Abstract
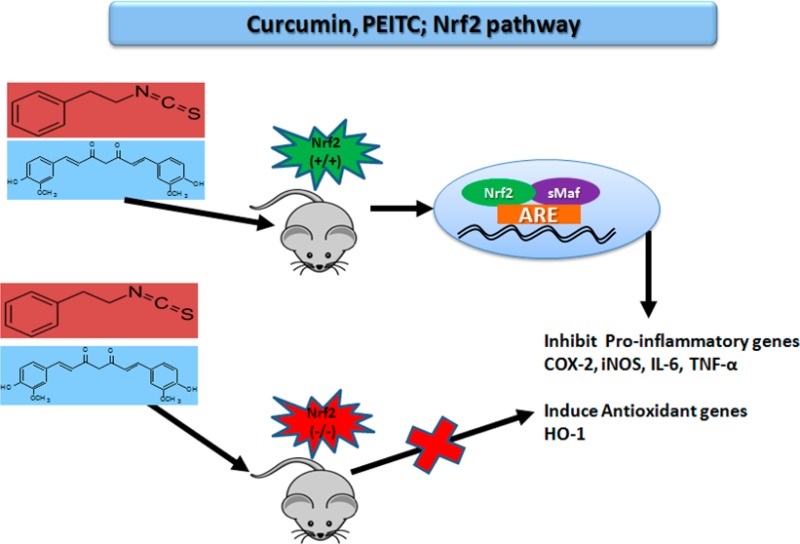
The role of phytochemicals in preventive and therapeutic medicine is a major area of scientific research. Several studies have illustrated the mechanistic roles of phytochemicals in Nrf2 transcriptional activation. The present study aims to examine the importance of the transcription factor Nrf2 by treating peritoneal macrophages from Nrf2+/+ and Nrf2–/– mice ex vivo with phenethyl isothiocyanate (PEITC) and curcumin (CUR). The peritoneal macrophages were pretreated with the drugs and challenged with lipopolysaccharides (LPSs) alone and in combination with PEITC or CUR to assess their anti-inflammatory and antioxidative effects based on gene and protein expression in the treated cells. LPS treatment resulted in an increase in the expression of inflammatory markers such as cycloxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in both Nrf2+/+ and Nrf2–/– macrophages, detected by quantitative polymerase chain reaction (qPCR). Nrf2+/+ macrophages treated with PEITC and CUR exhibited a significant decrease in the expression of these anti-inflammatory genes along with an increase in the expression of hemeoxygenase-1 (HO-1), which is an antioxidative stress gene downstream of the Nrf2 transcription factor battery. Although there was no significant decrease in the expression of the anti-inflammatory genes or an increase in HO-1 expression in Nrf2–/– macrophages treated with either PEITC or CUR, there was a significant decrease in the protein expression of COX-2 and an increase in the expression of HO-1 in Nrf2+/+ macrophages treated with PEITC compared to that with CUR treatment. No significant changes were observed in the macrophages from knockout animals. Additionally, there was a significant decrease in LPS-induced IL-6 and TNF-α production following PEITC treatment compared with that following CUR in Nrf2+/+ macrophages, whereas no change was observed in the macrophages from knockout animals. The results from qPCR, western blot, and ELISA analyses in macrophages from Nrf2+/+ and Nrf2 –/– mice indicate that Nrf2 plays an important role in the anti-inflammatory and antioxidative effects of PEITC and CUR, as observed by their decreased activities in Nrf2–/– macrophages.
Introduction
Inflammation is a cellular defense mechanism that protects cells from pathogens. However, an increased period of inflammation, leading to a chronic inflammatory state, has been established as a major driving force behind carcinogenesis. Specifically, increased pro-inflammatory cytokine production from macrophages and lymphocytes is known to promote various stages of tumorigenesis.1,2 Chronic inflammatory damage has a negative impact on the regulation of signal transduction pathways by causing aberrant protein expression that could lead to inflammation-driven carcinogenesis.3 Macrophages and monocytes act as a first line of defense against bacterial infections4 as well as other disease states. Previous studies have established that activation of macrophages by lipopolysaccharides (LPSs) increases the levels of inflammatory mediators, such as cytokines, prostaglandins, and nitric oxide, and provides a good model system to study inflammation.5,6 The nuclear factor-erythroid 2 (NF-E2)-related factor 2, commonly known as Nrf2, is a transcription factor that belongs to the cap ‘n’ collar subfamily containing a basic leucine zipper region. Nrf2 regulates downstream antioxidative stress genes, such as NAD(P)H quinine oxidoreductase (NQO1), hemeoxygenase-1 (HO-1), and phase II detoxifying enzymes, upon being challenged by cellular stress, and it plays a key regulatory role in cellular stress defense mechanisms7,8 by binding to the antioxidant redox element (ARE).
While the induction of antioxidant properties has been well-attributed to Nrf2, we and others have demonstrated the role of Nrf2 in the regulation of inflammation. Transcriptional regulation of Nrf2 induces HO-1, which has well-known antioxidant properties as well as anti-inflammatory functions, as shown in studies conducted in colon, skin, neural, and other tissues.9−12 It has been shown that the Nrf2 pathway plays an important role in lowering both acute and chronic inflammation. Several studies in different animal models have illustrated the role of inflammation in cancer. In particular, studies conducted in Nrf2 knockout mice have demonstrated increased inflammation and tumorigenesis in the liver,13 colon,14,15 brain,12 and lungs.16 Nrf2 confers protection against inflammation, as it has been shown that decreased Nrf2 expression leads to an increased inflammatory response in addition to increased electrophilic and oxidative stress.17
Recently, our group conducted several studies to examine the anti-inflammatory effects of phytochemicals and their role in chemoprevention.18,19 Phytochemicals such as curcumin (CUR), which is an ingredient in turmeric from India, and isothiocyanates, specifically phenethyl isothiocyanate (PEITC), which have been found in broccoli sprouts, water cress, and other vegetables, have shown potent anti-inflammatory and antioxidant effects.20−22 It has been well-documented that both of these phytochemicals are potent Nrf2 inducers.21,23,24 The anti-inflammatory effects of these compounds have been studied using the LPS-induced inflammation model, which is known to increase the expression of COX-2 in macrophages, endothelial cells, and fibroblasts.25 The anti-inflammatory role of phytochemicals, such as sulforaphane, which is an isothiocyanate present in broccoli, and eicosopentanoic acid and dococsahexaenoic acid, which are constituents of fish oil and belong to polyunsaturated fatty acids, has been well-documented in studies conducted in LPS-stimulated Nrf2–/– macrophages, in which the absence of Nrf2 caused an increase in pro-inflammatory markers, such as cycloxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), and interleukin-8 (IL-8), and a decrease in HO-1 expression, which is an antioxidative stress gene that also has anti-inflammatory effects.26−28 Our current study builds on previous work and aims to examine the role of Nrf2 in regulating the anti-inflammatory properties in LPS-induced peritoneal macrophages isolated from Nrf2+/+ and Nrf2–/– mice. We hypothesize that although CUR and PEITC are not structurally similar and do not belong to the same class of phytochemicals they act via the Nrf2 pathway. To evaluate our hypothesis, we compared the effects of CUR and PEITC in the presence and absence of Nrf2 in peritoneal macrophages. Furthermore, we examined the inhibitory effects of both of these phytochemicals on TNF-α, NO, and IL-6 production while also examining their regulatory role in the expression of COX-2, iNOS, IL-6, IL-8, and HO-1.
