Abstract
The rate of antigen binding by mouse lymphoid cells has been investigated with polymerized flagellin of Salmonella adelaide that had been biosynthetically labeled with tritium. Autoradiographs of lymphnode cells incubated with the tritiated antigen at 37° showed aggregation of antigen receptors to one cell pole. This was followed 4-5 hr later by the appearance of antigen receptors on the cell surface, at a density severalfold higher than at the time of first contact with the antigen. Antigen-binding cells exposed in vitro to antigen concentrations known to cause high zone tolerance induction failed to form polar antigen caps once they had entered the phase of increased receptor formation. The data suggest that sufficient crosslinking of receptors by the antigen to cause their aggregation triggers the cell to differentiate and to increase its density of antigen receptors. In the presence of antigen concentrations favoring high zone tolerance, receptors may become interlinked to such an extent that they are prevented from aggregation. Such a “frozen” state of the antigen recognition system would render the cell unresponsive to antigenic stimuli.
Keywords: mouse, lymphoid cells, flagellin, autoradiography, caps
Full text
PDF

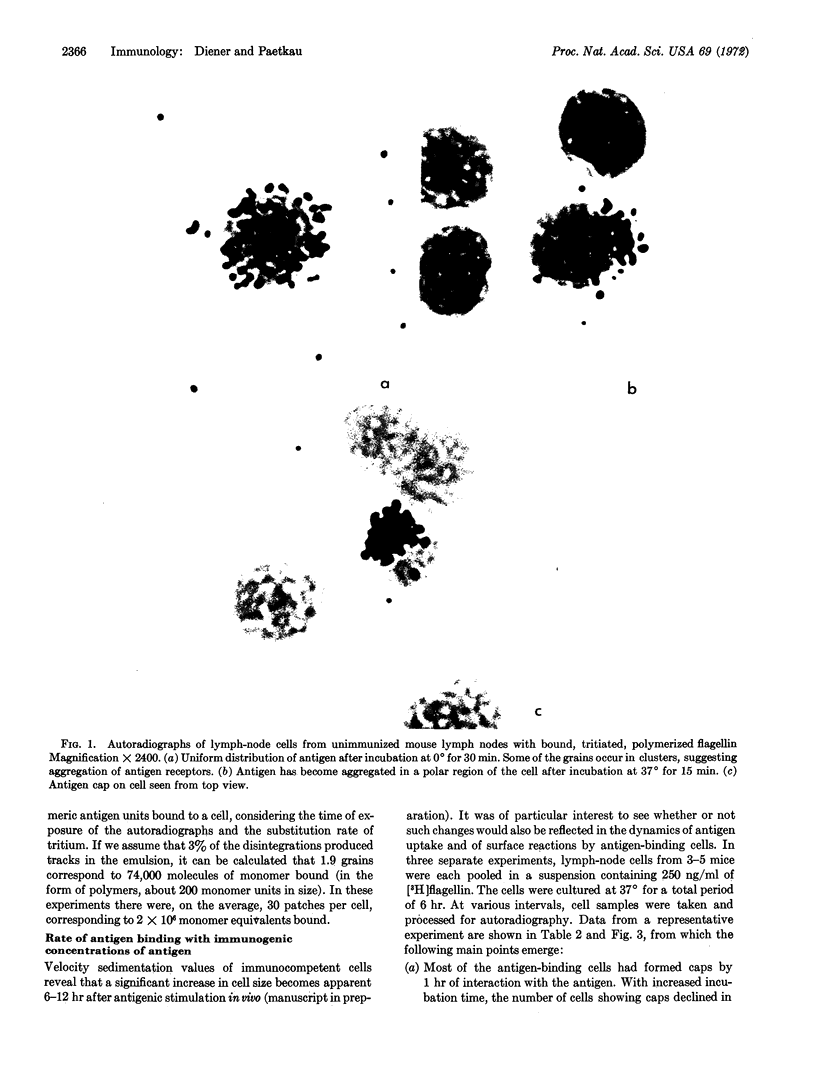
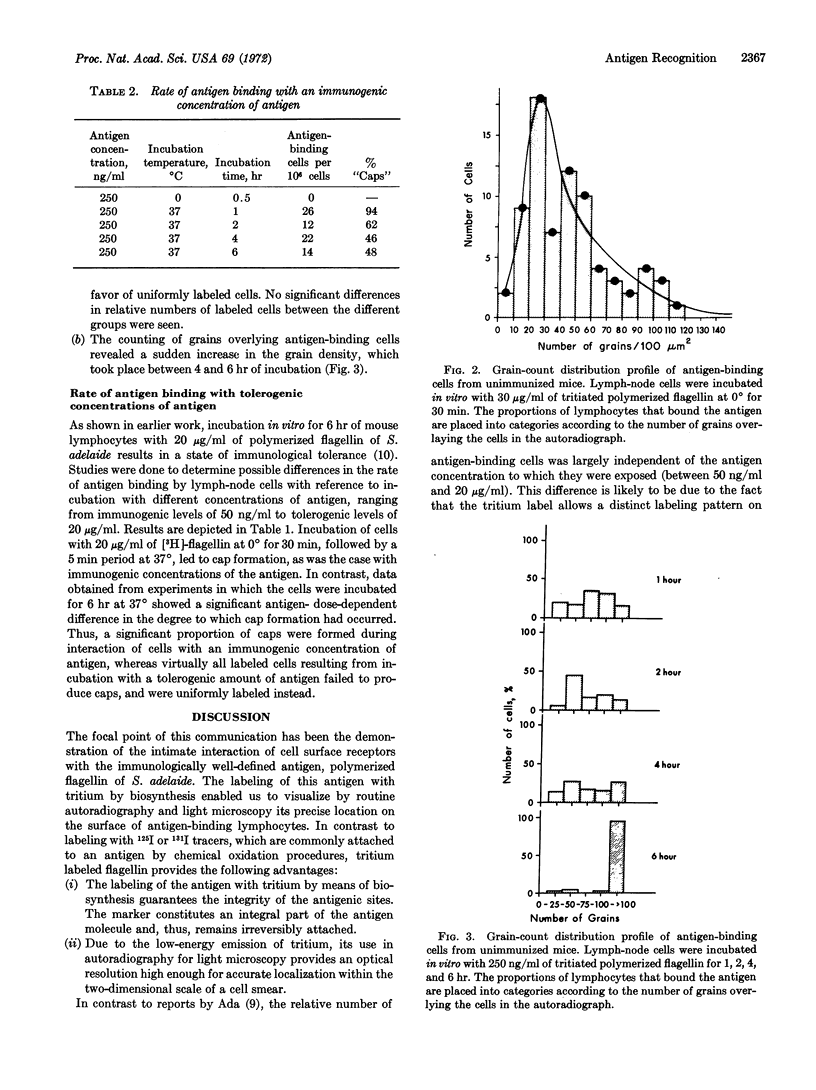

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. ANTIGENS IN IMMUNITY. I. PREPARATION AND PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA ADELAIDE. Aust J Exp Biol Med Sci. 1964 Jun;42:267–282. [PubMed] [Google Scholar]
