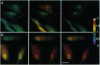
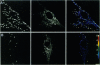
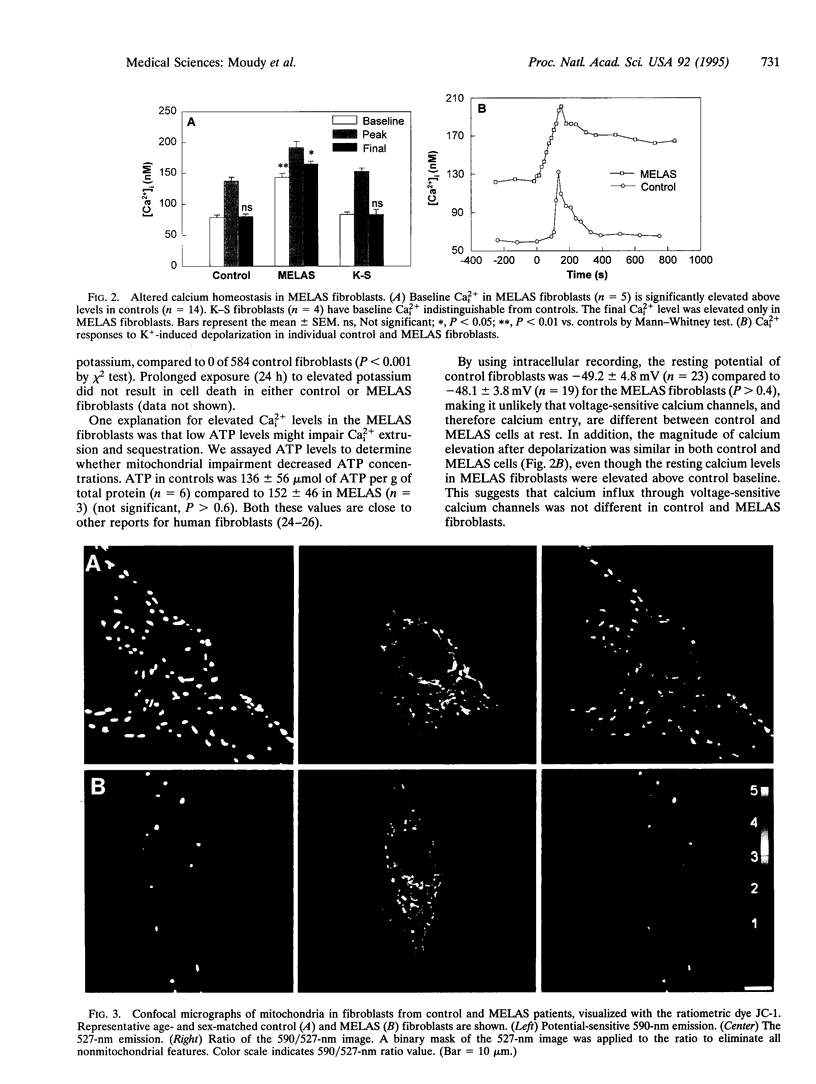
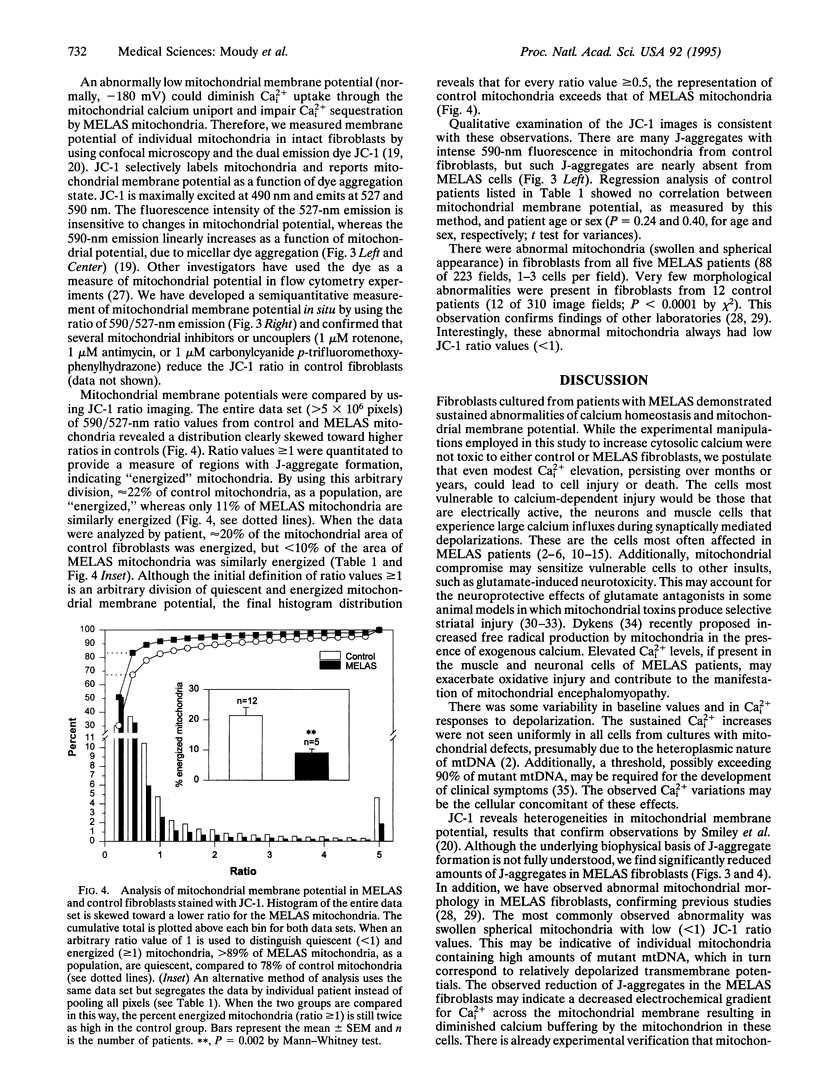
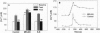Abstract
Patients with several inherited human encephalomyopathies exhibit systemic and neurological symptoms in association with specific mitochondrial mutations. The mechanisms by which these mitochondrial mutations result in cellular injury have not been elucidated. One potential cause of neuronal vulnerability is an inability to effectively buffer intracellular calcium. We report that fibroblasts from patients with one specific inherited encephalomyopathy, MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) syndrome, have elevated levels of ionized calcium and cannot normally sequester calcium influxes. Quantitative fluorescence imaging demonstrated that this abnormality was associated with a relative decrease in mitochondrial membrane potential compared to control fibroblasts. This documentation of pathological calcium homeostasis in a genetic neurological disease extends the calcium hypothesis of toxic cell injury to human mitochondrial encephalomyopathies.