Materials and Methods
Animals
The C57BL/6J mouse strain, which is homozygous WT for Nrf2, and Nrf2–/– mice were used as the control and treatment groups for the current study. Specifically, 10–12 week-old male mice from the second filial generation of Nrf2–/– mice and age-matched C57BL/6J male mice were used for the study. Nrf2–/– mice were generated by backcrossing the F1 generation of Nrf2–/– with C57BL/6J mice purchased from the Jackson Laboratory (Bar Harbor, ME) as described previously in studies by our group.26,27 The genotype of each animal was confirmed by performing tail DNA extraction followed by polymerase chain reaction (PCR). Bands for Nrf2–/– were visualized at 200 bp by agarose gel electrophoresis, whereas the WT mice displayed a band at 300 bp, as illustrated in our previous studies.26,27 All animals used in the study were housed and maintained at Rutgers following the guidelines established by IACUC and NIH guidelines for the care and use of laboratory animals.
Chemicals and Reagents
Dulbecco’s modified Eagle’s medium (DMEM), penicillin, streptomycin, and fetal bovine serum (FBS) were obtained from Invitrogen-Gibco (Grand Island, NY). Primary antibodies against HO-1, COX-2, iNOS, and actin and secondary antibodies were purchased from Santa Cruz (Santa Cruz, CA). Curcumin (CUR) and phenethyl isothiocyanate (PEITC, 99%) were purchased from Sigma-Aldrich (St. Louis, MO). Thioglycollate broth was purchased from Edge Biological (Memphis, TN). Lipopolysaccharide (LPS from Escherichia coli 0111:B4) was purchased from Sigma-Aldrich (St. Louis, MO). ELISA kits for the measurement of TNF-α (KMC 3012) and IL-6 (KMC 0062) were purchased from Invitrogen (Grand Island, NY). Taqman RT reagents were purchased from Life Technologies
Isolation of Peritoneal Macrophages
The peritoneal macrophages were isolated using a previously established procedure in our laboratory.26,27 A volume of 800 μL of thioglycollate broth (TG broth) was injected intraperitoneally into the animals on day 1. The animals were euthanized on day 4, and the macrophages were harvested by flushing the peritoneal cavity with 1× ice-cold PBS buffer (pH of 6.8 and 0.02% EDTA). The macrophages were then centrifuged at 1000 rpm for 10 min. Next, the cell pellet was resuspended with 0.83% ammonium chloride to remove any residual red blood cells.
Cell Culture and Treatment
The viability of the harvested macrophages was evaluated with the trypan blue exclusion method, and the cell viability was calculated from the cell population. Nrf2+/+ and Nrf2–/– cells (macrophages) were cultured for 6 h in DMEM using 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C and 5% CO2. The macrophages were then pretreated with the drugs CUR or PEITC alone at two nontoxic concentrations (5 and 10 μM, Supporting Information Figure S1) for 6 h followed by cotreatment with LPS (1 μg/mL) from E. coli to induce the pro-inflammatory cytokines for 8 h before RNA was extracted. For protein extraction, the cells were pretreated with CUR (5 and 10 μM) or PEITC (5 and 10 μM) alone for 6 h followed by cotreatment with LPS (1 μg/mL) for 18 h. LPS-only treatment served as a positive control. DMEM with 10% FBS served as a negative control.
Biological Assays for Measurement of Nitrite Production (NO) and Cytokine Concentrations
Nitrite production was assessed as described previously by measuring nitrite accumulation in the culture media fluorimetrically using an Infinite 200 PRO Tecan microplate reader.29 The cells were pretreated with drugs for 6 h and cotreated with (1 μg/mL) LPS for 8 h. A 50 μL solution of supernatant from the cultured media was added to 96-well black polystyrene flat-bottom plates (Whatman Inc., Piscataway, NJ), to which 10 μL of freshly prepared 2,3-diaminonaphthalene (0.05 mg/mL in 0.62 N HCl) was added and incubated for 10 min. This step serves as the start of the reaction. Next, 5 μL of 2.8 N sodium hydroxide was added to the solution to stop the reaction, which results in the production of 2,3-diaminonaphthotriazole. The reactions were measured at an excitation of 360 nm and emission of 460 nm with a gain setting of 80% using an Infinite 200 PRO Tecan microplate reader. The levels of cytokines, TNF-α and IL-6, produced as a response to the inflammatory state, were measured in the supernatant of the culture medium by enzyme-linked immunosorbent assay kits from Invitrogen (Grand Island, NY) following the manufacturer’s instructions.
Isolation of RNA, Reverse Transcription, and Quantitative Polymerase Chain Reaction
Total RNA from the treatment and control groups was extracted using RNeasy Mini Kit (Carlsbad, CA), and the mRNA was quantified using a NanoDrop 2000. Approximately 600 ng of mRNA was reverse-transcribed into cDNA using Taqman RT reagents. This cDNA was used to perform the quantitative PCR using SYBR Green PCR master mix from ABI on the ABI7900HT system (Life Technologies, Grand Island, NY). The primer sequences used for the different genes are listed in Table 1 and have been used in our previous studies.27
Table 1. Oligonucleotide Primer Sequences for Real-Time Quantitative Polymerase Chain Reaction (qPCR).
