Abstract
Introduction
This study aimed to assess the costs and benefits of three alternative second-line treatment strategies for Swedish patients with type 2 diabetes mellitus (T2DM) who fail to reach glycated hemoglobin (HbA1c) ≤ 7% with metformin treatment alone: glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and neutral protamine Hagedorn (NPH) insulin.
Methods
A previously developed cohort model for T2DM was applied over a 35-year time horizon. Data on T2DM patients on metformin monotherapy with HbA1c > 7% were collected from the Swedish National Diabetes Register. Treatment effects were taken from published studies. Costs and effects were discounted at 3% per annum, and the analysis was conducted from a societal perspective. The robustness of the results was evaluated using one-way and probabilistic sensitivity analyses.
Results
Treatment with GLP-1 agonists was associated with a discounted incremental benefit of 0.10 and 0.25 quality-adjusted life years (QALYs) and higher discounted costs of Swedish Krona (SEK) 34,865 and SEK 40,802 compared with DPP-4 inhibitors and NPH insulin, respectively. Assuming willingness-to-pay (WTP) of SEK 500,000 per QALY, treatment strategy with GLP-1 agonists was a cost-effective option with incremental cost-effectiveness ratios of SEK 353,172 and SEK 160,618 per QALY gained versus DPP-4 inhibitors and NPH insulin, respectively. The results were most sensitive to incidence rate of moderate/major hypoglycemia and disutilities associated with insulin treatment, body mass index (BMI), and hypoglycemia.
Conclusion
Assuming a WTP of SEK 500,000 per QALY, treatment strategy with GLP-1 agonists is a cost-effective strategy in comparison to DPP-4 inhibitors and NPH insulin among T2DM patients inadequately controlled with metformin alone in a Swedish setting.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-014-0080-0) contains supplementary material, which is available to authorized users.
Keywords: Cost–utility analysis, DPP-4 inhibitors, GLP-1 agonists, Insulin, Sweden, Type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a serious progressive disorder characterized by insulin resistance and relative insulin deficiency resulting in increased blood glucose level. The prevalence of T2DM is growing worldwide, mainly due to growth in urbanization and the resulting substantial changes in lifestyle [1]. The total number of adults aged 20–79 with diabetes is projected to increase from 382 million in 2013 to 592 million in 2035 [1]. It has been estimated that 6.4% of adults aged 20–79 had diabetes in Sweden in 2013 [1]. T2DM is the most common form of diabetes worldwide, and is an established risk factor for several fatal and non-fatal micro- and macrovascular complications. This translates into a significant economic burden for individuals and societies. In 2010, an estimated 8% of the total Swedish health expenditure went to diabetes-related healthcare [2].
Previous studies have shown that good glycemic control is crucial to decrease the risk of diabetes-related complications [3–6]. The American Diabetes Association recommends glycated hemoglobin (HbA1c) < 7% as treatment goal for most patients with T2DM [7], and the year 2010 guidelines from the National Board of Health and Welfare in Sweden advocates similar treatment goals for newly diagnosed, people who were diagnosed at younger age, and people with low risk of cardiovascular diseases [8]. A typical treatment pattern for T2DM, advocated by international and Swedish guidelines, begins with lifestyle modifications, then adds metformin monotherapy when blood glucose control is unsatisfactory, followed by the addition of other medication such as sulfonylurea or basal insulin including intermediate-acting neutral protamine Hagedorn (NPH) insulin. The progressivity of the disease implies that many patients eventually will need multiple dose insulin treatment if second-line treatment does not result in achievement of sufficient control of blood glucose levels [8–10].
Despite these guidelines and the availability of a range of drugs, a large proportion of T2DM patients fail to achieve and maintain the treatment goals, mainly due to the progressive nature of the disease and the inadequacy of conventional treatments [11]. A survey conducted in 2008 by the Swedish National Diabetes Register showed that while there was an improvement in the proportion of T2DM patients reaching HbA1c ≤ 7% compared with 1999, a substantial proportion of patients (48%) still did not achieve this treatment goal [12]. Similar values have been reported for years 2009–2012 [13]. In addition, conventional medications such as sulfonylurea or insulin are associated with side effects including weight gain and hypoglycemia [14, 15]. Hence, there is a need for new therapies with better efficacy and fewer side effects.
In response to this, incretin-based therapies have attracted growing interests during recent years, as clinical trials indicated that they might provide improved glycemic control with low risk of hypoglycemia and seem weight neutral, or even weight reducing [14, 16–18]. These therapies are mainly recommended as second-line therapy among patients who fail to achieve or maintain the blood glucose treatment goals on metformin alone [10]. Two classes of incretin-based therapies are available: glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors. Currently, two GLP-1 agonists (liraglutide and exenatide) and three DPP-4 inhibitors (sitagliptin, saxagliptin, and vildagliptin) have been approved for use in Sweden.
While the short-term treatment efficacy and safety of incretin-based therapies have been investigated, there is limited evidence on the long-term efficacy and cost-effectiveness of these therapies. Increased use of these newer, more expensive drugs in routine practice has substantial budgetary implications for health systems. Moreover, while there is consensus on using metformin as the first-line oral anti-hyperglycemic alongside lifestyle modifications in T2DM patients when lifestyle interventions are insufficient for glycemic control, there is disagreement over the preferred agent for second-line therapy [19]. Hence, it is of value to evaluate and compare the cost-effectiveness of these second-line therapies to aid well-informed decisions regarding second-line therapy for patients with T2DM inadequately controlled by metformin monotherapy. The aim of this study was to estimate the lifetime costs and benefits of three second-line treatment alternatives: adding GLP-1 agonists, DPP-4 inhibitors, or NPH insulin to metformin among T2DM patients failing to reach HbA1c ≤ 7% with metformin alone in Sweden. In addition, considering the treatment strategies included in this study, we implicitly evaluated the cost-effectiveness of starting NPH insulin as the second-line therapy or later as third-line therapy after applying incretin-based agents.
