Abstract
Recent studies indicate that, unlike glucose, fructose has little or no post-oral preference conditioning actions in C57BL/6J (B6) mice. The present study determined whether this is also the case for FVB mice, which overconsume fructose relative to B6 mice. In experiment 1, FVB mice strongly preferred a noncaloric 0.1% sucralose + 0.1% saccharin (S+S) solution to 8% fructose in a 2-day choice test but switched their preference to fructose after separate experience with the two sweeteners. Other FVB mice displayed a stronger preference for 8% glucose over S+S. In a second experiment, ad libitum-fed FVB mice trained 24 h/day acquired a significant preference for a flavor (CS+) paired with intragastric (IG) self-infusions of 16% fructose over a different flavor (CS−) paired with IG water infusions. IG fructose infusions also conditioned flavor preferences in food-restricted FVB mice trained 1 h/day. IG infusions of 16% glucose conditioned stronger preferences in FVB mice trained 24- or 1 h/day. Thus, fructose has post-oral flavor conditioning effects in FVB mice, but these effects are less pronounced than those produced by glucose. Further studies of the differential post-oral conditioning effects of fructose and glucose in B6 and FVB mice should enhance our understanding of the physiological processes involved in sugar reward.
Keywords: post-oral flavor conditioning, sucralose, saccharin, C57BL/6J mice
it is well established that inbred mouse strains differ in their taste responses to caloric and noncaloric sweeteners. This is due, in part, to allelic variations in the Tas1r3 gene that encodes the T1r3 sweet taste receptor protein (6, 25). Mouse strains with the SacB variant of the Tas1r3 gene are more sensitive to sugars (e.g., sucrose) and noncaloric sweeteners (e.g., saccharin) than are strains with the SacD variant. In addition, sugar intake and preference are stimulated by the post-oral actions of sugars, a process referred to as appetition to distinguish it from the satiation process that inhibits sugar intake (27). Appetition is demonstrated by the intake and preference-stimulating effects of intragastric (IG) sugar infusions in mice and rats (3, 21, 27, 30). Conceivably, strain differences in post-oral sugar appetition may contribute to variations in sugar intake in mice, but this has yet to be documented. One study reported mouse strain differences in sugar-conditioned flavor preferences, but because the sugars were orally consumed, the conditioning effects may have been mediated by the taste and/or post-oral actions of the sugars (24). In another study, we compared IG sucrose conditioning in C57BL/6J (B6) and 129 mice, which have the SacB and SacD variants of T1r3 receptor and, thus, are sweet sensitive and subsensitive, respectively (35). These strains were of interest in view of the discovery that the T1r3 taste receptor protein and other taste signaling elements are expressed in the gastrointestinal tract (12, 16, 40). However, while B6 mice consume more sucrose than do 129 mice in 24-h sugar vs. water tests, the two strains displayed similar flavor conditioning responses to IG sucrose infusions (26, 35). These results indicated that genetic variation in the T1r3 receptor does not influence post-oral sucrose appetition. This was confirmed by the finding of similar IG sucrose-conditioned flavor preferences in T1r3 knockout (KO) and wild-type (WT) mice (33).
The present study sought evidence for strain differences in the post-oral conditioning response to glucose and fructose, the constituent monosaccharide sugars of sucrose. B6 mice acquire strong preferences for flavors paired with IG glucose infusions but fail to prefer flavors paired with isocaloric fructose infusions (29, 43). Related findings were obtained with B6 mice given oral choice tests with 8% glucose or 8% fructose vs. a noncaloric 0.1% sucralose + 0.1% saccharin solution (S+S) (Sclafani A, Zukerman S, and Ackroff K, unpublished data). In 1-min choice tests that minimized post-oral effects, the mice significantly preferred the S+S solution to both sugar solutions, suggesting that S+S has a sweeter taste. The mice also preferred S+S to fructose in 24-h tests but developed a strong preference for glucose over S+S solution. In addition, they consumed substantially more glucose than fructose or S+S in 24-h sweetener vs. water tests. The enhanced intake and preference for glucose but not fructose vs. S+S was attributed to the post-oral conditioning actions of glucose. Earlier studies demonstrated that IG glucose infusions are much more effective than fructose infusions in conditioning flavor preferences in Sprague-Dawley (SD) rats (4, 31, 32). In particular, whereas IG glucose infusions conditioned a significant CS+ preference in SD rats trained 30 min/day, IG fructose infusions were completely ineffective (4, 32). However, unlike B6 mice, SD rats acquired a preference when the CS+ was paired with fructose self-infusions in 20- to 24-h sessions, although the fructose infusions were less effective than glucose infusions (2, 4). Thus, fructose has a post-oral preference conditioning action in SD rats in some training paradigms.
In this study, we determined whether FVB/NJ mice would acquire a CS+ preference when infused IG with fructose during 24 h or 1 h/day sessions. We selected this inbred strain based on an earlier observation that FVB mice consumed substantially more 10% fructose than did B6 mice when given ad libitum access to sugar solution, water, and food (15). The FVB mice also consumed more 10% sucrose than did B6 mice, but this difference was less pronounced than that observed with fructose. FVB mice have a similar, but not identical, form of the sweet-sensitive T1r3 receptor as that in B6 mice, so it appeared unlikely that differences in sweet taste sensitivity accounted for the greater fructose intakes of FVB mice (25). In experiment 1, we indirectly evaluated FVB mice for post-oral fructose conditioning by measuring their preference for fructose over S+S solution in two-bottle choice tests. Experiments 2 and 3 then directly assessed post-oral sugar conditioning in FVB mice using IG conditioning procedures. In all three experiments, we compared the sugar conditioning response to fructose with that to isocaloric glucose.
EXPERIMENT 1: FRUCTOSE AND GLUCOSE VS. SUCRALOSE + SACCHARIN PREFERENCES
Experiment 1 compared the preference of FVB mice for 8% fructose and 8% glucose vs. a palatable, noncaloric S+S solution in 2-day choice tests. In a recent study, B6 mice developed a significant preference for 8% glucose, but not for fructose, over S+S after they had separate sugar vs. water and S+S vs. water tests (Sclafani A, Zukerman S, and Ackroff K, unpublished data). These sweetener vs. water (“one sweetener”) tests allowed the mice to unambiguously associate the taste of each sweetener with its post-oral actions.
Materials and Methods
Animals.
Adult male FVB/NJ mice (n = 18, mean body weight 26.5 g) obtained from Jackson Laboratories (Bar Harbor, ME) were adapted to the laboratory for 1 wk. The animals were singly housed in plastic tub cages in a room maintained at 22°C with a 12:12-h light-dark cycle and given ad libitum access to chow (no. 5001, PMI Nutrition International, Brentwood, MO) and water except where noted. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Test solutions.
Sugar solutions (8%) were prepared using food-grade glucose and fructose (Tate and Lyle, Honeyville Food Products, Rancho Cucamonga, CA). The noncaloric sweetener solution (S+S) was prepared using 0.1% sucralose (Tate & Lyle, Dayton, OH) and 0.1% sodium saccharin (Sigma-Aldrich, St. Louis, MO). These sweetener concentrations were the same as those used in our recent B6 study (Sclafani A, Zukerman S, and Ackroff K, unpublished data). The 8% sugar concentration was used because it produces near-maximum 24-h intakes in B6 mice (45). The S+S noncaloric mixture was used because it was preferred to the 8% sugar solutions in brief access tests and had no post-oral intake-suppressive effects (Sclafani A, Zukerman S, and Ackroff K, unpublished data). The solutions were prepared with deionized water and served at room temperature; they were formulated on a wt/wt basis because intakes were measured by weight.
