET is enigmatic not only when comes to repellence activity, but also regarding its mode(s) of action. The sex pheromone of the silkworm moth, bombykol, was discovered almost a decade after DEET was already protecting the lives of war fighters and later being used by the public at large. Scientists have already unraveled the intricacies and molecular details of bombykol reception and perception. We now know how bombykol is transported inside insect antennae by a carrier pheromone-binding protein and delivered to a neuron-housed odorant receptor. The molecular details of the silkworm bombykol receptor, including putative binding sites, have been untangled. It has been unraveled how bombykol signals are processed in the brain and integrated with other stimuli to ultimately start odorant-guided navigation (reviewed in (Leal 2013)). By contrast, DEET is older than any investigator interested in its mode of action yet our understanding of DEET reception is still in its infancy. Our ignorance of the subject is due in part to the lack of appropriate funding, the challenging nature of the subject, the one-size-fits-all attempts to understand vector biology with surrogates, and, perhaps dogmatic positions against new insights that challenge old models. The latter is exacerbated by top tier journals interest in simple and across-the-board explanations to complex problems. Insects are diverse; they may have found different solutions for “common” problems. Although groundbreaking, the many discoveries by Sir Vincent Wigglesworth, a founder of the discipline of insect physiology, did not explain the physiology of other insect species. It is unlikely that the fruit fly would detect DEET in the same way that mosquitoes do. After all, for the latter DEET interferes with an essential step for reproduction: a blood meal to acquire essential nutrients for fertilization. DEET has no known ecological significance for the fruit fly. Flies may never encounter DEET, except at times of laboratory bioassays.
The three major mosquito species currently under investigation are the southern house mosquito, Cx. quinquefasciatus, the yellow fever mosquito, Ae. aegypti, and the malaria mosquito, An. gambiae. Their biology, behavior and chemical ecology differ in so many ways that it should not be entirely surprising if they have different modes of action for detection of this human-made compound.
DEET modes of action
It is well known that DEET generates electroantennographic (EAG) responses on mosquito antennae (Costantini et al. 2001; Leal 2007; Leal and Uchida 1998), and olfactory receptor neurons (ORNs) sensitive to DEET have been identified in the antennae of the southern house mosquito (Syed and Leal 2008) and the yellow fever mosquito (Boeckh et al. 1996; Stanczyk et al. 2010). It is known that ORNs on antennae express ORs along with an odorant receptor co-receptor (Orco) (reviewed in (Leal 2013)). The findings that DEET activates antennal neurons suggest a direct activation of antennal receptors.
One of the earliest hypotheses is that DEET interferes with detection of lactic acid (Davis 1985). The notion of DEET “jamming” the olfactory system was reinforced by the suggestion that this insect repellent attenuates the responses of the malaria mosquito to 1-octen-3-ol (Ditzen et al. 2008). Further evidence was provided by a natural polymorphism of an OR from the fruit fly, DmelOR59B, which is sensitive to 1-octen-3-ol. The Brazilian strain Boa Esperança, albeit sensitive to 1-octen-3-ol, was insensitive to DEET, and this observation led to identification of relevant residues in DmelOR59B associate with DEET interference in the fruit fly (Pellegrino et al. 2011).
A convincing evidence for direct detection of DEET was generated with mutations of the co-receptor orco gene in the yellow fever mosquito (DeGennaro et al. 2013). The rationale for this landmark paper was that mutations in the orco gene should eliminate signaling mediated by all ORs. Indeed, orco mutants did not respond to 1-octen-3-ol, but responded normally to CO2, which is detected by gustatory receptors. In contrast to starved wild-type (WT), starved male and female orco mutants showed little or no response to honey thus suggesting that the entire olfactory system of the orco mutants was impaired. Additionally, WT females showed moderate responses to human derived attractants devoid of CO2, but orco mutants did not. The lack of response could be compensated in the presence of CO2. More importantly to the current discussion, WT mosquitoes avoided the port of an olfactometer treated with DEET and accumulated in the control port, whereas orco mutants accumulated in both ports, thus, demonstrating insensitivity to DEET as an odorant (DeGennaro et al. 2013). These findings suggested that as a repellent sensu stricto (or special repellent) DEET is detected by odorant receptor(s).