Full text
PDF
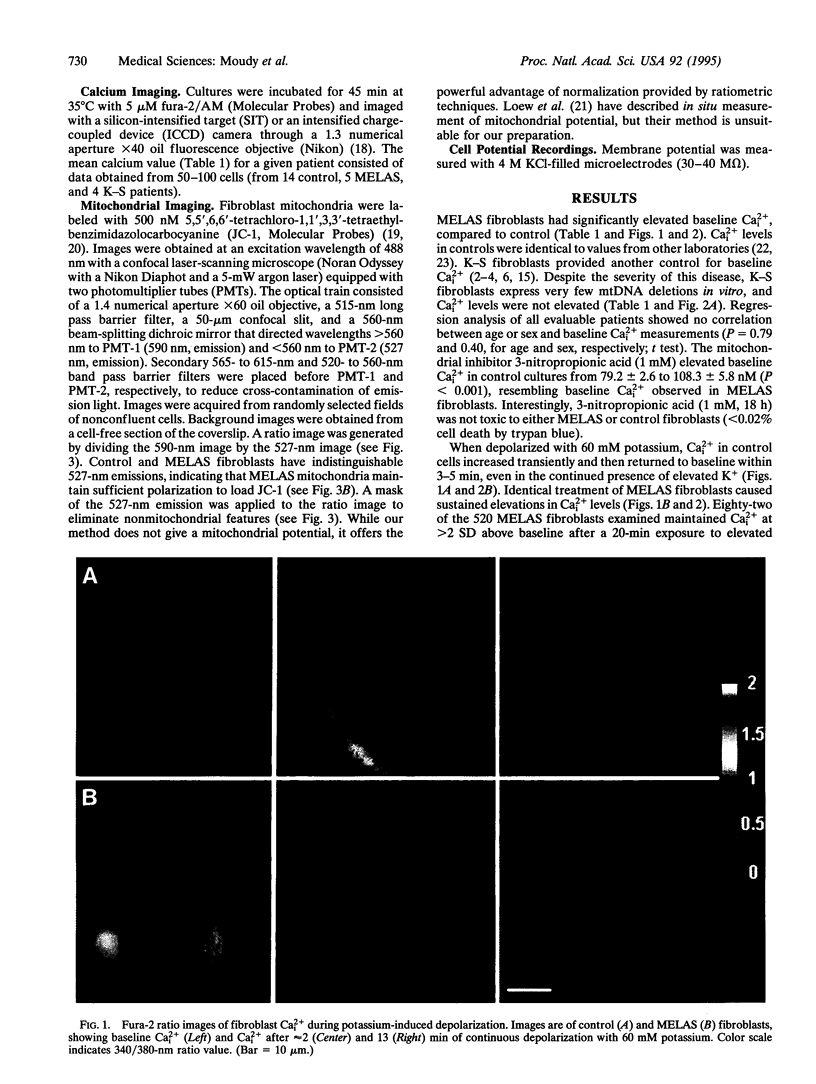

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agsteribbe E., Huckriede A., Veenhuis M., Ruiters M. H., Niezen-Koning K. E., Skjeldal O. H., Skullerud K., Gupta R. S., Hallberg R., van Diggelen O. P. A fatal, systemic mitochondrial disease with decreased mitochondrial enzyme activities, abnormal ultrastructure of the mitochondria and deficiency of heat shock protein 60. Biochem Biophys Res Commun. 1993 May 28;193(1):146–154. doi: 10.1006/bbrc.1993.1602. [DOI] [PubMed] [Google Scholar]
- Albin R. L., Greenamyre J. T. Alternative excitotoxic hypotheses. Neurology. 1992 Apr;42(4):733–738. doi: 10.1212/wnl.42.4.733. [DOI] [PubMed] [Google Scholar]
- Beal M. F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992 Feb;31(2):119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Hyman B. T., Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993 Apr;16(4):125–131. doi: 10.1016/0166-2236(93)90117-5. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Ciafaloni E., Ricci E., Shanske S., Moraes C. T., Silvestri G., Hirano M., Simonetti S., Angelini C., Donati M. A., Garcia C. MELAS: clinical features, biochemistry, and molecular genetics. Ann Neurol. 1992 Apr;31(4):391–398. doi: 10.1002/ana.410310408. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Intracellular calcium mobilization by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalization. Cell Calcium. 1993 Mar;14(3):185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Cossarizza A., Baccarani-Contri M., Kalashnikova G., Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem Biophys Res Commun. 1993 Nov 30;197(1):40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Moraes C. T. Mitochondrial encephalomyopathies. Arch Neurol. 1993 Nov;50(11):1197–1208. doi: 10.1001/archneur.1993.00540110075008. [DOI] [PubMed] [Google Scholar]
- Dykens J. A. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994 Aug;63(2):584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Ito E., Kim C. S., Alkon D. L. Soluble beta-amyloid induction of Alzheimer's phenotype for human fibroblast K+ channels. Science. 1994 Apr 8;264(5156):276–279. doi: 10.1126/science.8146663. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Ito E., Oka K., Tofel-Grehl B., Gibson G. E., Alkon D. L. Potassium channel dysfunction in fibroblasts identifies patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8209–8213. doi: 10.1073/pnas.90.17.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Fox I. H., Shefner R., Palmieri G. M., Bertorini T. E. Duchenne muscular dystrophy: normal ATP turnover in cultured cells. Neurology. 1985 Oct;35(10):1521–1524. doi: 10.1212/wnl.35.10.1521. [DOI] [PubMed] [Google Scholar]
- Goto Y., Horai S., Matsuoka T., Koga Y., Nihei K., Kobayashi M., Nonaka I. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA mutation. Neurology. 1992 Mar;42(3 Pt 1):545–550. doi: 10.1212/wnl.42.3.545. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A new mtDNA mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta. 1991 Oct 21;1097(3):238–240. doi: 10.1016/0925-4439(91)90042-8. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Pardee A. B., Goldberg A. L. The ATP dependence of the degradation of short- and long-lived proteins in growing fibroblasts. J Biol Chem. 1985 Mar 25;260(6):3344–3349. [PubMed] [Google Scholar]