Procedure.
Two-day choice tests were conducted in the home cages. The solutions were available through stainless-steel sipper spouts attached to 50-ml plastic tubes that were placed on the grid top of the cage. The two sipper spouts were inserted through holes positioned 3.7 cm apart, and the drinking tubes were fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking bottles on an electronic balance interfaced to a laptop computer. Intakes were corrected for spillage, which was estimated by recording the change in weight of two bottles that were placed on an empty cage. The mice were given two-bottle access to water for 5 days to familiarize them to the choice tests.
The mice were divided into two groups (n = 9 each) equated for mean 24-h water intake and body weight during the two days prior to sweetener testing. The Fructose Group was given a series of 2-day, two-bottle choice tests as follows (see Table 1): test 1 (days 1 and 2) 8% fructose vs. S+S, test 2 (days 3 and 4) 8% fructose vs. water, test 3 (days 5 and 6) S+S vs. water, and test 4 (days 8 and 9) 8% fructose vs. S+S. The mice were given water only on day 7 prior to test 4 to make it comparable to test 1, which was preceded by water-only access. The Glucose Group was similarly tested but with 8% glucose. The mice in both groups were next given water only for 4 days and then another series of 2-day tests as follows: test 5 (days 14 and 15) 8% fructose vs. water, test 6 (days 16 and 17) 8% glucose vs. water, and test 7 (days 19 and 20) 8% fructose vs. 8% glucose. Water only was available on day 18 between tests 6 and 7. Note that test 5 was the first exposure of the mice in the Glucose Group to fructose, and test 6 was the first exposure of the mice in the Fructose Group to glucose. The left-right position of the sweetener and water bottles were switched from the first to second day of each test.
Table 1.
Experimental procedures
| Condition | |
|---|---|
| Experiment 1. 2-Day Test Series, Ad Libitum Food | |
| Test 1 | 8% fructose or glucose vs. 0.1% sucralose +0.1% saccharin |
| Test 2 | 0.1% sucralose +0.1% saccharin vs. water |
| Test 3 | 8% fructose or glucose vs. water |
| Test 4 | 8% fructose or glucose vs. 0.1% sucralose +0.1% saccharin |
| Test 5 | 8% fructose vs. water |
| Test 6 | 8% glucose vs. water |
| Test 7 | 8% fructose vs. glucose |
| Experiment 2A. 24-h IG Conditioning, Ad Libitum Food | |
| Days 1, 3, 5 | One-bottle training: CS− with IG water infusions |
| Days 2, 4, 6 | One-bottle training: CS+ with IG 16% fructose or glucose infusions |
| Day 7 | One-bottle water with IG water infusion |
| Days 8–9 | Two-bottle reinforced test: CS+ with IG sugar vs. CS− with IG water infusion |
| Days 10–11 | Two-bottle nonreinforced test: CS+ vs. CS− with IG water infusions |
| Experiment 2B. 1-h IG Conditioning, Restricted Food | |
| Sessions 1, 3, 5 | One-bottle training: CS− with IG water infusions |
| Sessions 2, 4, 6 | One-bottle training: CS+ with IG 16% fructose or glucose infusions |
| Sessions 7–8 | Two-bottle reinforced test: CS+ with IG sugar vs. CS− with IG water infusions |
| Sessions 9–10 | Two-bottle nonreinforced test: CS+ vs. CS− with IG water infusions |
| Experiment 3: 1-h IG Conditioning, Restricted Food | |
| Sessions 1–4 | One-bottle training: CS− with IG water infusions |
| Sessions 5–7 | One-bottle training: CS+ with IG 16% fructose or glucose infusions |
| Sessions 8, 10 | One-bottle training: CS− with IG water infusions |
| Sessions 9, 11 | One-bottle training: CS+ with IG 16% fructose or glucose infusions |
| Sessions 12–15 | Two-bottle test CS+ vs. CS−, no infusions |
IG, intragastric; CS, conditioned stimulus.
Data analysis.
Daily solution intakes were averaged over the 2 days of each test, and sweetener preferences were expressed as percent solution intakes (e.g., fructose intake/total intake × 100). Data are presented as group means ± SE. Intakes were analyzed using a mixed model ANOVA with test and solution as repeated factors. One ANOVA included results from tests 1 and 4 and asked whether relative intakes of sugar and S+S changed across the two tests. A second ANOVA included results from tests 2 and 3 and compared the preference for each sweetener over water. Additional ANOVAs are described below. Percent sweetener intakes were analyzed with t-tests.
Results
Mice in the Fructose Group consumed significantly more S+S than fructose in test 1 but more fructose than S+S in test 4 [solution × test, F(1,8) = 15.5, P < 0.001] (Fig. 1A), and the mean group percent fructose preference increased from a low 10 ± 2.8% to 76 ± 11.0% from test 1 to 4 [t(8) = 5.8, P < 0.001]. All mice preferred S+S to fructose in test 1, while seven mice strongly preferred fructose in test 4 with a mean preference of 92%; the remaining two mice preferred S+S. In tests 2 and 3, the mice consumed more fructose and S+S than water [F (1,8) = 124.0, P < 0.001] and their intakes of and preferences for the two sweeteners did not differ.
Fig. 1.
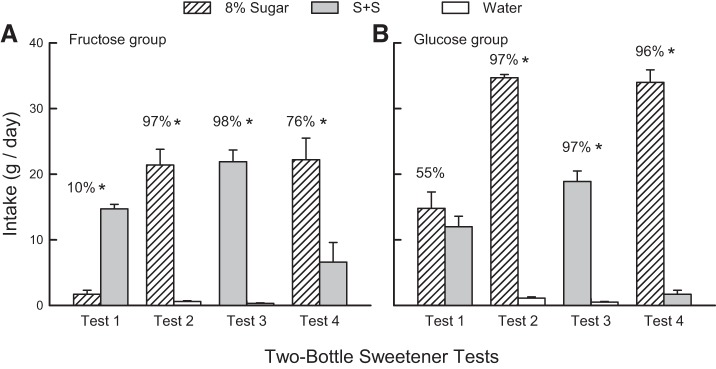
Experiment 1. Oral sweetener two-bottle preference tests. A: mice from the Fructose Group were given 2-day choice tests with 8% fructose vs. 0.1% sucralose + 0.1% saccharin (S+S) (test 1), 8% fructose vs. water (test 2), S+S vs. water (test 3), and 8% fructose vs. S+S (test 4). B: mice in the Glucose Group were similarly tested but with 8% glucose and S+S. Values are expressed as means ± SE daily intakes averaged across each 2-day test. Numbers atop bars represent mean percent preference for the solution represented by that bar. Significant intake differences (*P < 0.05) within two-bottle tests are shown.