Further behavioral assays with the orco mutants demonstrated that as a “contact disengagent” DEET is not detected by the olfactory system. In an arm-in-cage biting assay, DEET treatment prevented biting by both WT and orco mutants. The authors concluded their study by discussing three hypotheses. First, DEET may silence ORs tuned to attractive odorants. Second, DEET may activate one or a few ORs to trigger repulsion. Third, DEET may act as a “confusant” to modulate activity of many ORs. They refuted the first hypothesis and suggested that DEET may act by direct activation of ORs and modulation of the activity of other ORs (DeGennaro et al. 2013).
Despite the solid evidence derived from the above-described research with orco mutants, which supports the hypothesis that in mosquitoes DEET reception is mediated by direct activation of OR(s), and possibly modulation of other (ORs) a more recent literature suggests that DEET is detected by an ionotropic receptor (Kain et al. 2013). This is a separate family of receptors (Rytz et al. 2013), which are independent from the coreceptor Orco. It is a conundrum given that incapacitating Orco would not affect IRs. With an innovative approach, Kain et al. identified neurons in the sacculus, a pit-like structure in the antennae of the fruit fly, and an inonotropic receptor, IR40a, sensitive to DEET. Additionally, IR40a knockdown flies showed a significant loss of avoidance to DEET in a two-choice trap assay (Kain et al. 2013). Although the reported data were convincing that the fruit flies detected DEET with the identified neurons and receptor, there was no documented evidence for mosquitoes. It was suggested, however, that “IR40a can account for the widespread effect of DEET olfactory repellency because it is highly conserved in species that show strong avoidance to it including Drosophila, mosquitoes, head lice and trobolium, but not in the honey bee” (Kain et al. 2013). Our trascriptome analysis suggests that CquiIR40a is unlikely a DEET receptor in the southern house mosquito (Leal et al. 2013). Transcript levels of CquiIR40a in antennae are very low and the receptor gene is poorly enriched in olfactory as compared to non-olfactory tissue (moderated logtwofold change; female antennae/legs, 0.74) (Leal et al. 2013). It is, therefore, conceivable that DEET reception in the fruit flies and mosquitoes differ.
The hypothesis that IR 40a is involved in DEET reception in mosquitoes has been just tested in Cx. quinquefasciatus (Xu et al. 2014). Electrophysiological and behavioral responses of knockdown mosquitoes were compared with WT and control mosquitoes. Adult female mosquitoes emerging from pupae, which were injected with CquiIR40a-double-strand RNA (dsRNA), showed significant reduction in CquiIR40a transcript levels. Despite reduced CquiIR40a transcript levels, electroantennographic (EAG) and behavioral responses of these knockdown mosquitoes to DEET did not differ significantly from similar responses recorded with two groups of control mosquitoes, one of them prepared by injecting dsRNA of a control gene, β-galactosidade-dsRNA, and the other injected with water. The fact that reducing CquiIR40a transcript levels did not affect DEET-elicited behavior or EAG responses, led us to conclude that in the southern house mosquito IR40a is not involved in DEET reception.
Attempts to de-orphanize Cx. quinquefasciatus ORs highly enriched in female antennae (as compared to female legs) led us to an OR, CquiOR136, sensitive to DEET and other insect repellents (Xu et al. 2014). When co-expressed along with the obligatory coreceptor CquiOrco in Xenopus oocytes, CquiOR136 responded to DEET, picaridin, IR3535 and PMD in a dose-dependent manner. Knockdown experiments showed that EAG responses to DEET recorded from mosquitoes with reduced levels of CquiOR136 transcripts were dramatically lower than those recorded from control mosquitoes, whereas responses to control odorants, nonanal and octanal, did not differ significantly. More importantly, knockdown mosquitoes could not discriminate DEET from control in behavioral assays. Taken together, these results suggest that CquiOR136, not CquiIR40a, is involved in the direct detection of DEET in the southern house mosquito.
