Abstract
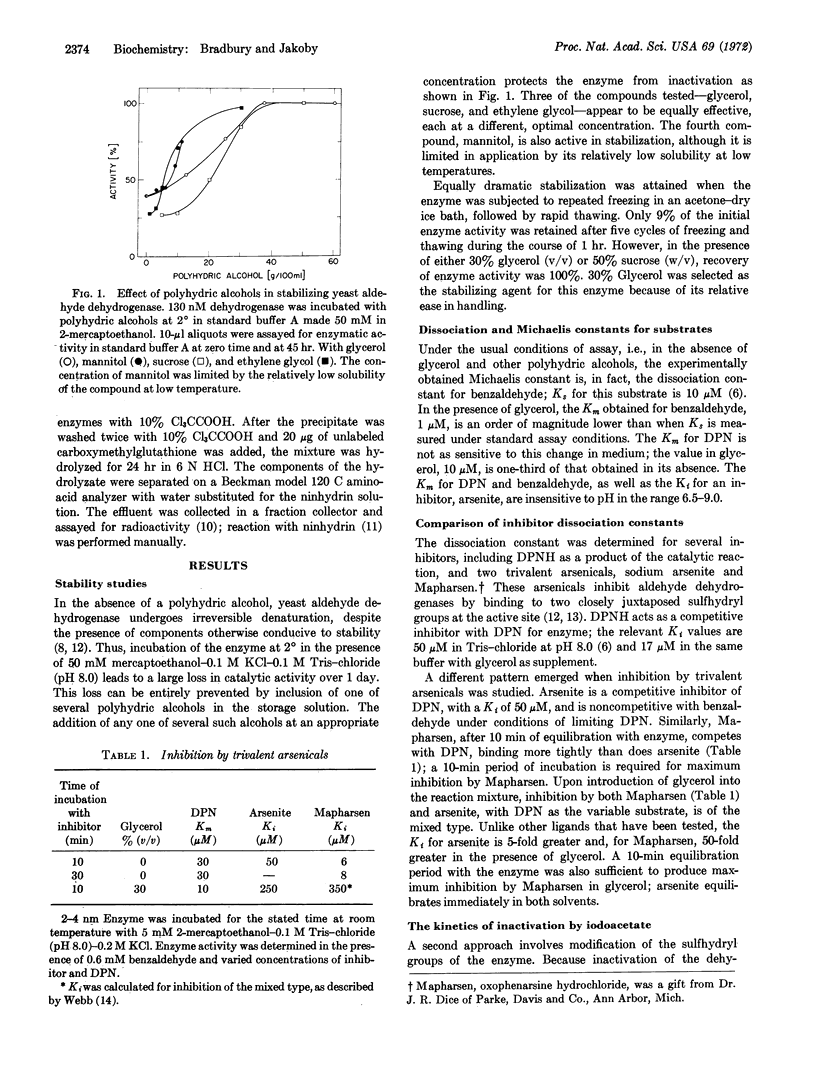
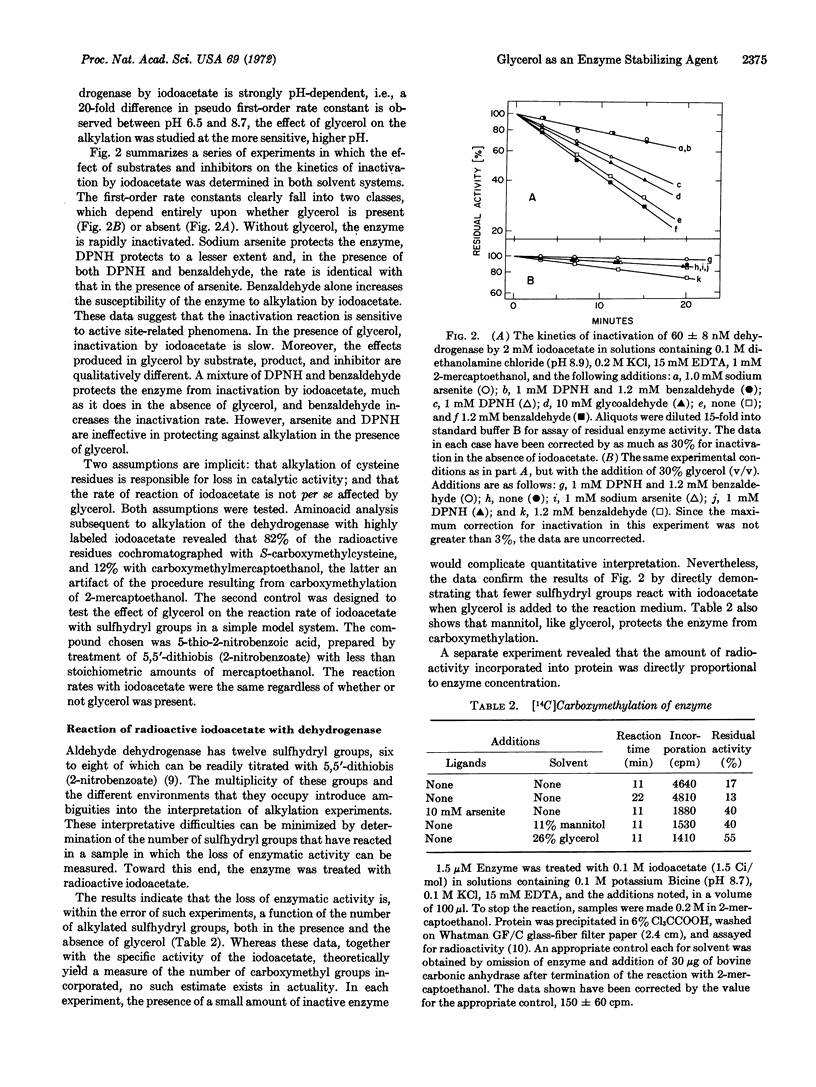
The potassium-dependent aldehyde dehydrogenase (EC 1.2.1.3), from yeast is markedly altered by the addition of high concentrations of glycerol or other polyhydric alcohols to aqueous buffers. Several lines of evidence suggest that the three-dimensional structure near the active site is involved: (i) The stability of the enzyme when stored at 2°, or when subjected to repeated freezing and thawing, depends upon the presence of at least 30% (v/v) glycerol. (ii) In the same solvent, the Km value for DPN and the binding constant for benzaldehyde decrease by 3- and 10-fold, respectively, compared with the values obtained for these substrates in fully aqueous media. (iii) Competitive inhibition by trivalent arsenicals with respect to DPN is no longer observed in glycerol; the inhibition becomes mixed and the Ki values increase by 5- and 50-fold, respectively, with arsenite and Mapharsen. (iv) Essential sulfhydryl groups, which are easily carboxymethylated in aqueous buffers, are not readily available in either glycerol or mannitol.
The data are consistent with a change in topography induced by polyhydric alcohols in which sulfhydryl groups near the DPN-binding site are displaced to a more protected environment, where their reactivity is reduced. Since the stabilizing effects of such alcohols are frequently encountered, these results may have application to other enzymes.
Keywords: thiol groups, polyhydric alcohols, arsenite