| gene | forward primer | reverse primer |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | 5′-TGA AGC AGG CAT CTG AGG G-3′ | 5′-CGA AGG TGG AAG AGT GGG AG-3′ |
| Hemeoxygenase-1 (HO-1) | 5′-CCT CAC TGG CAG GAA ATC ATC-3′ | 5′-CCT CGT GGA GAC GCT TTA CAT A-3′ |
| Inducible nitric oxide synthase 2 (iNOS) | 5′-CCT GGT ACG GGC ATT GCT-3′ | 5′-GCT CAT GCG GCC TCC TTT-3′ |
| Tumor necrosis factor-alpha (TNF-α) | 5′-TCT CAT GCA CCA CCA TCA AGG ACT-3′ | 5′-ACC ACT CTC CCT TTG CAG AAC TCA-3′ |
| Interleukin-6 (IL-6) | 5′-ATC CAG TTG CCT TCT TGG GAC TGA-3′ | 5′-TAA GCC TCC GAC TTG TGA AGT GGT-3′ |
| Cyclooxygenase-2 (COX-2) | 5′-TGC CTG GTC TGA TGA TGT ATG CCA-3′ | 5′-AGT AGT CGC ACA CTC TGT TGT GCT-3′ |
Protein Extraction and Western Blotting
Radio immune precipitation assay (RIPA) buffer containing a protease inhibitor cocktail (Sigma, St. Louis, MO) was used to harvest the cells from the control and treatment groups. The concentrations of protein lysates were determined using the bicinchoninic acid (BCA) method (Pierce, Rockford, IL). Samples containing 20 μg of total protein were loaded onto a 4–12% SDS-PAGE gel. The proteins from the gel were then transferred onto a PVDF membrane at 1.3 A and 25 V for 5 min using the Transblot Turbo transfer system from Bio-Rad. The PVDF membranes were then incubated with selected primary antibodies at 4 °C overnight, followed by detection using HRP-conjugated secondary antibodies. The signals were enhanced using ECL reagents (Thermo Scientific) and were visualized using a Bio-Rad gel documentation system. All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical Analysis
All experiments were performed at least three times, and similar results were obtained. Statistical tests were performed using Student’s t test. All p values were two-sided, and a p value < 0.05 was considered to be statistically significant.
Results
Curcumin and PEITC Inhibit Nitrite Production in Nrf2+/+ Macrophages
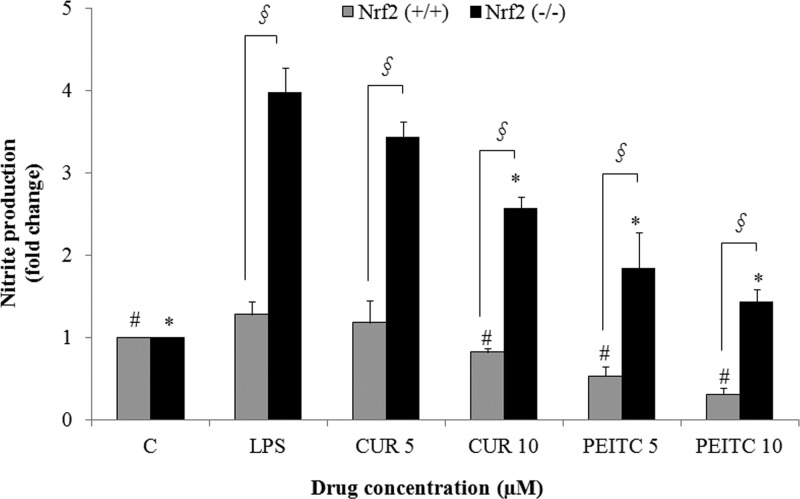
Nitrite production as a result of CUR and PEITC treatment was assessed in LPS-stimulated Nrf2+/+ macrophages. The results showed that nitrite production was lowered by CUR at 10 μM and PEITC in a dose-dependent manner at 5 and 10 μM compared with that in the LPS-only treatment in Nrf2+/+ macrophages (Figure 1). Nitrite production was also decreased by CUR and PEITC in Nrf2–/– macrophages in comparison to that from the LPS treatment, but the effect was not as pronounced as it was in Nrf2+/+ macrophages, where nitrite production was lower by more than than 0.5-fold (Figure 1). Overall, it can be observed that both CUR and PEITC lowered NO production in Nrf2+/+ macrophages in a dose-dependent manner in comparison to that from LPS treatment (p < 0.05). Although the lowering effect was also seen in Nrf2–/– macrophages, nitrite production itself was much higher than that in the WT cells, thus emphasizing the importance of Nrf2 (Figure 1).
Figure 1.
LPS-induced nitrite production is decreased by the treatment of Nrf2+/+ peritoneal macrophages with CUR and PEITC, but no significant decrease was seen in Nrf2–/– macrophages. The peritoneal macrophages were pretreated with the drugs for 6 h followed by cotreatment with the drug and LPS for 8 h. The results are shown as the mean ± SE of three independent experiments (n = 3). §, statistical significance between Nrf2+/+ and Nrf2–/– macrophages; #, statistical significance between LPS and treatment groups in Nrf2+/+ macrophages; *, statistical significance between LPS and treatment groups in Nrf2–/– macrophages.