Why does DEET work in the first place?
One of the most intriguing questions in olfaction, chemical ecology, insect behavior, and medical entomology is “why does DEET work?” Does it mimic anything in nature that conveys an across-the-board message to all insect species? Why is it so generic? The discovery of a DEET receptor in Cx. quinquefasciatus opened the door to test the hypothesis that DEET mimics a natural product with long insect-plant evolutionary history. Insect repellents like DEET, and particularly picaridin, share similar structural motifs with methyl jasmonate. Jasmonic acid elicited very small currents from CquiOR136/CquiOrco-expressing oocytes, but methyl jasmonate displayed dose-dependence responses, slightly stronger than those elicited by DEET. Behavioral assays confirmed that methyl jasmonate is a repellent for the southern house mosquito. We then hypothesized that methyl jasmonate is a natural ligand for CquiOR136 and that DEET might work by mimicking a plant defensive compound. The discovery of a DEET sensitive receptor paves the way for the development of better and more affordable insect repellents.
Conclusions
Repellents play a crucial role in reducing mosquito bites and, consequently, minimizing the transmission of vector-borne diseases. Molecular design led to the development of modern alternatives to DEET, such as IR 3535 and Picaridin, but DEET remains as the gold standard of insect repellents. DEET is undoubtedly a multitasking compound, which repels at a distance and also cause contact disengagement. There is unambiguous evidence in the literature that the olfactory system is involved in direct detection of DEET. Although it has been suggested that an ionotropic receptor, IR40a, can account for the widespread effect of DEET on various insects, the experimental evidence from mosquitoes suggests that odorant receptor(s) are involved. Identification of the molecular target(s) will pave the way for the development of better repellents, particularly more affordable products, which are sorely needed in areas where malaria, dengue, and other diseases are endemic.
Highlights.
Repellents and substances causing contact disengagement
DEET as an odorant and a tstant
Natural is not synonym of safe
An intact olfactory system is essential for DEET reception
DEET mimics a natural product
Acknowledgments
Mosquito research in my laboratory is funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award R01AI095514. I benefited greatly from discussion with past and current lab members, and with many colleagues in the field. I thank particularly Dr. Robert Bedoukian (Bedoukian Research Inc.) and lab members (Dr. Pingxi Xu, Mario Izidoro, and Garrison Buss) for their suggestions to improve an earlier draft of the manuscript. The content of the paper is solely the responsibility of the author and does not necessarily represent the official view of NIH or the opinion of the above colleagues who generously read the earlier draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boeckh J, Breer H, Geier M, Hoever F-P, Kruger BW, Nentwig G, Sass H. Acylated 1,3-aminopropanols as repellents against bloodsucking arthropods. Pest. Sci. 1996;48:359–373. [Google Scholar]
- Costantini C, Birkett MA, Gibson G, Ziesmann J, Sagnon NF, Mohammed HA, Coluzzi M, Pickett JA. Electroantennogram and behavioural responses of the malaria vector Anopheles gambiae to human-specific sweat components. Med Vet Entomol. 2001;15:259–66. doi: 10.1046/j.0269-283x.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- Davis EE. Insect repellents: concepts of their mode of action relative to potential sensory mechanisms in mosquitoes (Diptera: Culicidae) J Med Entomol. 1985;22:237–43. doi: 10.1093/jmedent/22.3.237. [DOI] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–91. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG, Browne LB, Smith CN. The designation of chemicals in terms of the responses they elicit from insects. J. Econ. Entomol. 1960;53:134–136. doi: 10.1603/029.102.0606. [DOI] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–42. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- Gertler SI. N,N-Diethylbenzamide as an insect repellent. Book N,N-Diethylbenzamide as an insect repellent. 1944 City. [Google Scholar]
- Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, Roberts DR. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS One. 2007;2:e716. doi: 10.1371/journal.pone.0000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Bhattacharjee AK. History of insect repellents. In: Debboun M, Frances SP, Strickmann D, editors. Insect repellents: principles, methods, and uses. CRC Press; Boca Raton, FL: 2007. pp. 195–228. [Google Scholar]
- Jackson D, Luukinen B, Buhl K, Stone D. Book DEET Technical Fact Sheet. National Pesticide Information Center, Oregon State University Extension Services; 2008. DEET Technical Fact Sheet. City. [Google Scholar]
- Kain P, Boyle SM, Tharadra SK, Guda T, Pham C, Dahanukar A, Ray A. Odour receptors and neurons for DEET and new insect repellents. Nature. 2013;502:507–12. doi: 10.1038/nature12594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Klier M, Kuhlow F. Neue Insektenabwehrmittel - am Stickstoff disubstitutierte ß-Alaninderivative. J. Soc. Cosmet. Chem. 1976;27:141–153. [Google Scholar]
- Leal WS. Molecular-based prospecting of mosquito attractants and repellents. In: Debboun M, Frances SP, Strickmann D, editors. Insect repellents: principles, methods, and uses. CRC Press; Boca Raton, FL: 2007. pp. 229–242. [Google Scholar]
- Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373–91. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- Leal WS, Choo YM, Xu P, da Silva CS, Ueira-Vieira C. Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proc Natl Acad Sci U S A. 2013;110:18704–9. doi: 10.1073/pnas.1316059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS, Uchida K. Application of GC-EAD to the determination of mosquito repellents derived from a plant Cymbopogon citratus. J. Asia-Pacific Entomol. 1998;1:217–221. [Google Scholar]
- Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–61. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe ET, Barthell WF, Gertler SI, Hall SA. Insect repellents: N,N-dimethylamides. J. Org. Chem. 1954;19:493–498. [Google Scholar]
- Miller JR, Siegert PY, Amimo FA, Walker ED. Designation of chemicals in terms of the locomotor responses they elicit from insects: an update of Dethier et al. J Econ Entomol. 2009;1960;102:2056–60. doi: 10.1603/029.102.0606. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Debboun M. History of insect repellents. In: Debboun M, Frances SP, Strickmann D, editors. Insect repellents: principles, methods, and uses. CRC Press; Boca Raton, FL: 2007. pp. 1–29. [Google Scholar]
- Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 2011;478:511–4. doi: 10.1038/nature10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Molec. Biol. 2013;43:888–897. doi: 10.1016/j.ibmb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Stanczyk NM, Brookfield JF, Ignell R, Logan JG, Field LM. Behavioral insensitivity to DEET in Aedes aegypti is a genetically determined trait residing in changes in sensillum function. Proc Natl Acad Sci U S A. 2010;107:8575–80. doi: 10.1073/pnas.1001313107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci U S A. 2008;105:13598–603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikar SN, Yadav R, Mendki MJ, Rao AN, Sukumaran D, Parashar BD. Oviposition deterrent activity of three mosquito repellents diethyl phenyl acetamide (DEPA), diethyl m-toluamide (DEET), and diethyl benzamide (DEB) on Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Parasitol Res. 2014;113:101–6. doi: 10.1007/s00436-013-3631-9. [DOI] [PubMed] [Google Scholar]
- Xu P, Choo YM, De La Rosa A, Leal WS. Mosquito odorant receptor for DEET and methyl jasmonate. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1417244111. PNAS Early Edition doi 10.1073/pnas.1417244111. [DOI] [PMC free article] [PubMed] [Google Scholar]