Mice in the Glucose Group consumed similar amounts of glucose and S+S in test 1 but substantially more glucose than S+S in test 4 [solution × test, F(1,8) = 35.5, P < 0.001] (Fig. 1B). Their mean percent glucose preference increased from 55 ± 7.8% % in test 1 to 96 ± 1.4% % in test 4 [t(8) = 5.9, P < 0.001]. There was a bimodal response to the sweeteners in test 1; four mice preferred the glucose, while five mice were indifferent or preferred the S+S. In tests 2 and 3, the mice consumed more glucose and S+S than water [F (1,8) = 813.0, P < 0.001] but also substantially more glucose than S+S [F (1,8) = 89.8, P < 0.001]. However, their mean preferences for the two sweeteners over water were similar at 97%. Between group comparisons indicated that mice in the Glucose Group consumed substantially more sugar than did the mice in the Fructose Group in test 2, but the two groups drank similar amounts of S+S in test 3 [F (1,16) = 42.4, P < 0.001].
The Fructose and Glucose Groups did not differ in their intakes in the last three tests, and their data were, therefore, combined. In tests 5 and 6, the mice consumed more sugar than water [28.8 ± 0.9 vs. 0.6 ± 0.1 g/day; F(1,17) = 91.2, P < 0.001] but substantially more glucose than fructose [35.3 ± 0.8 vs. 22.4 ± 1.3 g/day; F(1,16) = 105.6, P < 0.001]. They also strongly preferred glucose to fructose in test 7 [33.0 ± 1.3 vs. 3.1 ± 0.7 g/day, 91% preference; t(17) = 15.9, P < 0.001].
Discussion
The FVB mice consumed more S+S than fructose in test 1, indicating that the S+S had a more preferred (presumably sweeter) taste. However, after experiencing the fructose and S+S solutions separately in the sweetener vs. water tests 2 and 3, the mice switched their preference and significantly preferred fructose to S+S by 76% in test 4. This is in marked contrast to B6 mice, which significantly preferred S+S to fructose in both tests 1 and 4 (Sclafani A, Zukerman S, and Ackroff K, unpublished data) (see Table 2). The preference switch displayed by the FVB mice suggests that fructose had a post-oral conditioning action that enhanced the avidity of the mice for the sugar. Mice in the Glucose Group also greatly increased their preference for glucose over S+S from test 1 to 4. Unlike mice in the Fructose Group, however, they were indifferent to the two sweeteners in test 1 and displayed a near-total sugar preference in test 4. The mice in both groups consumed considerably more glucose than fructose in tests 5 and 6 and strongly preferred glucose to fructose in test 7. Conceivably, glucose has a sweeter taste than fructose for FVB mice, although B6 mice equally preferred the two sugars in 1-min choice tests (Sclafani A, Zukerman S, and Ackroff K, unpublished data). More likely, the greater intake and preference for glucose over fructose was due to the differential post-oral conditioning actions of the two sugars. This issue was addressed in experiment 2.
Table 2.
Sugar-conditioned preferences in FVB/NJ and C57BL/6J mice compared
| FVB/NJ | C57BL/6J | |
|---|---|---|
| Experiment 1: 8% Fructose vs. 0.1% Sucralose + 0.1% Saccharin, 2 Day | ||
| Test 1, fructose preference | 10% | 19%1 |
| Test 4, fructose preference | 76% | 22%1 |
| Experiment 1: 8% Glucose vs. 0.1% Sucralose, +0.1% Saccharin, 2 Day | ||
| Test 1, glucose preference | 55% | 40%1 |
| Test 4, glucose preference | 96% | 88%1 |
| Experiment 1: 8% Glucose vs. 8% Fructose, 2 day | ||
| Test 7, glucose preference | 91% | 98%2 |
| Experiment 2A: IG 16% Fructose, 24 h | ||
| Reinforced test, CS+ preference | 80% | 53%3 |
| Nonreinforced test, CS+ preference | 86% | 58%3 |
| Experiment 2A: IG 16% Glucose, 24 h | ||
| Reinforced test, CS+ preference | 98% | 86%3 |
| Nonreinforced test, CS+ preference | 95% | 89%3 |
| Experiment 3: IG 16% Fructose, 24 h | ||
| Nonreinforced test, CS+ preference | 67% | 53%*5 |
| Experiment 3: IG 16% Glucose, 1 h | ||
| Nonreinforced test, CS+ preference | 92% | 80%4 |
EXPERIMENT 2A: 24-H IG FRUCTOSE AND GLUCOSE FLAVOR CONDITIONING
The substantial increase in preference from tests 1 to 4 displayed by the Fructose and Glucose Groups indicates that both sugars had post-oral appetition effects that enhanced the preference for these sugars relative to S+S. The present experiment sought direct evidence that post-oral fructose and glucose condition flavor preferences by having FVB mice self-infuse 16% fructose or glucose IG as they drank a CS+ flavored solution. The infused 16% sugar solutions were diluted by the ingested CS+ solution to 8% sugar in the stomach, which is the sugar concentration used in experiment 1. The 24-h conditioning protocol was identical to that used in an earlier study of B6 mice, in which the mice displayed a significant preference for the glucose-paired CS+ flavor but no preference for the fructose-paired CS+ flavor (29).
Materials and Methods
Animals.
Adult male FVB mice (n = 22, mean body weight 27.1 g) were used. They were housed singly in plastic cages with ad libitum access to chow and water or flavored saccharin solutions.
Surgery.
The mice were anesthetized with 2% isoflurane inhalation and fitted with a gastric catheter, as described previously (34). Two weeks after surgery, the animals were briefly (5 min) anesthetized with isoflurane, fitted with a harness and a spring tether (CIH62; Instech Laboratories, Plymouth Meeting, PA), and transferred to infusion test cages.
Apparatus.
Testing occurred in infusion cages (15 × 15 × 32 cm high), as described previously (35). Fluid was available from one or two stainless-steel sipper spouts attached to 55-ml glass tubes. The sipper spouts were interfaced via electronic lickometers (Med Electronics, St. Albans, VT) to a computer that operated a syringe pump (model A-99; Razel Scientific, Stamford, CT), which infused liquid into the gastric catheters as the animals licked. The pump rate was nominally 0.5 ml/min, but the animal controlled the overall infusion rate and volume by its licking response; the oral-to-infusion intake ratio was maintained at ∼1:1 by computer software. In two-bottle tests, two infusion pumps were attached via a 20-gauge Y connector to the gastric catheters. Daily intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
Test solutions.
The CS solutions contained 0.2% (wt/wt) saccharin and were flavored with 0.05% (wt/wt) cherry or grape Kool-Aid flavor mix (General Foods, White Plains, NY). The IG infusates were water and 16% (wt/wt) glucose or fructose. For half of the mice, cherry-saccharin was the CS+ paired with IG sugar infusion and grape-saccharin was the CS− paired with water infusion; the flavor-infusate pairs were reversed for the remaining animals. The orally consumed CS+ mixed with the IG sugar infusion in the stomach, so that the carbohydrate concentration in the gut was ∼8%.
Procedure.