PEITC Decreases the TNF-α and IL-6 Levels in Nrf2+/+ Macrophages
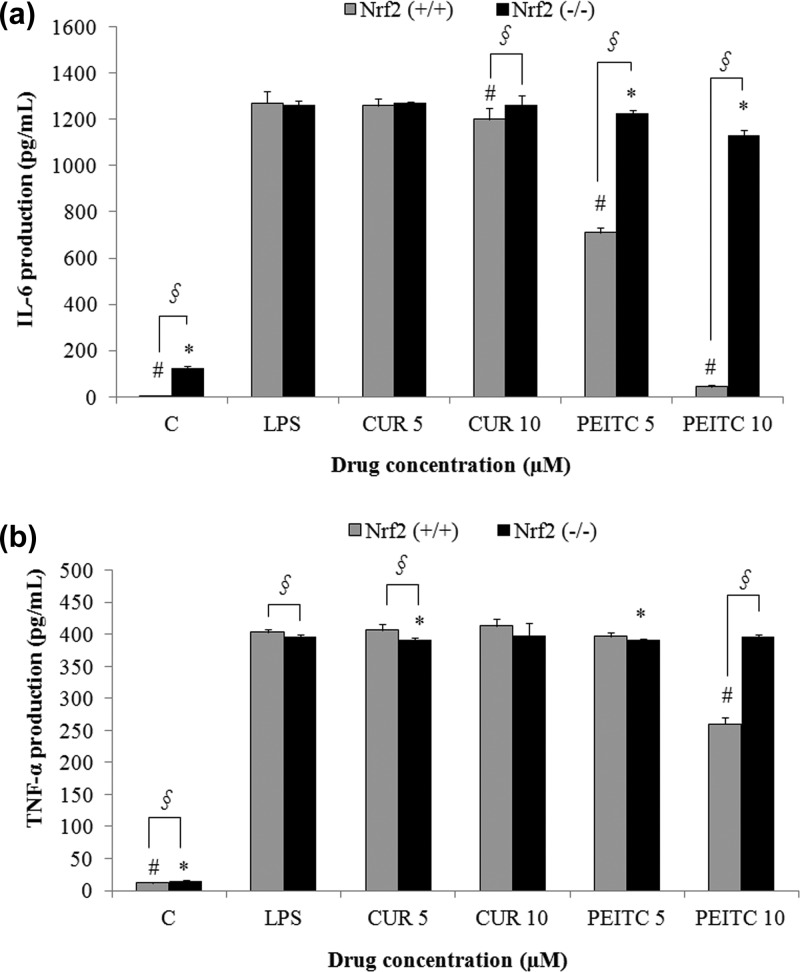
To assess cytokine production in LPS-stimulated Nrf2+/+ macrophages treated with CUR and PEITC, we performed ELISA analysis of TNF-α and IL-6. We found that the levels of TNF-α and IL-6 were significantly lower (p < 0.05) after PEITC treatment (10 μM) in Nrf2+/+ macrophages in comparison to that from LPS treatment only (Figure 2A,B). Conversely, cytokine production in Nrf2–/– macrophages treated with 10 μM of PEITC was not lowered. By contrast, the effect of CUR on the level of cytokines was not nearly as significant compared with that from PEITC and the LPS-only treatment in both groups for IL-6 and TNF-α, as shown in Figure 2A,B, respectively. PEITC and CUR show differential effects on cytokine production in Nrf2+/+ macrophages in terms of gene expression. The differential effects of these compounds could be attributed to post-transcriptional regulation. It was previously published that CUR promotes differential expression of cytokines TNF-α and IL-6 in adipocytes, which was due to post-transcriptional regulation.30
Figure 2.
Inhibition of IL-6 (A) and TNF-α (B) secretion in Nrf2+/+ macrophages upon treatment with PEITC is significantly higher than that with CUR. Both of the treatment groups were compared with the LPS-only treatment, which served as a positive control. The cells were pretreated with CUR or PEITC alone for 6 h and cotreated with LPS and CUR or PEITC for 8 h, and the supernatant was used for ELISA (n = 3). §, statistical significance between Nrf2+/+ and Nrf2–/– macrophages; #, statistical significance between LPS and treatment groups in Nrf2+/+ macrophages; *, statistical significance between LPS and treatment groups in Nrf2–/– macrophages.
Curcumin and PEITC Inhibit LPS-Induced Gene Expression of COX-2, iNOS, TNF-α, and IL-6 and Induced HO-1 Gene Expression in Nrf2+/+ Macrophages
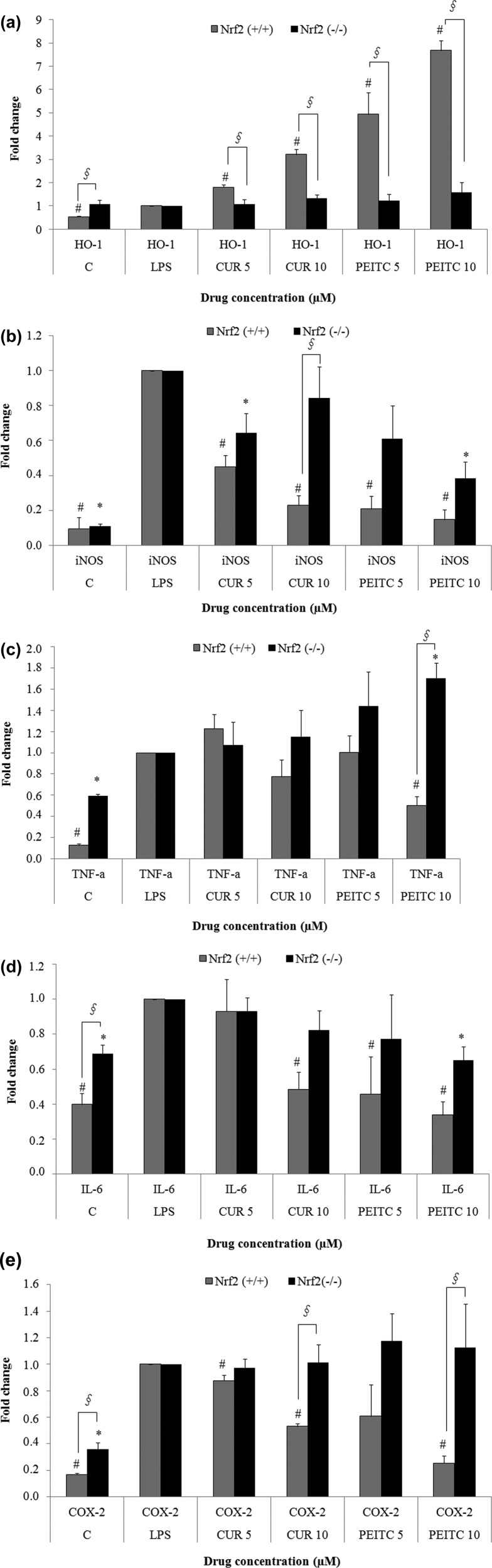
It is known the LPS stimulation increases the expression of pro-inflammatory markers such as COX-2, TNF-α, and IL-6.5,6 The qPCR results illustrate that CUR exerted an anti-inflammatory effect by lowering the LPS-induced expression of iNOS (Figure 3B), TNF-α (Figure 3C), IL-6 (Figure 3D), and COX-2 (Figure 3E). Similarly, treatment with PEITC showed a significant dose-dependent decrease at 5 and 10 μM in the gene expression of LPS-induced pro-inflammatory markers such as COX-2, iNOS, TNF-α, and IL-6 in Nrf2+/+ macrophages, whereas no change was observed in Nrf2–/– macrophages for TNF-α and COX-2 (Figure 3C,E). iNOS gene expression was also decreased by PEITC in Nrf2–/– macrophages in comparison to that from the LPS treatment, but the effect itself was not as pronounced as it was in Nrf2+/+ macrophages. The fold change in Nrf2+/+ macrophages was 0.2 and 0.15 when they were treated with 5 and 10 μM PEITC, whereas the same treatments in Nrf2–/– macrophages showed fold changes of 0.6 and 0.4, respectively, as shown in Figure 3B. Both CUR and PEITC also induced the expression of HO-1 (Figure 3A) at 5 and 10 μM in Nrf2+/+ peritoneal macrophages in a dose-dependent manner. At the same time, there was no significant decrease in pro-inflammatory markers or an increase in HO-1 induction in the Nrf2–/– macrophages (Figure 3A–E).