Methods
Model Description
The authors conducted this cost–utility analysis using the Swedish Institute for Health Economics Cohort Model for T2DM (IHECM-T2DM). This model was previously described and used to compare cost-effectiveness of liraglutide versus sulphonylurea or sitagliptin in Sweden [20]. The IHECM-T2DM is a cohort model consisting of two parallel Markov chains covering 120 microvascular health states and 100 macrovascular health states. The microvascular health states include three subgroups: retinopathy, neuropathy, and nephropathy based on the work of Eastman et al. [21], Brown et al. [22], and Bagust et al. [23]. The macrovascular health states include four subgroups: ischemic heart disease, myocardial infarction, stroke and, heart failure based on United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Models [24, 25] and the Swedish National Diabetes Register (NDR) equations [26]. For macrovascular complications, in the current study, the authors used equations from the NDR [26] in the base case and from the UKPDS Outcome Model-1 [24] in a sensitivity analysis. With this structure, patients can develop multiple complications within each model cycle and over the simulation period. The IHECM-T2DM has a yearly cycle and a time horizons of up to 40 years can be used. In addition, the user can choose between two sets of mortality risk equations [24, 25]. The authors used the UKPDS Outcome Model-1 [24] for mortality in this study.
The model also includes evolution of biomarkers over time, treatment algorithms, and treatment-related side effects such as hypoglycemia. Eight biomarkers are included in the model and evolution of these biomarkers over time is determined by the initial treatment effects and an annual drift. Treatment algorithms include up to eight changes in anti-hyperglycemic treatment composition to account for possible combinations of glucose-lowering agents and treatment intensifications; this algorithm depends on a user-defined switching threshold of HbA1c. The model starts with assigning baseline clinical and demographic characteristics of the cohort, history of complications before diagnosis, and prevalence of diabetes-related complications.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Simulation Cohort, Treatment Effects and Scenarios
The baseline characteristics of the simulated cohort were based on a survey conducted by the Swedish National Diabetes Register [27]. As in this study, the authors evaluated the three second-line therapies as add-on to metformin, they used the data on a sample of patients on metformin monotherapy with HbA1c > 7% consisting 30% of patients on metformin monotherapy in Ekstrom et al.’s study [27] (Table 1, data were provided by the Swedish National Diabetes Register).
Table 1.
Baseline characteristics of patients on metformin monotherapy with HbA1c > 7% in the Swedish National Diabetes Register (NDR)
| Variable | N | Value (±SD) |
|---|---|---|
| Age (years) | 12,172 | 64.7 ± 11.6 |
| Diabetes duration (years) | 10,988 | 5.6 ± 4.6 |
| Male (%) | 7,000 | 57.5 |
| Smoker (%) | 1,710 | 17.5 |
| HbA1c (%) | 12,172 | 7.7 ± 0.8 |
| Systolic BP (mmHg) | 11,649 | 137 ± 16 |
| Diastolic BP (mmHg) | 11,649 | 79 ± 9 |
| Total cholesterol (mmol/l) | 9,381 | 4.9 ± 1.1 |
| HDL (mmol/l) | 8,322 | 1.2 ± 0.3 |
| LDL (mmol/l) | 7,355 | 2.8 ± 0.9 |
| Triglycerides (mmol/l) | 8,200 | 2.1 ± 1.3 |
| BMI | 10,385 | 30.9 ± 5.3 |
BMI body mass index, BP blood pressure, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein, SD standard deviation
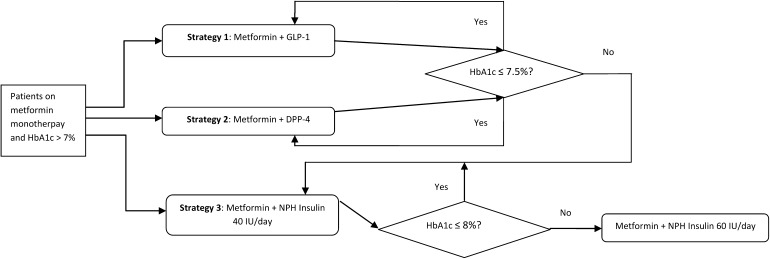
Three treatment strategies evaluated in the study are presented in Fig. 1. In strategies 1 and 2, patients received the GLP-1 receptor agonists and the DPP-4 inhibitors as add-on to metformin, respectively. In both these strategies, patients progressed to NPH insulin 40 insulin units (IU)/day + metformin when HbA1c exceeded 7.5% and to intensified NPH insulin 60 IU/day + metformin when HbA1c ≥ 8% (the base case analysis). In sensitivity analyses, these HbA1c threshold values changed to 8% (switch to NPH insulin 40 IU/day) and 8.5/9% (switch to NPH insulin 60 IU/day). In strategy 3, patients received NPH insulin 40 IU/day + metformin as initial second-line treatment, then progressed to NPH insulin 60 IU/day + metformin on reaching the HbA1c threshold value of 8% (the base case analysis) and 8.5/9% (the sensitivity analyses). Comparing strategy 3 with strategies 1 and 2 would provide more insight about timing of insulin initiation in T2DM patients. In the current study, the GLP-1 receptor agonists included liraglutide 1.2 mg daily and exenatide 2 mg once weekly, and the DPP-4 inhibitors are sitagliptin 100 mg daily, saxagliptin 5 mg daily, and vildagliptin 100 mg daily.
Fig. 1.
Schematic of treatment strategies applied in the base case analysis. DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, HbA1c glycated hemoglobin, IU insulin units
Treatment effects were considered as absolute change from baseline in HbA1c and weight and the rates of mild, moderate, and major hypoglycemia (Table 2) [28–34]. The treatment effects for each drug class were extracted from the literature; where data at drug class level were not available, the authors used data from head-to-head randomized controlled trials for a single agent in each drug class. The model considers non-severe daytime hypoglycemia as mild and non-severe nocturnal hypoglycemia as moderate hypoglycemia.
Table 2.