The mice were adapted to drink water paired with matched IG water infusions for 5 days. They were then given one-bottle training sessions with the CS− solution paired with IG water infusions (days 1, 3, 5) and the CS+ solution paired with IG sugar infusions (days 2, 4, 6) (see Table 1). The mice were divided into two groups equated for their CS− intake on day 1; the Fructose (n = 10) and Glucose (n = 12) Groups were infused with 16% fructose and glucose, respectively, on CS+ training days. Following the last CS+ training day, the mice were given water (paired with IG water) for 1 day. A two-bottle “reinforced” choice test with the CS+ vs. CS− was conducted for 2 days with the intakes of the CS+ and CS− paired with IG infusions of sugar and water, respectively. Then a “nonreinforced” two-bottle test was conducted for 2 days with both the CS+ and CS− now paired with IG water infusions. The left-right position of the CS+ and CS− solutions were alternated daily throughout training and testing. Chow (#5001) was available ad libitum throughout the experiment.
Data analysis.
Total fluid intake (oral intake plus IG infusate) during the one-bottle training days and two-bottle test days was calculated. The training data for each group were evaluated with a two-way ANOVA, using CS solution (CS+ vs. CS−) and training days as within factors. The two-bottle test data were averaged over the two reinforced days and two nonreinforced days and analyzed in a two-way ANOVA with CS solution and test as within factors. Additional between-group analyses compared the mean CS+ and CS− intakes of the two groups during training, reinforced tests, and nonreinforced tests. The two bottle intakes of the individual mice were also expressed as percent CS+ intakes (CS+ intake/total intake × 100) and evaluated with both within-group and between-group ANOVAs. Drinking patterns during one-bottle training were evaluated with a bout defined as a period of drinking containing at least 30 licks and with interlick intervals no longer than 5 min (14). Mean bout size (g) was determined by dividing the total 24-h CS intakes by the number of bouts.
Results
During one-bottle training, mice in the Fructose Group increased their CS+ intake over days and consumed more (P < 0.05) CS+ than CS− during the last training session with each CS (CS × sessions interaction) [F(2, 18) = 3.8, P < 0.05] (Fig. 2A). In the two-bottle tests mice in the Fructose Group consumed significantly more CS+ than CS− in both the reinforced and nonreinforced tests [F(1,9) = 13.6, P < 0.01] (Fig. 2A). The tests differed in that CS+ intake increased from the reinforced to the nonreinforced test [CS × test interaction: F(1,9) = 10.2, P < 0.05], as did the percent CS+ preference [80% to 86%, t(9) = 2.4, P < 0.05].
Fig. 2.
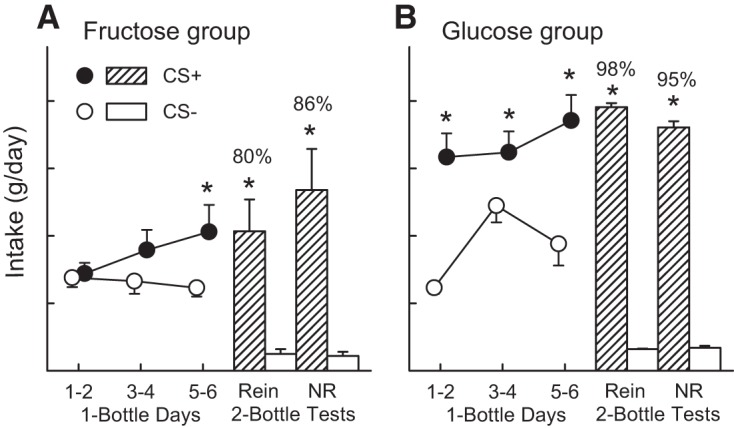
Experiment 2A. Intragastric (IG) sugar conditioning in ad libitum-fed FVB mice trained 24 h/day. Intake of the conditioned stimulus (CS)+ solution was paired with IG infusions of either 16% fructose (A) or 16% glucose (B). Intake of the CS− solution was paired with IG infusions of water. The left side of each panel shows total daily fluid intake (oral CS intake plus IG infusate) during each of the six one-bottle training days. The CS− solution was offered on days 1, 3, and 5, and the CS+ solution was offered on days 2, 4, and 6. The right side of each panel shows mean total daily intake during the reinforced (Rein) two-bottle preference test, in which the CS+ was paired with IG sugar infusion, and mean daily intake during the nonreinforced (NR) two-bottle test, in which the CS+ was paired with IG water infusion. Values are expressed as means ± SE total daily intakes. Numbers atop bars represent mean percent preference for the CS+ solutions. Significant differences (*P < 0.05) between CS+ and CS− intakes during one-bottle training and two-bottle tests are shown.
Mice in the Glucose Group consumed more CS+ than CS− in all one-bottle training sessions [F(2,22) = 34.5, P < 0.001] (Fig. 2A). They increased their CS intakes over sessions, particularly their CS− intake [CS × sessions interaction, F(2,22) = 5.4, P < 0.001], which may represent a generalization from the CS+ to the CS− flavor. In the two-bottle tests, mice in the Glucose Group consumed substantially more CS+ than CS− in both the reinforced and nonreinforced tests [F(1,11) = 125.8, P < 0.001], and their intakes and percent CS+ preferences did not differ in the two tests.
Analysis of drinking bout patterns during one-bottle training revealed that the Fructose Group mice did not differ in their CS+ and CS− bouts/day but CS+ bout sizes were larger (P < 0.05) than CS− bouts during the last four training days [F(2,16) = 4.8, P < 0.05] (data not shown). In contrast, mice in the Glucose Group took more CS+ than CS− bouts/day with the difference being significant (P < 0.001) for the first and last pair of training days [CS × days interaction, F(2,18) = 5.0, P < 0.05], and their mean CS+ bout sizes exceeded CS− bout sizes on all training days [F(1,9) = 21.3, P < 0.001] (data not shown).
Between-group comparisons indicated that mice in the Glucose Group consumed significantly more CS+ during one-bottle training than did mice in the Fructose Group, but CS− intakes did not differ [Group × CS interaction, F(1,20) = 11.2, P < 0.01]. Overall, mice in the Glucose and Fructose Groups consumed 85% and 30%, respectively, more CS+ than CS− during training [t(20) = 2.6, P < 0.05]. In addition, CS+ bout number and size were greater in mice in the Glucose than Fructose Group [50.7 ± 3.0 vs. 34.4 ± 3.5 bouts/day; 0.73 ± 0.1 vs. 0.51 ± 0.1 g/bout; F(1,17) ≥ 8.2, P < 0.05]. Mean CS− bout size was also greater in the Glucose Group [0.56 ± 0.1 vs. 0.41 ± 0.1 g/bout, F(1,17) = 16.6, P < 0.01]; the groups did not differ in their CS− bout numbers (34.2 ± 3.4 vs. 32.7 ± 3.0 bouts/day). In the two-bottle tests, mice in the Glucose Group consumed more CS+ than did the mice in the Fructose Group, while their CS− intakes did not differ [Group × CS interaction, F(1,20) = 13.5, P < 0.01]. The percent CS+ preference was greater in the Glucose than Fructose Group, although this difference was significant only in the reinforced test [F(1,20) = 6.7, P < 0.05] (Fig. 2).