Figure 3.
qPCR analyses (A, HO-1; B, iNOS; C, TNF-α; D, IL-6; E, COX-2) showing that LPS-induced gene expression of pro-inflammatory markers such as iNOS, COX-2, IL-6, and TNF-α is significantly reduced by PEITC in Nrf2+/+ macrophages in a dose-dependent manner. Although the effect was comparably less in response to CUR treatment, it still lowered LPS-induced gene expression of those genes at 10 μM. However, the same effect of PEITC and CUR was not seen in the Nrf2–/– macrophages. PEITC and CUR also significantly increased the expression of HO-1 in Nrf2+/+ macrophages, whereas the effect was not seen in the Nrf2–/– macrophages. The results are shown as the mean ± SE of three independent experiments (n = 3). §, statistical significance between Nrf2+/+ and Nrf2–/– macrophages; #, statistical significance between LPS and treatment groups in Nrf2+/+ macrophages; *, statistical significance between LPS and treatment groups in Nrf2–/– macrophages.
PEITC Induces HO-1 Protein Expression and Inhibits LPS-Induced COX-2 and iNOS Protein Expression
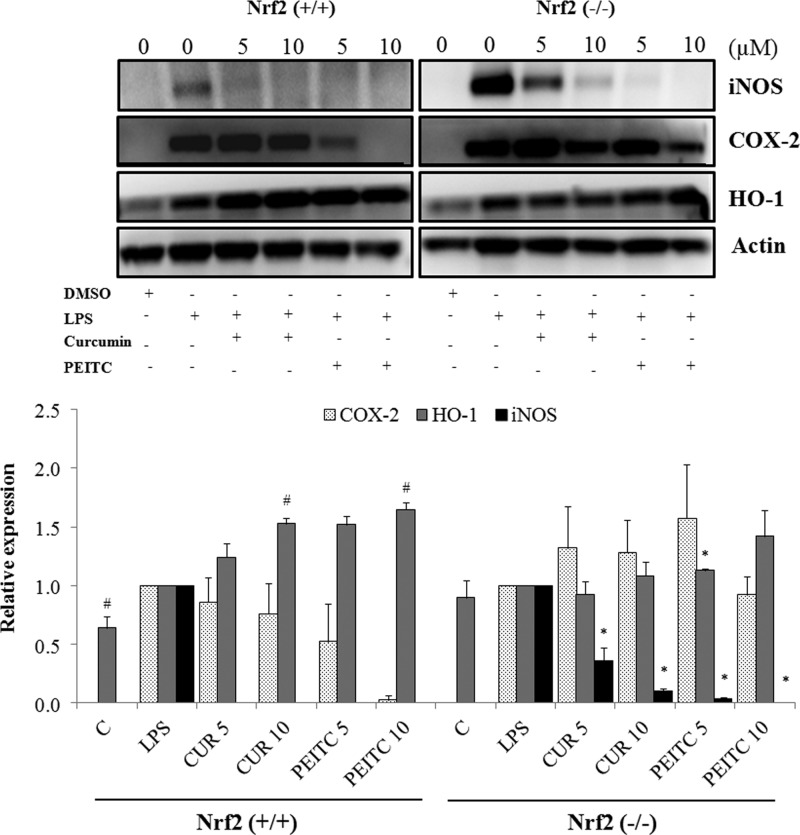
The protein expression of COX-2, iNOS, and HO-1 in macrophages upon pretreatment with the drugs for 6 h and cotreatment with LPS for 18 h to induce an inflammatory response were analyzed by western blotting. The results show that PEITC caused a dose-dependent decrease in COX-2 protein expression and a dose-dependent increase in HO-1 protein expression in Nrf2+/+ macrophages, whereas the effect was not similar in Nrf2–/– macrophages, as shown in Figure 4A,B. The protein expression was consistent with the mRNA expression, as shown by qPCR analyses. Additionally, CUR caused a decrease in COX-2 expression; however, this decrease was not significant compared with that induced by PEITC. This result demonstrates that PEITC has a more potent anti-inflammatory effect, which is mediated by the Nrf2 pathway. Additionally, the presence of Nrf2 has more attenuating effects on COX-2 and iNOS.
Figure 4.
(Top) Western blot and (Bottom) quantitation showing that PEITC significantly reduced LPS-induced protein expression of COX-2 in a dose-dependent manner, whereas the effect was lower with CUR in Nrf2+/+ macrophages. There was no significant lowering of COX-2 protein expression in Nrf2–/– macrophages upon treatment with either PEITC or CUR. HO-1 protein expression was significantly increased in Nrf2+/+ macrophages in a dose-dependent manner upon treatment with PEITC and CUR, whereas the induction was not as pronounced in Nrf2–/– macrophages. The macrophages were pretreated with the drugs for 6 h and cotreated with drugs and LPS for 18 h. The results are shown as the mean ± SE of three independent experiments (n = 3). #, statistical significance between LPS and treatment groups in Nrf2+/+ macrophages; *, statistical significance between LPS and treatment groups in Nrf2–/– macrophages.