Efficacy of treatments used in the study model
| Variable | GLP-1 + Met | DPP-4 + Met | NPH insulin 40 IU/day + Met | NPH insulin 60 IU/day + Met |
|---|---|---|---|---|
| Change in HbA1c (%) | −0.96 [28] | −0.69 [28] | −1.12 [28] | −0.69 |
| Change in body weight (kg) | −1.80 [28] | 0.00 [28] | 3.30 [28] | 1.80 |
| Mild hypoglycemia (per patient per year) | 0.14 [29] | 0.14 [29] | 3.16 [30] | 3.16 [30] |
| Moderate hypoglycemia (per patient per year) | 0.05 [31] | 0.07 [30] | 1.014 [32] | 1.014 [32] |
| Major hypoglycemia (per patient per year) | 0.006 [29] | 0.01 [30] | 0.06 [33] | 0.06 [33] |
| Systolic blood pressure (mmHg) | −3.20 [34] | −4.00 [30] | −2.00 [30] | 0.00 |
| Diastolic blood pressure (mmHg) | −1.60 [34] | −1.47 [29] | −1.00 [30] | 0.00 |
| Total cholesterol (mmol/l) | −0.01 [29] | 0.00 [30] | −0.20 [30] | 0.00 |
| HDL cholesterol (mmol/l) | 0.01 [29] | 0.01 [29] | 0.00 [30] | 0.00 |
| LDL cholesterol (mmol/l) | 0.09 [29] | 0.09 [29] | −0.10 [30] | 0.00 |
| Triglyceride (mmol/l) | −0.10 [29] | −0.23 [29] | −0.40 [30] | 0.00 |
DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, HbA1c glycated hemoglobin, HDL high-density lipoprotein, IU insulin units, LDL low-density lipoprotein, Met metformin, NPH neutral protamine Hagedorn
The authors used data from the literature to estimate the treatment effects due to intensification of insulin from 40 IU/day to 60 IU/day [35, 36]. A recent meta-analysis found no direct association between dosage of insulin and risk of hypoglycemia [37], and so the authors applied the same rate of hypoglycemia events for both insulin treatments in this study. To account for association between hypoglycemic events and changes in HbA1c, the reported event rate from a study is transferred to expected event rate using coefficient (1.43) from a previous study [38].
As in previous studies, no treatment effect on other biomarkers was assumed in the base case analysis [39–42]. This assumption was relaxed in the sensitivity analysis. When data on treatment effects of NPH insulin were not available, the authors used the results from glargine insulin, since previous studies reported no significant difference in treatment effects between NPH and glargine insulin [43–45].
Treatment effects were applied for the first year after treatment, and then a constant annual drift was assumed for different treatment strategies. An annual drift of 0.15% unit for HbA1c was assumed for all treatments [46]. The annual drifts in weight were 0.42 kg for insulin and 0.23 kg for other treatments in the base case analysis [47]. In the sensitivity analyses, the authors considered 0.23 kg and 0.1 kg change in weight for all treatments [48]. They assumed 0.3 mmHg and 0.03 mg/dl annual drifts in blood pressure and lipid levels, respectively [49]. Prevalence of T2DM-related complications at baseline was extracted from national and international published sources [26, 50, 51].
Costs and Utilities
In the base case, costs were accounted from a societal perspective (2013 Swedish krona, SEK, 1 SEK = 0.115 Euros) and included healthcare costs, productivity losses, and net consumption losses. Costs for drugs, self-monitoring blood glucose (SMBG) test strips, and SMBG lancets were collected through the Dental and Pharmaceutical Benefits Agency in Sweden (TLV) [52]. In the base case analysis, the authors calculated a weighted average cost for GLP-1 agonists and DPP-4 inhibitors according to consumption of single agents in each class in Sweden in 2013, while in a sensitivity analysis, they applied the price of the lowest cost agent for each drug class (liraglutide for GLP-1 agonists class and vildagliptin for DPP-4 inhibitors class). In the base case analysis, based on recommendations by Owens et al. [53], the authors assumed that no SMBG is required for patients on DPP-4 inhibitors and GLP-1 agonists regimens, and daily monitoring is required for patients on NPH insulin regimens. It was assumed that patients use a new strip and lancet for each SMBG. Costs associated with T2DM-related complications and treatment side effects were derived from Sweden-specific published sources adjusted for 2013 using the Swedish Consumer Price Index (Table 1 in supplement).
Productivity losses were measured using human capital approach. Due to lack of data on productivity losses due to T2DM-related complications, we assumed that only severe stages of micro- and macrovascular complications caused loss of production. Annual cost of consumption includes costs in added life years and is calculated as the difference in market consumption and production during gained life years. Including these costs is required by the TLV in Sweden. For the current study, these costs were extracted from a previous study in Sweden [54] inflated to year 2012 using consumer price index (see supplement for more details).
Baseline utility was modeled using data from a recent study on EQ-5D scores in Swedish T2DM patients with no complications [55]. Health state utilities for T2DM-related complications and utility decrements associated with age, gender, duration of diabetes, and BMI were derived, whenever possible, from Sweden-specific published sources, supplemented with data from other sources if necessary (Table 2 in supplement). The same utility decrement was applied for subsequent events as for the initial one. No utility decrement associated with modality of treatment was applied in the base case analysis. Decrement associated with hypoglycemia was extracted from a previous study [56] which used data from the Swedish respondents in a multinational study [57]. A 3% annual discount rate for costs and quality-adjusted life years (QALYs) was applied in the base case analysis over a 35-year time horizon. While there is no formal willingness-to-pay (WTP) threshold for cost-effectiveness ratios in Sweden, interventions with less than 500,000 SEK per QALY gained are generally considered as cost-effective [58, 59].
Sensitivity Analyses
The authors conducted a series of one-way sensitivity analyses to assess the impact of variation in the model inputs and assumptions on the results of the base case analysis. In a sensitivity analysis, they excluded the net consumption and productivity losses (applying healthcare payer perspective). The number of weekly SMBG performed by patients in the GLP-1 agonists + metformin and DPP-4 inhibitors + metformin groups was varied (3, 5, and 7), as were the time horizons (10 and 20 years) and the discount rate for costs and QALYs (0 and 5%). A utility decrement of 0.049 for insulin therapy was applied [60]. The annual drift in HbA1c was set to 0.10% and 0.20% unit, the cost of one SMBG was varied by 10% and 25%, and the cost of T2DM-related complications varied by ±20%. No major hypoglycemia and no moderate/major hypoglycemia were assumed for all treatment groups. The treatment effects on HbA1c were changed to the lower and upper limits of 95% CI of the main estimate [28]. In addition, the treatment effect on HbA1c for NPH insulin 60 IU/day was set to −0.56%, −0.35% [35], and −0.18% [37].