Discussion
These findings provide direct evidence that fructose has a post-oral appetition effect in FVB mice, although fructose was less effective than glucose. In particular, IG glucose infusions stimulated CS+ training and test intakes more than did IG fructose infusions and conditioned a stronger CS+ preference. The two groups consumed more CS+ than CS− in both the reinforced and nonreinforced preference tests, which indicates that elevated CS+ intakes were not driven by concurrent sugar infusions but represented a learned preference for the CS+ flavor. The groups differed, however, in that mice in the Fructose Group consumed more CS+ in the nonreinforced than reinforced test, suggesting that IG fructose infusions limited their 24-h CS+ intakes. Overall, these results are consistent with the findings of experiment 1 that mice in the Glucose Group consumed more sugar and displayed a greater sugar preference over S+S than did mice in the Fructose Group, and that all mice preferred glucose to fructose in the final choice test.
EXPERIMENT 2B: 1-H IG FRUCTOSE AND GLUCOSE CONDITIONING
The significant flavor preference conditioned by the IG fructose infusions is in marked contrast to the failure of fructose to condition flavor preferences in B6 mice trained 24 h/day (29). The FVB fructose results, however, are similar to earlier findings that showed SD rats learned to prefer a saccharin-sweetened CS+ flavor paired with IG 16% fructose with 24-h training (2). Nevertheless, fructose is much less effective than glucose in conditioning flavor preferences in rats (2, 4, 31, 32). In particular, IG fructose conditioned no or at best a weak CS+ preference in food-restricted rats trained in short daily sessions (0.5 or 2 h), whereas IG glucose produced robust preferences (4, 31, 32). Even rats that had acquired a significant preference for a fructose-paired CS+ flavor in long training sessions failed to learn a preference for a new CS+ flavor paired with IG fructose in short daily sessions (4). The present experiment determined whether this is also the case with FVB mice. The mice from experiment 2A were food-restricted and trained with new CS flavors paired with IG fructose or glucose self-infusions during daily 1-h sessions.
Materials and Methods
At the end of experiment 2A, mice in the Fructose (n = 10) and Glucose (n = 12) Groups were transferred to separate home cages similar to the test cages. They were given daily food rations that maintained them at ∼90% of their ad libitum body weight. Body weights were measured daily and fixed-size chow pellets (0.5 or 1 g, F0171, F0173; Bio-Serv, Frenchtown, NJ) were used to precisely adjust the daily rations. Beginning 1 day after the start of food restriction, the mice were adapted to drink unflavored saccharin solutions paired with IG water infusions during daily 1-h sessions in the test cages. The saccharin concentration was initially 0.2% (days 1 and 2) but was reduced to 0.05% (days 3–5), which equated the 1- h total intakes (oral + IG) of the Fructose and Glucose Groups at 3.1 g/h. The mice were then trained with new flavors (0.05% orange and lemon-lime Kool Aid in 0.05% saccharin) with the CS+ and CS− paired with IG infusions of 16% sugar and water, respectively. The flavor-infusion pairs were counterbalanced across subjects, and individual mice were infused with the same sugar they received in experiment 2A. The CS− flavor was presented in training sessions 1, 3, and 5, and the CS+ flavor was presented in sessions 2, 4, and 6 (see Table 1). The mice were then given reinforced two-bottle test sessions (7 and 8) with the CS+ and CS− paired with IG infusions of sugar and water, respectively, and nonreinforced sessions (9 and 10) with both CSs paired with IG water infusions.
Results
During one-bottle training, mice in the Fructose Group reduced their total intake (oral + IG) from CS− session 1 to CS+ session 2 and then consumed similar amounts of the two CS solutions; only the overall decline in CS intakes over sessions was significant [F(2,18) = 4.1, P < 0.05] (Fig. 3A). In the two-bottle tests, the Fructose Group mice consumed significantly more CS+ than CS− in both the reinforced and nonreinforced tests [F(1,9) = 91.2, P < 0.001] (Fig. 3A), and their CS intakes and CS+ preferences were similar in the two tests.
Fig. 3.
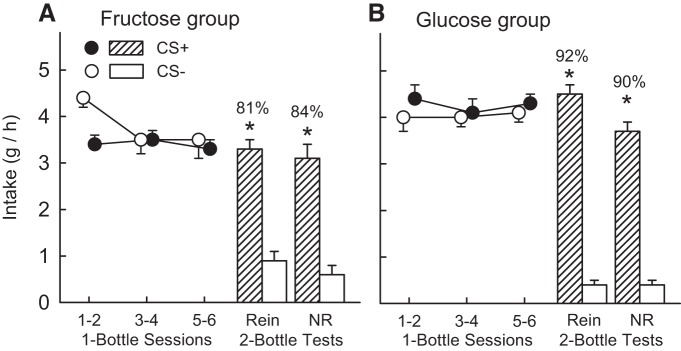
Experiment 2B. IG sugar conditioning in food-restricted FVB mice trained 1 h/day. Intake of the CS+ solution was paired with IG infusions of either 16% fructose (A) or 16% glucose (B). Intake of the CS− solution was paired with IG infusions of water. The left side of each panel shows total 1-h fluid intake (oral CS intake plus IG infusate) during each of the six one-bottle training sessions. The CS− solution was offered in sessions 1, 3, and 5, and the CS+ solution was offered in sessions 2, 4, and 6. The right side of each panel shows mean total daily intake during the reinforced (Rein) two-bottle preference test, in which the CS+ was paired with IG sugar infusion, and mean daily intake during the nonreinforced (NR) two-bottle test, in which the CS+ was paired with IG water infusion. Values are expressed as means ± SE total daily intakes. Numbers atop bars represent mean percent preference for the CS+ solutions. Significant differences (*P < 0.05) between CS+ and CS− intakes during two-bottle tests are shown.
Mice in the Glucose Group consumed similar amounts of CS+ and CS− during one-bottle training (Fig. 3B). In the two-bottle tests, the Glucose Group mice consumed significantly more CS+ than CS− in both the reinforced and nonreinforced tests [F(1,11) = 254.5, P < 0.01], although their CS+ intake declined from the reinforced to nonreinforced test [CS × test interaction, F(1,11) = 13.4, P < 0.001] (Fig. 3B).
Between-group comparisons indicated that the Glucose group consumed significantly more CS+ than did the Fructose group during one-bottle training (4.3 ± 0.2 vs. 3.4 ± 0.1 g/h), while the groups did not differ in their CS− intakes [group × CS interaction, F(1,20) = 5.2, P < 0.05]. In the two-bottle tests, the Glucose Group mice consumed significantly more CS+ and somewhat less CS− than did the mice in the Fructose Group [F(1,20) = 13.5, P < 0.01]. In addition, the percent CS+ preference in the reinforced and nonreinforced tests was greater in the Glucose group than in the Fructose group [91 ± 2.2% vs. 82 ± 2.4%, F(1,20) = 5.4, P < 0.05].
Discussion
These findings demonstrate that IG fructose and glucose infusions are both effective in conditioning CS+ preferences in food-restricted FVB mice trained 1 h/day. This contrasts with the ineffectiveness of IG fructose to condition flavor preferences in SD rats trained in short daily sessions (4, 32). However, as in experiment 2A, the mice consumed less CS+ fructose than CS+ glucose in one-bottle training and two-bottle testing and displayed a weaker preference for the CS+ fructose than for the CS+ glucose.