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury S. L., Jakoby W. B. Ligand interactions with yeast aldehyde dehydrogenase. J Biol Chem. 1971 Nov 25;246(22):6929–6932. [PubMed] [Google Scholar]
- Bradbury S. L., Jakoby W. B. Ordered binding of substrates to yeast aldehyde dehydrogenase. J Biol Chem. 1971 Mar 25;246(6):1834–1840. [PubMed] [Google Scholar]
- Clark J. F., Jakoby W. B. Yeast aldehyde dehydrogenase. 3. Preparation of three homogeneous species. J Biol Chem. 1970 Nov 25;245(22):6065–6071. [PubMed] [Google Scholar]
- Clark J. F., Jakoby W. B. Yeast aldehyde dehydrogenase. IV. Dissociation and reassociation of native and hybrid forms. J Biol Chem. 1970 Nov 25;245(22):6072–6077. [PubMed] [Google Scholar]
- FRIDOVICH I. Inhibition of acetoacetic decarboxylase by anions. The Hofmeister lyotropic series. J Biol Chem. 1963 Feb;238:592–598. [PubMed] [Google Scholar]
- Karow A. M., Jr Cryoprotectants--a new class of drugs. J Pharm Pharmacol. 1969 Apr;21(4):209–223. doi: 10.1111/j.2042-7158.1969.tb08235.x. [DOI] [PubMed] [Google Scholar]
- Ruwart M. J., Suelter C. H. Activation of yeast pyruvate kinase by natural and artificial cryoprotectants. J Biol Chem. 1971 Oct 10;246(19):5990–5993. [PubMed] [Google Scholar]
- STEIN W. H., MOORE S. The free amino acids of human blood plasma. J Biol Chem. 1954 Dec;211(2):915–926. [PubMed] [Google Scholar]
- Steinman C. R., Jakoby W. B. Yeast aldehyde dehydrogenase. I. Purification and crystallization. J Biol Chem. 1967 Nov 10;242(21):5019–5023. [PubMed] [Google Scholar]
- Steinman C. R., Jakoby W. B. Yeast aldehyde dehydrogenase. II. Properties of the homogeneous enzyme preparations. J Biol Chem. 1968 Feb 25;243(4):730–734. [PubMed] [Google Scholar]