A probabilistic sensitivity analysis (PSA) was conducted to assess the joint uncertainty of the input parameters using a Monte Carlo simulation with 1,000 iterations. Non-parametric bootstrapping with 1,000 bootstrap samples was then used to calculate the mean and bootstrap bias-corrected (BBC) 95% confidence interval (CI) of costs and QALYs as well as incremental cost-effectiveness ratios (ICERs). In the PSA, standard errors for utility decrement and treatment effects were collected from related sources. Costs of treatments and T2DM-related complications varied within a range of 20% from the base case values. Microsoft Excel and STATA 13 (StataCorp LP, College Station, TX, USA) were used for the analyses.
Results
The simulated profiles of HbA1c change over time for three treatments as add-on to metformin are shown in Fig. 2. It can be seen that using the base case threshold values, before switching to insulin therapy, patients on strategies 1 and 2 were expected to receive GLP-1 agonists and DPP-4 inhibitors, respectively, for 6 and 4 years. Figure 1 in the supplement displays the 35-year cumulative incidence of some major micro- and macrovascular events projected by the IHECM-T2DM. The model predicted a higher incidence of macrovascular events compared with microvascular events. For all events, the lowest and highest incidences were observed in the strategies 1 and 3, respectively.
Fig. 2.
Simulated profiles of HbA1c in the treatment strategies over the modeled time horizon. HbA1c glycated hemoglobin
The results of cost–utility analysis for the base case are given in Table 3. Treatment strategy 1 was associated with an 0.10 and 0.25 units improvement in discounted QALYs compared with strategies 2 and 3, respectively. In addition, treatment strategy 2 was associated with an improvement of 0.15 discounted QALYs compared with strategy 3. On the other hand, from a societal perspective, the treatment strategy 1 was associated with SEK 34,865 and SEK 40,802 higher discounted costs compared with strategies 2 and 3, respectively. The ICERs for strategy 1 versus strategies 2 and 3 were SEK 353,172 and SEK 160,618, respectively, and the ICER for strategy 2 versus strategy 3 was SEK 36,050. Assuming a WTP of SEK 500,000 per QALY gained in Sweden, strategy 1 could be considered cost-effective compared to either strategies 2 and 3.
Table 3.
The results of the base-case cost–utility analysis
| Strategy 1 | Strategy 2 | Strategy 3 | Difference strategy 1 versus strategy 2 | Difference strategy 1 versus strategy 3 | Difference strategy 2 versus strategy 3 | |
|---|---|---|---|---|---|---|
| Discounted QALYs | 4.75 | 4.65 | 4.50 | 0.10 | 0.25 | 0.15 |
| Discounted costs | ||||||
| Healthcare costs | 501,594 | 470,084 | 471,996 | 31,510 | 29,598 | −1,912 |
| Productivity losses | 8,972 | 9,473 | 10,599 | −501 | −1,627 | −1,126 |
| Net consumption losses | 1,652,341 | 1,648,485 | 1,639,510 | 3,857 | 12,831 | 8,974 |
| Total costs | 2,162,907 | 2,128,042 | 2,122,105 | 34,865 | 40,802 | 5,936 |
| ICER (SEK) | 353,172 | 160,618 | 36,050 | |||
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life years, SEK Swedish Krona
Sensitivity Analysis
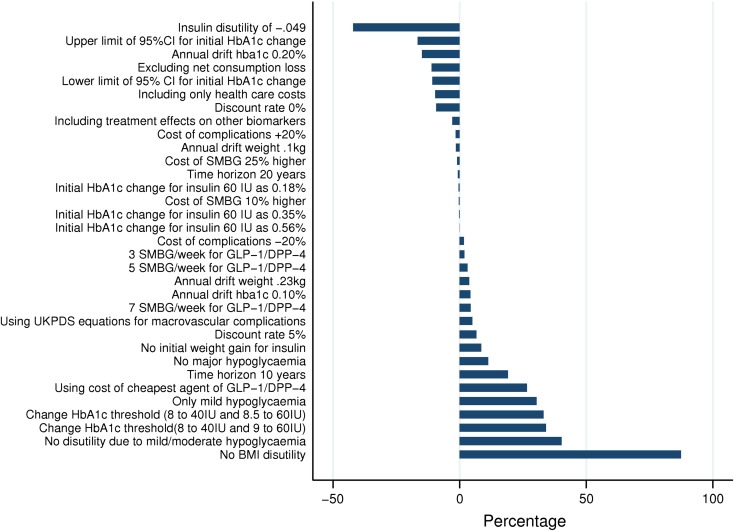
Overall, the univariate analyses showed that our base case analysis was robust to variation in the inputs and assumptions applied in the model. Figure 3 presents the results of the univariate sensitivity analyses as percentage changes in the ICER from the base case for comparison of strategy 1 versus strategy 2. The disutility associated with every unit of BMI over 25 (0.006 in the base case) was a key driver of the results, and excluding it from the analysis caused the ICER to rise to SEK 661,917, an 87.3% increase from the base case. While the result of the base case was sensitive to HbA1c threshold values, disutility due to mild/moderate hypoglycemia and insulin treatment, and incidence of moderate/major hypoglycemia, in all these cases the ICER remained below SEK 500,000 (range: SEK 204,517–495,368). In the PSA, the estimated ICER was SEK 319,217 (BBC 95% CI: SEK 309,849–330,212). Assuming a WTP of SEK 500,000 per QALY gained, strategy 1 had a 74.7% likelihood of being considered cost-effective in comparison to strategy 2.
Fig. 3.