EXPERIMENT 3: 1-H IG FRUCTOSE AND GLUCOSE STIMULATION OF INTAKE AND FLAVOR CONDITIONING
While the 1-h IG sugar infusions conditioned significant CS+ preferences in experiment 2B, they failed to stimulate CS+ intakes during training as they did in the 24-h sessions of experiment 2A. Total 1-h intakes (oral + IG) were high (∼4 g/h) in the first CS− training session, which may have precluded stimulation of intake by the IG sugar infusions in subsequent sessions. In a recent study, using a different 1-h training procedure, which produced low CS− baseline intakes (∼2 g/h), we observed rapid stimulation of CS+ intake in B6 mice self-infusing glucose but not in mice self-infusing fructose (43). In addition, only glucose-trained B6 mice displayed a CS+ preference in the two-choice test. The present experiment used this 1-h protocol to compare the effectiveness of glucose and fructose to stimulate CS+ intakes and condition preferences in naïve FVB mice.
Materials and Methods
Animals.
Adult male FVB mice (n = 21, mean body weight 27.3 g) were fitted with IG catheters as in experiment 2A. They were housed singly in plastic cages with ad libitum access to chow and water prior to flavor conditioning.
Test solutions.
The CS solutions contained 0.025% saccharin flavored with 0.02% ethyl acetate or propyl acetate (Sigma) as in prior studies (42, 44). For half of the animals, the CS+ paired with IG 16% fructose or glucose solutions contained ethyl acetate, and the CS− solution contained propyl acetate; the flavors were reversed for the remaining animals. The low saccharin concentration was used to keep baseline CS− intakes relatively low so that stimulation of CS+ by IG sugar infusions could be expressed.
Procedure.
The mice were trained (1 h/day) in the test cages to drink unflavored 0.025% saccharin solution paired with matched volume infusions of water for six sessions. Before the first two sessions, the mice were water-deprived for 22 h. They were given access to water in their home cage for 1 h following the first session and then ad libitum access to water but restricted food rations thereafter as in experiment 2B. The mice were then given four 1 h/day test sessions with a CS− solution paired with IG water infusions. They were then divided into two groups matched for their intakes during the last two CS− sessions. The mice in the Fructose group (n = 11) and Glucose group (n = 10) were given three sessions with the CS+ solution paired with IG infusions of 16% fructose and glucose, respectively (see Table 1). The mice were next given four alternating sessions with the CS−, CS+, CS−, and CS+, in that order, with each solution paired with its respective infusion (IG water or IG sugar). In the final CS− and CS+ training sessions, a second sipper tube containing water not paired with IG infusions was available to familiarize the mice to the presence of two sipper tubes in the subsequent two-bottle test. The two-bottle test, with the CS+ vs. CS− solutions no longer paired with IG infusions, was conducted over four 1 h/day sessions.
CS− licks and total intakes (oral + IG infusate) during the last two 1 h/day sessions were averaged. The data from these two sessions, referred to as CS− test 0, and the licks and intakes during the subsequent three CS+ sessions (tests 1–3) were analyzed using a mixed-model ANOVA with a group factor (fructose, glucose) and repeated-measures factor (tests 0–3). The mean CS− and CS+ licks during the two alternating one-bottle sessions and the four two-bottle tests were compared in separate ANOVAs. Additional analyses are described in the results. As in prior studies (42–44), the results section emphasizes the lick rather than intake data because the lick data provide a more detailed analysis of the sugar-conditioning effects.
Results
When switched from the CS− in test 0 to the CS+ in tests 1–3, the Fructose Group mice did not change their 1-h licks, whereas the Glucose Group mice significantly increased their licks (Fig. 4). The groups did not differ in their CS− licks, but overall, the Glucose group licked more for the CS+ than did the Fructose group [group × test interaction, F(3,57) = 18.9, P < 0.001]. A within-group analysis indicated that the mice in the Glucose Group increased their licks from CS− test 0 to CS+ tests 1 and 2, and then from CS+ tests 2 to 3. Analysis of the 1-h intake data revealed a similar pattern of results. Mice in the Glucose Group increased their intakes (oral + IG) from CS− test 0 to CS+ test 3 (2.3 ± 0.2 to 4.3 ± 0.2 g/h, P < 0.01), whereas the mice in the Fructose Group did not significantly increase their CS intakes from test 0 to test 3 (2.2 ± 0.2 to 2.5 ± 0.2 g/h) [group × test interaction, F(3,57) = 11.3, P < 0.001]. Consequently, the mice in the Glucose Group consumed more (P < 0.01) in CS+ tests 1–3 than did the Fructose Group. In the alternating CS training sessions, the mice in the Glucose Group also consumed more CS+ than CS− (3.8 ± 0.3 vs. 2.6 ± 0.3 g/h, P < 0.01), whereas CS+ and CS− intakes did not differ in the Fructose Group mice (2.3 ± 0.1 vs. 2.5 ± 0.1 g/h) [group × CS interaction, F(1,19) = 45.2, P < 0.001].
Fig. 4.
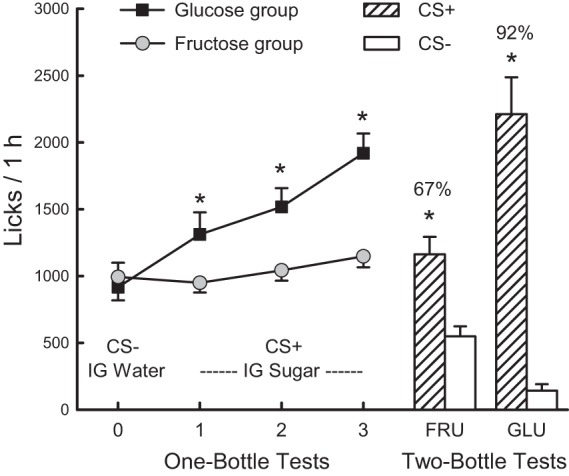
Experiment 3. IG sugar conditioning in food-restricted FVB mice trained 1 h/day. Mice in the Fructose Group drank (1 h/day) a CS-flavored saccharin solution paired with IG water infusions in test 0 (mean of last two CS− sessions) before being switched to a CS+ flavored saccharin solution paired with IG 16% fructose infusions in tests 1–3. Mice in the Glucose Group were similarly trained but with the CS+ paired with IG 16% glucose infusions. Left: 1-h licks (means ± SE) are plotted for 1-bottle tests 0–3. Right: 1-h licks (means ± SE) are plotted for CS+ and CS− flavored saccharin solutions during the two-bottle preference test for Fructose (FRU) and Glucose (GLU) Groups. CS+ and CS− intakes were not paired with IG infusions in the two-bottle test. Number above bar represents mean percent preference for the CS+ solution. *Significant differences (P < 0.05) between test 0 and tests 1–3 licks and between CS+ and CS− licks.
In the two-bottle tests conducted without IG infusions, both groups licked more for the CS+ than the CS− [F(1,19) = 63.7, P < 0.01] with the difference being more pronounced for the Glucose group [group × CS interaction, F(1,19) = 18.6, P < 0.01] (Fig. 4). Mice in the Glucose Group also licked more CS+ (P < 0.01) and somewhat less CS− (P < 0.07) than did the mice in the Fructose Group, and consequently, their CS+ preference was greater than that of mice in the Fructose Group [92 ± 3.1% vs. 67 ± 3.4%, t(19) = 5.0, P < 0.001]. The analysis of the CS intake data revealed a similar pattern of results.