Discussion
The Nrf2 signaling pathway plays an important role in the regulation of oxidative stress and the inflammatory state both in vitro(31) and in vivo.17,32−34 It has been well-documented that Nrf2 induction leads to increased expression of antioxidative stress genes, such as HO-1, that also confer anti-inflammatory effects by induction of IL-10 as an anti-inflammatory cytokine.35 Several studies have demonstrated the anti-inflammatory effects of bioactive phytochemicals, including flavonoids such as sappanchalcone,36 chalcones such as 4,2′,5′-trihydroxy-4′-methoxychalcone,37 diterpenoids such as kaurenoic acid,38 triterpenoids such as celastrol,39 and sesqueterpenoids such as costunolide,40 among others. These phytochemicals are regulated by the activation of the Nrf2 transcription factor and its target genes.41 It has been shown that the lack of Nrf2 can lead to lethality by septic shock, as evidenced by comparative studies performed in Nrf2+/+ and Nrf2–/– peritoneal neutrophils; these studies demonstrated that Nrf2 confers protection from LPS-induced inflammation.42 The importance of Nrf2 as an anti-inflammatory target for phytochemicals has been illustrated in previous studies performed in Nrf2–/– mice from our group26,27 as well as from others who showed that the lack of the Nrf2 gene makes these mice more susceptible to immune and inflammatory disorders.43 Multiple studies have suggested that Nrf2-deficient mice are more prone to inflammatory, cytotoxic, and genotoxic effects induced by oxidants as well as electrophiles.17,44 For instance, the lack of Nrf2 in the lungs led to reduced expression of Nrf2 target genes and an increase in pro-inflammatory cytokines such as IL-12 and IL-13.45
We have previously reported that curcumin and PEITC in combination with sulforaphane have a strong inhibitory effect on inflammation in RAW 264.7 cells by reducing iNOS and COX-2 expression.46 We also showed that the combination of curcumin and PEITC reduces the growth of PC3 cells in a xenograft model in vivo.(47,48) In this study, we provide mechanistic insight into the role of the Nrf2 pathway in ameliorating inflammation by using these phytochemicals to explore the anti-inflammatory and antioxidant effects of PEITC and curcumin mediated by Nrf2. Our results indicate that PEITC acts via the Nrf2 pathway to reduce inflammation, as demonstrated by its regulation of pro-inflammatory cytokines, decreased IL-6 and TNF-α, as revealed by ELISA in Nrf2+/+ macrophages compared with that in Nrf2–/– macrophages (Figure 2A,B). However, the same phenomenon was not as clear in CUR-treated macrophages, indicating an alternative pathway for attenuating inflammation, for instance, by inhibiting TLR4-mediated NF-κB signaling pathways.49 Although there was an increase in the expression of HO-1 in the Nrf2+/+ macrophages by both CUR and PEITC, differential cytokine production was observed, suggesting that there is a lack of direct correlation between Nrf2 expression and these cytokines. This was also observed in our previous work, where EPA and DHA showed changes in cytokine production that were not of the same magnitude as that of HO-1 expression;27 others reported that tBHQ induced the expression of Nrf2 and HO-1 in a dose-dependent manner while not having similar effects on the expression of cytokines like TNF-α.50 Although low levels of NO are required to maintain normal homeostasis in the body, one of the direct consequences of an inflammatory state is the increased expression of iNOS in macrophages and other immune cells, which exacerbates chronic inflammation by changing the normal tissue environment;51 curcumin and PEITC were shown to have anti-inflammatory effects due to the suppression of iNOS and consequent reduction nitrite production.51−54 In this context, our results show that PEITC treatment exhibits a dose-dependent suppression of nitrite production, as demonstrated in the NO assay of Nrf2+/+ macrophages. By contrast, the lack of Nrf2 reduces the impact of the drug on the reduction of NO production (Figure 1). Hemeoxygenase breaks down heme as the rate-limiting step and produces several active biological molecules that can serve as secondary messengers for cellular processes, such as inflammation.55 It has also been observed that lack of Nrf2 in macrophages decreases the expression of HO-1, which confers protection against inflammation. Additionally, LPS-induced iNOS expression in the macrophages was also lowered by an increase in HO-1 expression in Nrf2+/+ mice.56,57 The antioxidant gene HO-1 was clearly regulated by PEITC and CUR in Nrf2+/+ macrophages, as demonstrated by quantitative PCR (Figure 3A) and western blot analysis (Figure 4AB). Previous studies from our group as well as others showed that increased expression of HO-1 has anti-inflammatory effects.26,27,58 Accordingly, our results in the present study clearly indicate that PEITC and CUR increase the gene (Figure 3A) and protein expression (Figure 4A,B) of HO-1 in LPS-induced Nrf2+/+ macrophages in a dose-dependent manner, whereas this effect was not observed in Nrf2–/– macrophages. There have been several reports on the anti-inflammatory effects of CUR and PEITC acting via induction of HO-146 and suppression of NF-kB.59−61 It is logical to conclude that the induction of HO-1 and suppression of inflammatory cytokines as well as prostaglandins such as COX-2 is mediated via the Nrf2 pathway, which is further supported by the induction of HO-1 gene and protein expression. Furthermore, it was clearly observed that PEITC inhibited IL-6 and TNF-α production, as measured by ELISA (Figure 2A,B). Additionally, the mRNA expression of the corresponding genes was clearly attenuated by CUR and PEITC, as illustrated in (Figure 3C,D). Nevertheless, the effect was not prominent in the CUR-treated macrophages, as shown in (Figure 2A,B), which depicts cytokine measurements by ELISA. In addition to the cytokines, the mRNA and protein expression of COX-2, which is a classic inflammatory mediator, was lowered upon treatment by CUR and PEITC in a dose-dependent manner, as shown in Figures 3E and 4A,B.
In conclusion, our study indicates that Nrf2 plays a critical role in mediating the anti-inflammatory properties of PEITC and CUR, as demonstrated by their effects in Nrf2+/+ and Nrf2–/– macrophages upon induction with LPS. This result further strengthens the hypothesis that Nrf2, apart from regulating phase II and III drug metabolizing enzymes and transporters, also plays a crucial role in attenuating inflammation, which contributes to many acute and chronic diseases, including autoimmune, neurological, and cardiovascular diseases and cancer.
Acknowledgments
We thank all of the members of the Kong lab for their help and support in preparation of this manuscript.