Results of one-way sensitivity analyses: strategy 1 versus strategy 2. BMI body mass index, CI confidence interval, DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, HbA1c glycated hemoglobin, IU insulin units, SMBG self-monitoring blood glucose
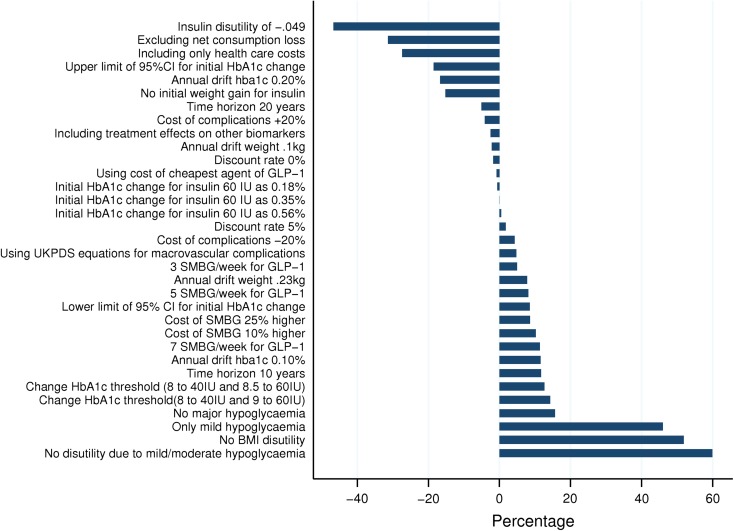
The results of the univariate sensitivity analyses for strategy 1 versus strategy 3 are shown in Fig. 4 as percentage changes in the ICER from the base case. The key model drivers were disutility of mild/moderate hypoglycemia, the disutility of insulin and BMI over 25, and incidence of moderate/major hypoglycemia. However, in all these cases, the ICER remained below SEK 500,000 (range: SEK 85,629–256,830). The PSA showed that the ICER was SEK 153,277 (BBC 95% CI: SEK 150,788–155,766). Assuming a WTP of SEK 500,000 per QALY gained, strategy 1 was expected to have a 100% probability of being cost-effective in comparison to strategy 3.
Fig. 4.
Results of one-way sensitivity analyses: strategy 1 versus strategy 3. BMI body mass index, CI confidence interval, DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, HbA1c glycated hemoglobin, IU insulin units, SMBG self-monitoring blood glucose
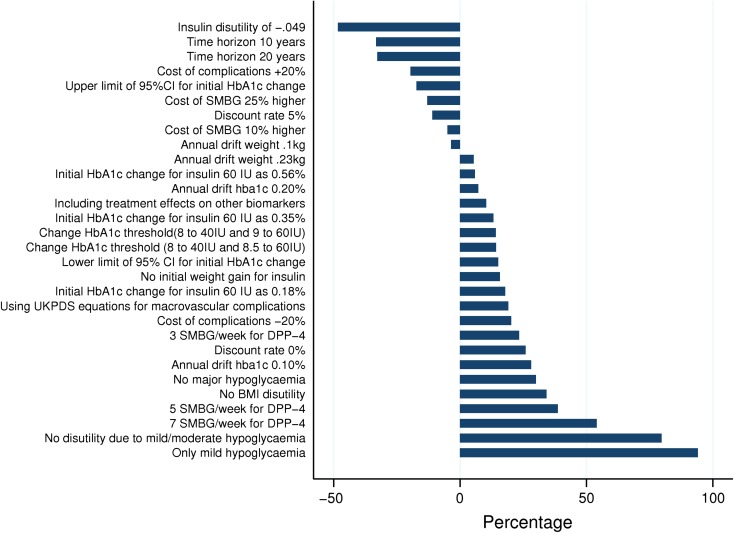
Figure 5 displays the results of the univariate sensitivity analyses for strategy 2 versus strategy 3 as percentage changes in the ICER from the base case. The key model drivers were incidence of moderate/major hypoglycemia, the disutility of mild/moderate hypoglycemia, including costs of 7 SMBG per week for the DPP-4 inhibitors, and the disutility of insulin. None of these changes had a significant impact on the cost–utility conclusions. Moreover, excluding net consumption and productivity loss, and applying the price of the cheapest DPP-4 agent, translated into dominance of strategy 2 versus strategy 3. The estimated ICER ranged from SEK 18,630 to SEK 69,976. The ICER from the PSA was SEK 40,277 (BBC 95% CI: SEK 37,436–43,119). Assuming a WTP of SEK 500,000 per QALY gained, strategy 2 was expected to have a 98.1% probability of being cost-effective in comparison to strategy 3.
Fig. 5.
Results of one-way sensitivity analyses: strategy 2 versus strategy 3. BMI body mass index, CI confidence interval, DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, HbA1c glycated hemoglobin, IU insulin units, SMBG self-monitoring blood glucose
Discussion
The authors applied a cohort model (IHECM-T2DM) to examine the long-term costs and health benefits of three different treatment strategies as add-on to metformin monotherapy in a representative sample of T2DM patients not achieving HbA1c < 7% on metformin monotherapy in Sweden. The results show that, assuming a WTP value of 500,000 SEK, the treatment strategy with GLP-1 agents is cost-effective in comparison to treatment strategies with either DPP-4 inhibitors or NPH insulin from both a societal and a healthcare payer perspective. Compared with treatment strategies of DPP-4 inhibitors and NPH insulin, the cost per QALY gained with GLP-1 agonists was approximately SEK 353,000 and SEK 161,000, respectively. The results from this study indicate that later transition to NPH insulin (i.e., as third-line therapy after providing incretin-based therapies) is cost-effective with the ICERs less than 200,000 SEK.
While there was no significant difference in hypoglycemic episodes between GLP-1 agonists and DPP-4 inhibitors, GLP-1 agonists were associated with a higher reduction in HbA1c and greater weight loss. These effects influence health outcomes in two ways: first, there is a direct effect in terms of lower cumulative incidence of T2DM-related complications, and second, there is an indirect effect through delay in switching to NPH insulin. The higher reduction in HbA1c meant that patients on GLP-1 agonists initiated NPH insulin therapy later than patients on DPP-4 inhibitors, resulting in fewer expected episodes of hypoglycemia in the lifetime perspective.