The one-bottle lick data for CS training sessions were analyzed further to identify when licking was elevated within the CS+ sessions. Fig. 5 presents the CS licks from tests 0 to 3 expressed as licks/10-min bin and as cumulative lick curves. Statistical analysis was performed on the 10-min data, and the cumulative lick curves are included to show the evolution of the licking response during the 1-h sessions. Mice in the Glucose Group licked more in CS+ test 1 than CS− test 0 and although the group × bin interaction was not significant, the difference in CS− and CS+ licks was most pronounced in 10-min bins 2 and 3 (Fig. 5B). In CS+ test 2, the Glucose Group mice licked more (P < 0.5) in bins 1, 2, and 5 than they did in CS− test 0 [test × bin interaction, F(5,45) = 5.9, P < 0.001]. In CS+ test 3, the mice in the Glucose Group licked more (P < 0.05) in bins 1 and 5 than they did in CS− test 0 [test × bin interaction, F(5,45) = 41.8, P < 0.001].
Fig. 5.
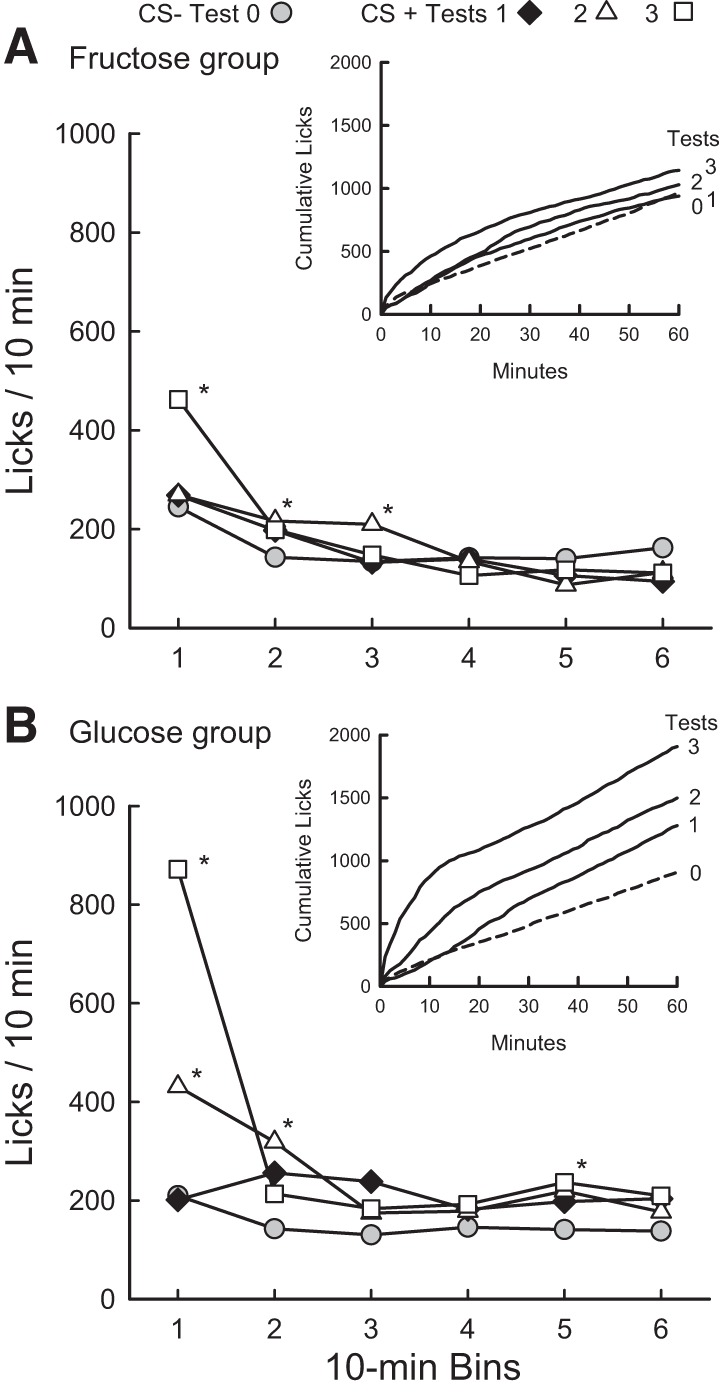
Experiment 3. IG sugar conditioning in food-restricted FVB mice trained 1 h/day. Mean licks per 10-min bin are plotted for test 0 (mean of last two 1-h sessions) with CS− flavored saccharin solution paired with IG water self-infusions, and for tests 1–3 with CS+ flavored saccharin solution paired with IG self-infusions of 16% fructose (A) or 16% glucose (B). Graph insets plot mean cumulative lick curves for tests 0–3. For the Fructose Group, 10-min licks in bins 2 and 3 of test 2 and licks in bin 1 of test 3 significantly (*P < 0.05) exceeded those of test 0. For the Glucose Group, 10-min licks in bins 1, 2 and 5 of test 2 and licks in bins 1 and 5 of test 3 exceeded those of test 0 (*P < 0.05).
Although the mice in the Fructose Group did not increase their total 1-h licks from tests 0 to 3, they did differ in their within-session distribution of licks. In particular, in CS+ test 2, they licked more (P < 0.05) in bins 2 and 3 than they did in CS− test 0 [test × bin interaction, F(5,50) = 4.3, P < 0.01], whereas in CS+ test 3, they licked more (P < 0.001) in bin 1 than they licked in CS− test 0 [test × bin interaction, F(5,50) = 13.8, P < 0.001] (Fig. 5A).
Discussion
As in experiment 2B, IG fructose and glucose infusions conditioned flavor preferences in FVB mice trained 1 h/day, with the preference being greater in mice in the Glucose than the Fructose Group. The new finding here is that only the glucose infusions stimulated the mice to drink more CS+ during one-bottle training. Also, whereas IG glucose conditioned similar preferences in this experiment and in experiment 2B (∼90%), the IG fructose infusions produced a weaker preference in the present compared with the prior experiment (67% vs. 84%). This is likely due to the fact that the mice in the Fructose Group consumed more CS+ and, thus, self-infused more fructose during training in experiment 2B than in the present experiment (total intakes: 3.4 g/h vs. 2.3 g/h). This, in turn, can be attributed to the sweeter CS solutions used in the previous experiment (0.05% vs. 0.025% saccharin). In addition, the conditioning experience of the mice in experiment 2A may have enhanced their attraction to the saccharin solutions (44), and the 24-h fructose infusions may have facilitated intestinal fructose absorption (10).
General Discussion
It is well documented that mouse strains differ in their taste preferences for sugars, and the present FVB findings along with prior results with B6 mice [29, 43, 44; and (Sclafani A, Zukerman S, and Ackroff K, unpublished data)] provide evidence for strain differences in post-oral sugar conditioning. FVB mice, like B6 mice, acquired strong preferences for glucose and glucose-paired flavors, but only FVB mice acquired preferences for fructose and fructose-paired flavors based on the post-oral actions of the sugars (see Table 2). This conclusion is based on four different measures of sugar conditioning in FVB mice.