Glossary
Abbreviations
- PEITC
phenethyl isothiocyanate
- CUR
curcumin
- Nrf2
nuclear factor-erythroid 2 (NF-E2)-related factor 2
- COX-2
cycloxygenase-2
- iNOS
inducible nitric oxide synthase
- TNF-α
tumor necrosis factor-α
- IL-1
interleukin-1
- IL-6
interleukin-6
- IL-8
interleukin-8
- HO-1
hemeoxygenase-1
- NQO1
NAD(P)H quinine oxidoreductase
- LPS
lipopolysaccharides
- RIPA
radio immune precipitation assay
- BCA
bicinchoninic acid
- ELISA
enzyme-linked immunosorbent assay
Supporting Information Available
Cell viability of isolated Nrf2+/+ and Nrf2–/– macrophages upon treatment with CUR, PEITC, and LPS for 24 h. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported in part by institutional funds and by R01-CA118947, R01-CA152826, from the National Cancer Institute (NCI), and R01AT007065, from the National Center for Complementary and Alternative Medicines (NCCAM) and the Office of Dietary Supplements (ODS).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Aggarwal B. B.; Shishodia S.; Sandur S. K.; Pandey M. K.; Sethi G. (2006) Inflammation and cancer: how hot is the link?. Biochem. Pharmacol. 72, 1605–1621. [DOI] [PubMed] [Google Scholar]
- Condeelis J.; Pollard J. W. (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266. [DOI] [PubMed] [Google Scholar]
- Colotta F.; Allavena P.; Sica A.; Garlanda C.; Mantovani A. (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T.; Brade H. (1992) Bacterial endotoxins. Sci. Am. 267, 54–61. [DOI] [PubMed] [Google Scholar]
- Fujihara M.; Muroi M.; Tanamoto K.; Suzuki T.; Azuma H.; Ikeda H. (2003) Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol. Ther. 100, 171–194. [DOI] [PubMed] [Google Scholar]
- Mosser D. M.; Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M.; Johnson J. A. (2004) An important role of Nrf2–ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 37, 139–143. [DOI] [PubMed] [Google Scholar]
- Nguyen T.; Nioi P.; Pickett C. B. (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 284, 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath K.; Varga C.; Berko A.; Posa A.; Laszlo F.; Whittle B. J. (2008) The involvement of heme oxygenase-1 activity in the therapeutic actions of 5-aminosalicylic acid in rat colitis. Eur. J. Pharmacol. 581, 315–323. [DOI] [PubMed] [Google Scholar]
- Pae H. O.; Ae Ha Y.; Chai K. Y.; Chung H. T. (2008) Heme oxygenase-1 attenuates contact hypersensitivity induced by 2,4-dinitrofluorobenzene in mice. Immunopharmacol. Immunotoxicol. 30, 207–216. [DOI] [PubMed] [Google Scholar]
- Syapin P. J. (2008) Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br. J. Pharmacol. 155, 623–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R.; Bains S. K.; Pitchumony T. S.; de Castro Bras L. E.; Drago F.; Dubois-Rande J. L.; Bucolo C.; Motterlini R. (2013) Small molecule activators of the Nrf2–HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 76, 132–148. [DOI] [PubMed] [Google Scholar]
- Osburn W. O.; Yates M. S.; Dolan P. D.; Chen S.; Liby K. T.; Sporn M. B.; Taguchi K.; Yamamoto M.; Kensler T. W. (2008) Genetic or pharmacologic amplification of Nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 104, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn W. O.; Karim B.; Dolan P. M.; Liu G.; Yamamoto M.; Huso D. L.; Kensler T. W. (2007) Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int. J. Cancer 121, 1883–1891. [DOI] [PubMed] [Google Scholar]
- Khor T. O.; Huang M. T.; Prawan A.; Liu Y.; Hao X.; Yu S.; Cheung W. K.; Chan J. Y.; Reddy B. S.; Yang C. S.; Kong A. N. (2008) Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev. Res. 1, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y.; Itoh K.; Morishima Y.; Kimura T.; Kiwamoto T.; Iizuka T.; Hegab A. E.; Hosoya T.; Nomura A.; Sakamoto T.; Yamamoto M.; Sekizawa K. (2005) Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J. Immunol. 175, 6968–6975. [DOI] [PubMed] [Google Scholar]
- Kim J.; Cha Y. N.; Surh Y. J. (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 690, 12–23. [DOI] [PubMed] [Google Scholar]
- Su Z. Y.; Khor T. O.; Shu L.; Lee J. H.; Saw C. L.; Wu T. Y.; Huang Y.; Suh N.; Yang C. S.; Conney A. H.; Wu Q.; Kong A. N. (2013) Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and radix Angelica sinensis via promoter CpG demethylation. Chem. Res. Toxicol. 26, 477–485. [DOI] [PubMed] [Google Scholar]
- Saw C. L.; Wu Q.; Su Z. Y.; Wang H.; Yang Y.; Xu X.; Huang Y.; Khor T. O.; Kong A. N. (2013) Effects of natural phytochemicals in Angelica sinensis (Danggui) on Nrf2-mediated gene expression of phase II drug metabolizing enzymes and anti-inflammation. Biopharm. Drug. Dispos. 34, 303–311. [DOI] [PubMed] [Google Scholar]
- Rose P.; Won Y. K.; Ong C. N.; Whiteman M. (2005) Beta-phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide 12, 237–243. [DOI] [PubMed] [Google Scholar]
- Balogun E.; Hoque M.; Gong P.; Killeen E.; Green C. J.; Foresti R.; Alam J.; Motterlini R. (2003) Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 371, 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. V. (2007) Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 595, 213–226. [DOI] [PubMed] [Google Scholar]
- Saw C. L.; Huang M. T.; Liu Y.; Khor T. O.; Conney A. H.; Kong A. N. (2011) Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinog. 50, 479–486. [DOI] [PubMed] [Google Scholar]
- Huang C. S.; Lin A. H.; Liu C. T.; Tsai C. W.; Chang I. S.; Chen H. W.; Lii C. K. (2013) Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFkappaB activation. Mol. Nutr. Food Res. 57, 1918–1930. [DOI] [PubMed] [Google Scholar]
- Hempel S. L.; Monick M. M.; Hunninghake G. W. (1994) Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J. Clin. Invest. 93, 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.; Wu R. T.; Wu T.; Khor T. O.; Wang H.; Kong A. N. (2008) Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 76, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Khor T. O.; Saw C. L.; Lin W.; Wu T.; Huang Y.; Kong A. N. (2010) Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol. Pharmaceutics 7, 2185–2193. [DOI] [PubMed] [Google Scholar]
- Malhotra D.; Thimmulappa R. K.; Mercado N.; Ito K.; Kombairaju P.; Kumar S.; Ma J.; Feller-Kopman D.; Wise R.; Barnes P.; Biswal S. (2011) Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J. Clin. Invest. 121, 4289–4302. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Misko T. P.; Schilling R. J.; Salvemini D.; Moore W. M.; Currie M. G. (1993) A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 214, 11–16. [DOI] [PubMed] [Google Scholar]