- Harding A. E. Neurological disease and mitochondrial genes. Trends Neurosci. 1991 Apr;14(4):132–138. doi: 10.1016/0166-2236(91)90081-5. [DOI] [PubMed] [Google Scholar]
- Herzberg N. H., Middelkoop E., Adorf M., Dekker H. L., Van Galen M. J., Van den Berg M., Bolhuis P. A., Van den Bogert C. Mitochondria in cultured human muscle cells depleted of mitochondrial DNA. Eur J Cell Biol. 1993 Aug;61(2):400–408. [PubMed] [Google Scholar]
- Karpati G., Arnold D., Matthews P., Carpenter S., Andermann F., Shoubridge E. Correlative multidisciplinary approach to the study of mitochondrial encephalomyopathies. Rev Neurol (Paris) 1991;147(6-7):455–461. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loew L. M., Tuft R. A., Carrington W., Fay F. S. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993 Dec;65(6):2396–2407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Jansen C., Hirano M., Rao N., Lovelace R. E., Rowland L. P., Schon E. A., DiMauro S. Two novel pathogenic mitochondrial DNA mutations affecting organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest. 1993 Dec;92(6):2906–2915. doi: 10.1172/JCI116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Moudy A. M., Yamada K. A., Rothman S. M. Rapid desensitization determines the pharmacology of glutamate neurotoxicity. Neuropharmacology. 1994 Aug;33(8):953–962. doi: 10.1016/0028-3908(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Muggleton-Harris A. L., Defuria R. Age-dependent metabolic changes in cultured human fibroblasts. In Vitro Cell Dev Biol. 1985 May;21(5):271–276. doi: 10.1007/BF02620941. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. A role for the mitochondrion in the protection of cells against calcium overload? Prog Brain Res. 1985;63:97–106. doi: 10.1016/S0079-6123(08)61978-0. [DOI] [PubMed] [Google Scholar]
- Pavlakis S. G., Phillips P. C., DiMauro S., De Vivo D. C., Rowland L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984 Oct;16(4):481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- Reers M., Smith T. W., Chen L. B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991 May 7;30(18):4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Griffiths T., Evans M. C., Swan J. H., Meldrum B. S. Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: an electron microscopy study in the rat. J Cereb Blood Flow Metab. 1984 Sep;4(3):350–361. doi: 10.1038/jcbfm.1984.52. [DOI] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler H. J., Medori R. Mitochondrial DNA alterations as a source of human disorders. Neurology. 1993 Feb;43(2):280–288. doi: 10.1212/wnl.43.2.280. [DOI] [PubMed] [Google Scholar]
- Turski L., Turski W. A. Towards an understanding of the role of glutamate in neurodegenerative disorders: energy metabolism and neuropathology. Experientia. 1993 Dec 15;49(12):1064–1072. doi: 10.1007/BF01929915. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Werth J. L., Thayer S. A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994 Jan;14(1):348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]