These health benefits partly offset the higher price of GLP-1 agonists compared with DPP-4 inhibitors, and this is in line with previous modeling studies comparing liraglutide with sitagliptin [20, 61–63]. The one-way sensitivity analyses in this study showed that this finding was most sensitive to the disutility due to BMI: assuming no disutility due to BMI >25 resulted in an ICER higher than SEK 500,000 per QALY gained. This is mainly due to significant difference between these two drug classes in weight changes (1.8 kg reduction versus no change) and implies that besides the effects on improved glycemic control, the effects on weight also play important role in cost-effectiveness of treatments in the T2DM context. In line with a previous study [62], this finding suggests that there should be a sub-group of patients with BMI >25 where treatment strategy with GLP-1 agonists is even more cost-effective in comparison to DPP-4 inhibitors. In particular, a previous study in Sweden showed that BMI is a significant predictor of other biomarkers in T2DM patients [64]. This implies that taking this association into account might decrease the ICER in favor of GLP-1 agonists, due to its effect on weight loss. The results were robust to other assumptions, and the ICER remained below SEK 500,000.
The ICER reported in the current study is higher than in a previous study in Sweden [20] that used the same model to compare liraglutide versus sitagliptin. Differences in utility decrement, the baseline characteristics, and treatment effects might be potential explanations for different ICER values. The ICER is also higher than in previous studies in other countries [61–63]. Beside differences mentioned earlier, there are differences in perspective (societal versus healthcare payer), and applied model that limits comparability of this study with previous ones. All these studies applied the CORE diabetes model [65], which mainly uses risk equations from the UKPDS Outcome Model-1 [24] for macro- and microvascular complications. The authors used a different model (i.e., the IHECM-T2DM), which applies different risk equations including Swedish-specific macrovascular risk equations [21–23, 26].
In the comparison of incretin-based therapies against NPH insulin, while NPH insulin was associated with higher HbA1c reduction, it resulted in weight gain and a higher number of hypoglycemic episodes. The estimated ICER of both incretin-based second-line treatment strategies (1 and 2) remained below SEK 500,000. This finding is in line with a previous cost–utility analysis comparing the DPP-4 inhibitor saxagliptin against NPH insulin as add-on to metformin in Poland [39]. In these treatment strategies, patients on incretin-based therapies benefit from an extra treatment compared with patients who initiate NPH insulin as second-line treatment, and this finding implies that the health benefits of providing second-line treatment prior to insulin introduction offset the higher prices of these medications.
However, it should be noted that the cost-effectiveness of incretin-based therapies decreased as the HbA1c switching threshold value to NPH insulin increased. This implies that as the number of years on these treatments increases, the marginal costs of the treatments outweigh the marginal benefits, and the ICER rises. The similar finding was observed in a previous study in Sweden using the same model [20]. The one-way sensitivity analyses showed that the results were most sensitive to the assumptions related to incidence of hypoglycemia and disutility of hypoglycemia and insulin. However, this base case conclusion was robust against all these variations.
The main strengths of the current study include: applying the baseline characteristics from a large sample of T2DM patients from routine practice in Sweden, using utility decrements for a number of complications from a Swedish sample with T2DM, applying Swedish-specific risk equations for macrovascular complications, and extracting treatment effects on the main biomarkers (i.e., HbA1c and weight) from a meta-analysis instead of a single clinical trial. In addition, the authors used HbA1c threshold to determine the treatment pathway, reflecting clinical practice and national guidelines. These strengths improve the external validity and relevance of these results for policy making in the Swedish setting.
The results of the current study should be interpreted in light of a number of cautions. The authors included only hypoglycemia as a treatment-related adverse event in the model. The results of the 1860-LIRA-DPP-4 (liraglutide dipeptidyl peptidase-4 inhibitor) trial showed no significant difference in other adverse events between liraglutide and sitagliptin [29]. On the other hand, previous studies [30, 66] reported higher episodes of non-hypoglycemia-related adverse events such as nausea for GLP-1 and DPP-4 agents compared with insulin glargine. If insulin glargine and NPH insulin are comparable with respect to adverse events, then the authors’ reported ICERs in comparing GLP-1/DPP-4 against NPH insulin are underestimated to some extent. However, the authors do not expect this to have influenced the final conclusion, as these ICERs are long way from our assumed SEK 500,000 WTP value. As they had no clinical trial data regarding the effects of treatment on diabetes-related complications, the authors applied the commonly used method of using biomarkers (e.g., HbA1c and weight) as surrogate markers for these complications. Due to lack of data, the authors did not take into account the possibilities of treatment discontinuation by patients and different annual drifts for different treatments. This limits the generalizability of these findings. However, these are common modeling practices, and the authors tried to consider the possibility of different annual drifts in the PSA, assuming a normal distribution for annual drift in biomarkers. It should be mentioned that their conclusions are based on a baseline HbA1c of 7.7% ± 0.8, and generalizability to patients with lower and higher HbA1c values at baseline is limited. Especially, as it was recommended by the American Diabetes Association and the European Association for the study of Diabetes, starting treatment with insulin should be strongly considered for patients with dramatically high HbA1c or significant hyperglycemic symptoms [10]. Pooling agents into classes (i.e., GLP-1 and DPP-4) implies that agents within a drug class have similar efficacy. The results of meta-analysis on individual agents in the authors’ main source (i.e., Ref. [28]) supported this assumption and conducting the class-level analysis.
Conclusion
From both a societal and a healthcare payer perspective, treatment with GLP-1 agonists is a cost-effective treatment strategy in comparison to DPP-4 inhibitors and NPH insulin as second-line therapy among T2DM patients inadequately controlled with metformin alone in a Swedish setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. A.A. Kiadaliri received funds from the Health Ministry of Iran to perform this research as a part of his Ph.D. thesis. The Health Economics Programme at Lund University receives core funding from the Swedish Research Council for Health, Working Life, and Welfare (ref: 2006–1660); a Government Grant for Clinical Research (ALF) and the Skåne Region (UG Gerdtham). The authors would like to thank Ann-Marie Svensson and Mervete Miftaraj for their help in obtaining data on patients’ characteristics from the Swedish National Diabetes Register.