- Gonzales A. M.; Orlando R. A. (2008) Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. Y.; Khor T. O.; Saw C. L.; Loh S. C.; Chen A. I.; Lim S. S.; Park J. H.; Cai L.; Kong A. N. (2011) Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen A. L.; Inkala M.; Heikura T.; Jauhiainen S.; Jyrkkanen H. K.; Kansanen E.; Maatta K.; Romppanen E.; Turunen P.; Rutanen J.; Yla-Herttuala S. (2007) Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler., Thromb., Vasc. Biol. 27, 741–747. [DOI] [PubMed] [Google Scholar]
- Itoh K.; Mochizuki M.; Ishii Y.; Ishii T.; Shibata T.; Kawamoto Y.; Kelly V.; Sekizawa K.; Uchida K.; Yamamoto M. (2004) Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Δ12,14-prostaglandin J2. Mol. Cell. Biol. 24, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T.; Cho C. Y.; Thimmulappa R. K.; Zhen L.; Srisuma S. S.; Kensler T. W.; Yamamoto M.; Petrache I.; Tuder R. M.; Biswal S. (2004) Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 114, 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi C. A.; Withers C. M.; Bartz R. R.; MacGarvey N. C.; Fu P.; Sweeney T. E.; Welty-Wolf K. E.; Suliman H. B. (2011) Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 286, 16374–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong G. S.; Lee D. S.; Li B.; Lee H. J.; Kim E. C.; Kim Y. C. (2010) Effects of sappanchalcone on the cytoprotection and anti-inflammation via heme oxygenase-1 in human pulp and periodontal ligament cells. Eur. J. Pharmacol. 644, 230–237. [DOI] [PubMed] [Google Scholar]
- Lee D. S.; Li B.; Im N. K.; Kim Y. C.; Jeong G. S. (2013) 4,2′,5′-Trihydroxy-4′-methoxychalcone from Dalbergia odorifera exhibits anti-inflammatory properties by inducing heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 16, 114–121. [DOI] [PubMed] [Google Scholar]
- Lyu J. H.; Lee G. S.; Kim K. H.; Kim H. W.; Cho S. I.; Jeong S. I.; Kim H. J.; Ju Y. S.; Kim H. K.; Sadikot R. T.; Christman J. W.; Oh S. R.; Lee H. K.; Ahn K. S.; Joo M. (2011) Ent-kaur-16-en-19-oic acid, isolated from the roots of Aralia continentalis, induces activation of Nrf2. J. Ethnopharmacol. 137, 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo W. Y.; Goh A. R.; Ju S. M.; Song H. Y.; Kwon D. J.; Jun J. G.; Kim B. C.; Choi S. Y.; Park J. (2011) Celastrol induces expression of heme oxygenase-1 through ROS/Nrf2/ARE signaling in the HaCaT cells. Biochem. Biophys. Res. Commun. 407, 535–540. [DOI] [PubMed] [Google Scholar]
- Pae H. O.; Jeong G. S.; Kim H. S.; Woo W. H.; Rhew H. Y.; Kim H. S.; Sohn D. H.; Kim Y. C.; Chung H. T. (2007) Costunolide inhibits production of tumor necrosis factor-alpha and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflammation Res. 56, 520–526. [DOI] [PubMed] [Google Scholar]
- Kumar H.; Kim I. S.; More S. V.; Kim B. W.; Choi D. K. (2013) Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 31, 109–139. [DOI] [PubMed] [Google Scholar]
- Thimmulappa R. K.; Scollick C.; Traore K.; Yates M.; Trush M. A.; Liby K. T.; Sporn M. B.; Yamamoto M.; Kensler T. W.; Biswal S. (2006) Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-imidazolide. Biochem. Biophys. Res. Commun. 351, 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q.; Battelli L.; Hubbs A. F. (2006) Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am. J. Pathol. 168, 1960–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.; Lu R.; Chang J. C.; Kan Y. W. (1996) NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U.S.A. 93, 13943–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. J.; Takizawa H.; Azuma A.; Kohyama T.; Yamauchi Y.; Takahashi S.; Yamamoto M.; Kawada T.; Kudoh S.; Sugawara I. (2008) Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin. Immunol. 128, 366–373. [DOI] [PubMed] [Google Scholar]
- Cheung K. L.; Khor T. O.; Kong A. N. (2009) Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm. Res. 26, 224–231. [DOI] [PubMed] [Google Scholar]
- Cheung K. L.; Kong A. N. (2010) Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 12, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor T. O.; Keum Y. S.; Lin W.; Kim J. H.; Hu R.; Shen G.; Xu C.; Gopalakrishnan A.; Reddy B.; Zheng X.; Conney A. H.; Kong A. N. (2006) Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 66, 613–621. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Gao R.; Cao Y.; Guo M.; Wei Z.; Zhou E.; Li Y.; Yao M.; Yang Z.; Zhang N. (2014) Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-kappaB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 20, 54–58. [DOI] [PubMed] [Google Scholar]
- Rockwell C. E.; Zhang M.; Fields P. E.; Klaassen C. D. (2012) Th2 skewing by activation of Nrf2 in CD4+ T cells. J. Immunol. 188, 1630–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschek C. V.; Schnorr O.; Kolb-Bachofen V. (2004) The role of iNOS in chronic inflammatory processes in vivo: is it damage-promoting, protective, or active at all?. Curr. Mol. Med. 4, 763–775. [DOI] [PubMed] [Google Scholar]
- Menon V. P.; Sudheer A. R. (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 595, 105–125. [DOI] [PubMed] [Google Scholar]
- Chen Y. H.; Dai H. J.; Chang H. P. (2003) Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 69, 696–700. [DOI] [PubMed] [Google Scholar]
- Murakami A. (2009) Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum Nutr. 61, 193–203. [DOI] [PubMed] [Google Scholar]
- Ryter S. W.; Alam J.; Choi A. M. (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86, 583–650. [DOI] [PubMed] [Google Scholar]
- Surh Y. J.; Kundu J. K.; Li M. H.; Na H. K.; Cha Y. N. (2009) Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 32, 1163–1176. [DOI] [PubMed] [Google Scholar]
- Ashino T.; Yamanaka R.; Yamamoto M.; Shimokawa H.; Sekikawa K.; Iwakura Y.; Shioda S.; Numazawa S.; Yoshida T. (2008) Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol. Immunol. 45, 2106–2115. [DOI] [PubMed] [Google Scholar]
- Otterbein L. E.; Soares M. P.; Yamashita K.; Bach F. H. (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 24, 449–455. [DOI] [PubMed] [Google Scholar]
- Biswas S. K.; McClure D.; Jimenez L. A.; Megson I. L.; Rahman I. (2005) Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid. Redox Signaling 7, 32–41. [DOI] [PubMed] [Google Scholar]
- Murakami A.; Song M.; Ohigashi H. (2007) Phenethyl isothiocyanate suppresses receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis by blocking activation of ERK1/2 and p38 MAPK in RAW264.7 macrophages. Biofactors 30, 1–11. [DOI] [PubMed] [Google Scholar]
- Xu C.; Shen G.; Chen C.; Gelinas C.; Kong A. N. (2005) Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene 24, 4486–4495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.