- Ada G. L. Antigen binding cells in tolerance and immunity. Transplant Rev. 1970;5:105–129. doi: 10.1111/j.1600-065x.1970.tb00358.x. [DOI] [PubMed] [Google Scholar]
- BURNET M. A DARWINIAN APPROACH TO IMMUNITY. Nature. 1964 Aug 1;203:451–454. doi: 10.1038/203451a0. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Wilson J. D. Detection of antigen-binding cells by combined rosette formation and autoradiography. Nat New Biol. 1971 Dec 1;234(48):154–156. doi: 10.1038/newbio234154b0. [DOI] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- Diener E., Armstrong W. D. Immunological tolerance in vitro: kinetic studies at the cellular level. J Exp Med. 1969 Mar 1;129(3):591–603. doi: 10.1084/jem.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E., O'Callaghan F., Kraft N. Immune response in vitro to Salmonella H-antigens, not affected by anti-theta serum. J Immunol. 1971 Dec;107(6):1775–1777. [PubMed] [Google Scholar]
- Feldmann M., Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D., Sulitzeanu D. Affinity of radioiodinated bovine serum albumin for lymphoid cells. Binding of I-125-BSA to lymphoid cells of immune mice. Isr J Med Sci. 1969 Mar-Apr;5(2):217–229. [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Endocytosis by lymphocytes of complexes of anti-Ig with membrane-bound Ig. J Immunol. 1972 Feb;108(2):569–572. [PubMed] [Google Scholar]