Conflict of interest
A.A. Kiadaliri, U.G. Gerdtham and K. Steen Carlsson declare no conflicts of interest. B. Eliasson has participated in Advisory Board for Eli Lilly, Sanofi, MSD; and received payment for lectures by Sanofi, MSD, and Novo Nordisk.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(3):293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, Li Q, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517–523. doi: 10.1038/ki.2012.401. [DOI] [PubMed] [Google Scholar]
- 5.Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–643. doi: 10.1007/s00125-011-2404-1. [DOI] [PubMed] [Google Scholar]
- 6.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4–S10. doi: 10.2337/dc11-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Board of Health and Welfare. National guidelines for diabetes care. 2nd edn. Vasteras, Sweden, 2010. http://www.socialstyrelsen.se/nationalguidelines/nationalguidelinesfordiabetescare. Accessed Feb 20, 2014.
- 9.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract. 2013;19(3):536–557. doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 11.Russell S. Incretin-based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm. 2013;35(2):159–172. doi: 10.1007/s11096-012-9729-9. [DOI] [PubMed] [Google Scholar]
- 12.Cederholm J, Nilsson P. A review of risk factors and cardiovascular disease in diabetes care—2011. Eur J Cardiovasc Med. 2011;3(1):21–25. [Google Scholar]
- 13.The Swedish National Diabetes Register. https://www.ndr.nu. Accessed June 2, 2014.
- 14.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303(14):1410–1418. doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- 15.Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 16.Deacon CF, Mannucci E, Ahren B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab. 2012;14(8):762–767. doi: 10.1111/j.1463-1326.2012.01603.x. [DOI] [PubMed] [Google Scholar]
- 17.Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15(3):204–212. doi: 10.1111/dom.12012. [DOI] [PubMed] [Google Scholar]
- 18.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5(1):e35–e48. [PMC free article] [PubMed] [Google Scholar]
- 20.Steen Carlsson K, Persson U. Cost-effectiveness of add-on treatments to metformin in a Swedish setting: liraglutide vs sulphonylurea or sitagplitin. J Med Econ 2014;1–23. doi:10.3111/13696998.2014.933110. [DOI] [PubMed]
- 21.Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Zbrozek AS, Dong F, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–734. doi: 10.2337/diacare.20.5.725. [DOI] [PubMed] [Google Scholar]
- 22.Brown JB, Russell A, Chan W, Pedula K, Aickin M. The global diabetes model: user friendly version 3.0. Diabetes Res Clin Pract. 2000;50(Suppl 3):S15–S46. doi: 10.1016/S0168-8227(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 23.Bagust A, Hopkinson PK, Maier W, Currie CJ. An economic model of the long-term health care burden of Type II diabetes. Diabetologia. 2001;44(12):2140–2155. doi: 10.1007/s001250100023. [DOI] [PubMed] [Google Scholar]
- 24.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47(10):1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 25.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–1933. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad Kiadaliri A, Gerdtham UG, Nilsson P, Eliasson B, Gudbjornsdottir S, Carlsson KS. Towards renewed health economic simulation of type 2 diabetes: risk equations for first and second cardiovascular events from Swedish register data. PLoS One. 2013;8(5):e62650. doi: 10.1371/journal.pone.0062650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekstrom N, Miftaraj M, Svensson AM, Andersson Sundell K, Cederholm J, Zethelius B, et al. Glucose-lowering treatment and clinical results in 163,121 patients with type 2 diabetes: an observational study from the Swedish national diabetes register. Diabetes Obes Metab. 2012;14(8):717–726. doi: 10.1111/j.1463-1326.2012.01591.x. [DOI] [PubMed] [Google Scholar]
- 28.Canadian Agency for Drugs and Technologies in Health, Second- and Third-Line Pharmacotherapy for Type 2 Diabetes: Update. Vol. Ottawa: The Agency; 2013. (CADTH optimal use report; vol. 3, no. 1a). 2013, Ottawa (ON).
- 29.Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397–407. doi: 10.1111/j.1742-1241.2011.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aschner P, Chan J, Owens DR, Picard S, Wang E, Dain MP, et al. Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379(9833):2262–2269. doi: 10.1016/S0140-6736(12)60439-5. [DOI] [PubMed] [Google Scholar]
- 31.Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P. Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin. J Diabetes Complications. 2003;17(6):307–313. doi: 10.1016/S1056-8727(03)00076-X. [DOI] [PubMed] [Google Scholar]
- 33.Milicevic Z, Hancu N, Car N, Ivanyi T, Schwarzenhofer M, Jermendy G. Effect of two starting insulin regimens in patients with type II diabetes not controlled on a combination of oral antihyperglycemic medications. Exp Clin Endocrinol Diabetes. 2009;117(5):223–229. doi: 10.1055/s-0028-1128126. [DOI] [PubMed] [Google Scholar]
- 34.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 35.Monnier L, Colette C. Addition of rapid-acting insulin to basal insulin therapy in type 2 diabetes: indications and modalities. Diabetes Metab. 2006;32(1):7–13. doi: 10.1016/S1262-3636(07)70241-0. [DOI] [PubMed] [Google Scholar]
- 36.Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(11):1008–1019. doi: 10.1111/j.1463-1326.2011.01433.x. [DOI] [PubMed] [Google Scholar]
- 37.Pontiroli AE, Miele L, Morabito A. Metabolic control and risk of hypoglycaemia during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2012;14(5):433–446. doi: 10.1111/j.1463-1326.2011.01543.x. [DOI] [PubMed] [Google Scholar]
- 38.Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 1991;90(4):450–9. [PubMed]
- 39.Grzeszczak W, Czupryniak L, Kolasa K, Sciborski C, Lomon ID, McEwan P. The cost-effectiveness of saxagliptin versus NPH insulin when used in combination with other oral antidiabetes agents in the treatment of type 2 diabetes mellitus in Poland. Diabetes Technol Ther. 2012;14(1):65–73. doi: 10.1089/dia.2011.0092. [DOI] [PubMed] [Google Scholar]
- 40.Erhardt W, Bergenheim K, Duprat-Lomon I, McEwan P. Cost effectiveness of saxagliptin and metformin versus sulfonylurea and metformin in the treatment of type 2 diabetes mellitus in Germany: a Cardiff diabetes model analysis. Clin Drug Investig. 2012;32(3):189–202. doi: 10.2165/11597060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Woehl A, Evans M, Tetlow AP, McEwan P. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled type 2 diabetes in the United Kingdom. Cardiovasc Diabetol. 2008;7:24. doi: 10.1186/1475-2840-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith-Palmer J, Fajardo-Montanana C, Pollock RF, Ericsson A, Valentine WJ. Long-term cost-effectiveness of insulin detemir versus NPH insulin in type 2 diabetes in Sweden. J Med Econ. 2012;15(5):977–986. doi: 10.3111/13696998.2012.692340. [DOI] [PubMed] [Google Scholar]
- 43.Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49(3):442–451. doi: 10.1007/s00125-005-0132-0. [DOI] [PubMed] [Google Scholar]
- 44.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ. 2009;180(4):385–397. doi: 10.1503/cmaj.081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;2:CD005613. doi: 10.1002/14651858.CD005613.pub3. [DOI] [PubMed] [Google Scholar]
- 46.U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 1995;44(11):1249–58. [PubMed]
- 47.Chaudhry ZW, Gannon MC, Nuttall FQ. Stability of body weight in type 2 diabetes. Diabetes Care. 2006;29(3):493–497. doi: 10.2337/diacare.29.03.06.dc05-1703. [DOI] [PubMed] [Google Scholar]
- 48.NICE. Technology appraisal guidance 288—Dapagliflozin in combination therapy for treating type 2 diabetes. http://guidance.nice.org.uk/TA288/PublicInfo/pdf/English. Accessed April 4, 2014.
- 49.NICE. Canagliflozin for the Treatment of Type 2 Diabetes Mellitus. http://www.nice.org.uk/guidance/index.jsp?action=download&o=66677. Accessed April 4, 2014.
- 50.Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837–53. [PubMed]
- 51.Ringborg A, Martinell M, Stalhammar J, Yin DD, Lindgren P. Resource use and costs of type 2 diabetes in Sweden—estimates from population-based register data. Int J Clin Pract. 2008;62(5):708–716. doi: 10.1111/j.1742-1241.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 52.Dental and Pharmaceutical Benefits Agency in Sweden. http://www.tlv.se. Accessed April 11, 2014.
- 53.Owens D, Barnett A, Pickup J, Kerr D, Bushby P, Hicks D, et al. Blood glucose self-monitoring in type 1 and type 2 diabetes: reaching a multidisciplinary consensus. Diabetes Prim Care. 2004;6(1):8–16. [Google Scholar]
- 54.Ekman M, Consumption and production by age in Sweden. Basic facts and health economic implications. In: Ekman M, editor. Studies in health economics: modelling and data analysis of costs and survival. Stockholm; 2002.
- 55.Kiadaliri AA, Gerdtham U, Eliasson B, Gudbjörnsdottir S, Svensson AM, Carlsson KS. Health utilities of type 2 diabetes-related complications: a cross-sectional study in Sweden. Int J Environ Res Public Health. 2014;11(5):4939–4952. doi: 10.3390/ijerph110504939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ericsson A, Pollock RF, Hunt B, Valentine WJ. Evaluation of the cost–utility of insulin degludec vs insulin glargine in Sweden. J Med Econ. 2013;16(12):1442–1452. doi: 10.3111/13696998.2013.852099. [DOI] [PubMed] [Google Scholar]
- 57.Evans M, Khunti K, Mamdani M, Galbo-Jorgensen CB, Gundgaard J, Bogelund M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11:90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlsson P, Anell A, Eliasson M. Hälsoekonomi får allt större roll för sjukvårdens prioriteringar. Läkartidningen. 2006;103(46):3617–3623. [PubMed] [Google Scholar]
- 59.Granström O, Bergenheim K, McEwan P, Sennfält K, Henriksson M. Cost-effectiveness of saxagliptin (Onglyza®) in type 2 diabetes in Sweden. Prim Care Diabetes. 2012;6(2):127–136. doi: 10.1016/j.pcd.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–230. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 61.Davies MJ, Chubb BD, Smith IC, Valentine WJ. Cost–utility analysis of liraglutide compared with sulphonylurea or sitagliptin, all as add-on to metformin monotherapy in Type 2 diabetes mellitus. Diabet Med. 2012;29(3):313–320. doi: 10.1111/j.1464-5491.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee WC, Samyshkin Y, Langer J, Palmer JL. Long-term clinical and economic outcomes associated with liraglutide versus sitagliptin therapy when added to metformin in the treatment of type 2 diabetes: a CORE Diabetes Model analysis. J Med Econ. 2012;15(Suppl 2):28–37. doi: 10.3111/13696998.2012.716111. [DOI] [PubMed] [Google Scholar]
- 63.Mezquita Raya P, Perez A, Ramirez de Arellano A, Briones T, Hunt B, Valentine WJ. Incretin therapy for type 2 diabetes in Spain: a cost-effectiveness analysis of liraglutide versus sitagliptin. Diabetes Ther. 2013;4(2):417–430. doi: 10.1007/s13300-013-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AhmadKiadaliri A, Clarke PM, Gerdtham UG, Nilsson P, Eliasson B, Gudbjornsdottir S, et al. Predicting changes in cardiovascular risk factors in Type 2 diabetes in the post-UKPDS era: longitudinal analysis of the Swedish National Diabetes Register. J Diabetes Res. 2013;2013:241347. doi: 10.1155/2013/241347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 66.Li WX, Gou JF, Tian JH, Yan X, Yang L. Glucagon-like peptide-1 receptor agonists versus insulin glargine for type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Curr Ther Res Clin Exp. 2010;71(4):211–238. doi: 10.1016/j.curtheres.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.