In experiment 1, the FVB mice initially consumed much more of the noncaloric S+S mixture than 8% fructose, but after separate experience with the two sweeteners in tests 2 and 3, they significantly preferred fructose to the S+S solution. The FVB mice also consumed more fructose than S+S in tests 2 and 3. This is in marked contrast to B6 mice, which strongly preferred S+S to fructose in tests 1 and 4 (Table 2) and did not consume more fructose than S+S in the sweetener vs. water in tests 2 and 3. The two strains were similar, however, in showing no preference for glucose and S+S in test 1 but robust glucose preferences in test 4 (Table 2). The initial indifference for glucose and S+S in the first test can be attributed to some mice preferring the S+S solution based on its sweeter taste, while other mice rapidly learned to prefer glucose based on its post-oral appetition effects. All mice then acquired a strong avidity to glucose when they experienced the sugar by itself in test 2. The FVB and B6 mice were similar in consuming more glucose than fructose in the sugar vs. water tests, as well as strongly preferring glucose to fructose in a two-bottle choice test (Table 2).
On the basis of the preference reversal from S+S to fructose observed in first experiment, we predicted that FVB mice would learn to prefer a CS+ flavor paired with IG fructose infusions. This was confirmed in experiment 2A, in which FVB mice displayed an ∼80% preference for the CS+ Fructose flavor and also consumed more CS+ than CS− in one-bottle training sessions. This contrasts with the failure of B6 mice to acquire a CS+ Fructose preference (Table 2) and their reduced rather than increased training intakes of the CS+ Fructose flavor. Yet, while IG fructose stimulated CS+ intake and preference in FVB mice, IG glucose was much more effective. In particular, IG glucose significantly increased CS+ intake on the first training day, whereas IG fructose did not have this effect until the last training day; overall CS+ Glucose training intakes were almost twice those of CS+ Fructose intakes. Mice in the Glucose Group also displayed a stronger CS+ preference than did the mice in the Fructose Group. These findings are consistent with the greater intakes and preferences for glucose than fructose observed in experiment 1 and demonstrate that the post-oral actions of the two sugars can account for these differences.
Experiment 2B demonstrated that FVB mice learned to prefer a new CS+ flavor paired with 1 h/day IG fructose infusions, although this preference was less robust than that produced by IG glucose infusions. The CS+ Fructose preference is notable, given our earlier findings that SD rats did not acquire preferences for CS+ flavors paired with IG fructose infusions in short-term sessions, even after they had already learned a preference for a CS+ flavor paired with IG fructose in long-term sessions (4, 32). Thus, FVB mice are more sensitive than SD rats as well as B6 mice to the post-oral conditioning actions of fructose.
The third experiment further investigated short-term sugar conditioning in FVB mice using a training protocol that is sensitive to post-oral stimulation of intake by sugars (42–44). With this protocol, both IG fructose and glucose self-infusions conditioned flavor preferences, but the fructose conditioned a much weaker preference than did glucose (67% vs. 92% CS+ preferences). Furthermore, while IG glucose infusions significantly increased CS+ licks during the very first training session and more so in subsequent sessions, IG fructose infusions failed to increase 1-h CS+ licks relative to the water-paired CS−. In this respect, the FVB mice were similar to B6 mice, which showed rapid stimulation of CS+ licking by IG glucose infusions but no stimulation by IG fructose infusions. The two strains differ, however, in that only the FVB mice developed a significant preference for the fructose-paired CS+ flavor (Table 2). The B6 mice were trained with IG infusions of 8% or 12% fructose, and it is possible they would have acquired a CS+ Fructose preference if infused with 16% sugar. This seems unlikely though, given that B6 mice trained 24 h/day with IG 16% fructose failed to learn a CS+ preference (29) (Table 2).
The stimulation of CS+ intake and conditioned preference produced by IG glucose but not fructose in B6 mice suggested that post-oral sugar appetition in this strain is mediated by glucose-selective sensors such as SGLT1 and SGLT3 (43). Supporting this hypothesis, infusions of the nonmetabolizable glucose analog, α-methyl-d-glucopyranoside (MDG), which is a ligand for SGLT1/SGLT3, also stimulated CS+ licking and preference in B6 mice (43). The MDG findings implicated an intestinal site of action for the flavor-conditioning response, since MDG is not actively transported out of intestinal cells. This is further supported by the findings that duodenal and jejunal glucose infusions, but not gastric-limited, ileal, hepatic portal, or intraperitoneal glucose infusion-conditioned preferences for flavored saccharin solutions in rats or mice (5, 44). Other studies indicate, however, that stimulation of glucose sensors in the hepatic-portal region can condition food or spout location preferences in rats (22, 39).
The site and mechanism of action for fructose-conditioned flavor preferences in FVB mice remain to be determined. The findings that IG fructose did not stimulate CS+ licking in the 1-h training sessions in experiment 3 and stimulated CS+ licking only on the third CS+ training day in experiment 1 suggest that this sugar produces a relatively weak and/or slowly appearing appetition signal compared with glucose. This signal may be generated by the postabsorptive metabolism of the sugar in the liver (38) or conceivably by a fructose-specific sensor as found in the fly (19). Whether IG fructose infusions fail to generate an appetition signal in B6 mice or the mice are less sensitive than FVB mice to such a signal is not clear. Supporting the second possibility, B6 mice display somewhat weaker glucose-conditioned preferences than do FVB mice (see Table 2). Further studies comparing the behavioral and physiological responses of FVB and B6 mice to fructose may reveal the process by which post-oral fructose conditions flavor preferences in mice. Further research is also needed to evaluate how fructose-conditioned preferences in FVB mice vary as a function of sugar concentration. We previously reported differences in flavor preference conditioning in B6 mice trained with 2 to 32% glucose infusions (44).
As previously noted, SD rats can learn to prefer CS+ flavors paired with IG fructose infusions in long training sessions, although conditioning response varies as a function of fructose concentration and CS flavors (2, 4, 31, 32). However, with short training sessions, SD rats display little or no flavor conditioning with IG fructose infusions. Yet they acquire significant preferences for a CS+ flavor mixed into a fructose solution over a flavor mixed into a less preferred saccharin solution in short training sessions (1, 28). This conditioned preference was attributed to the sweet taste of the fructose (flavor-taste learning) rather than the post-oral actions of the sugar, given the ineffectiveness of IG fructose infusions. Consistent with this interpretation, orally consumed fructose, like noncaloric saccharin, does not support flavor conditioning when there is a delay between the consumption of the CS+ and the sweetener, whereas oral glucose supports flavor conditioning over delays (17, 28).
In a recent study, Pinhas et al. (24) investigated preference conditioning by orally consumed flavored sucrose, fructose, and saccharin solutions in nine mouse strains. All strains learned to prefer the sucrose-paired flavor over the saccharin-paired flavor, although they varied in the magnitude of their preferences (FVB mice were not included in the study). Interestingly, only B6 and SJL mice failed to prefer a fructose-paired flavor. These strains consumed more flavored saccharin than flavored fructose during training, which may have attenuated sugar conditioning, although this did not prevent two other strains (SWJ, C57BL/10) from acquiring a fructose-conditioned preference. In a subsequent experiment, we observed fructose-conditioned flavor preferences in B6 mice trained with flavored fructose vs. flavored water in 24 h/day training sessions (unpublished data). We attributed the fructose-conditioned flavor preferences observed in the B6 mice and other strains to flavor-taste learning. However, in view of the IG fructose conditioning results obtained with FVB mice, this interpretation needs to be reconsidered for the non-B6 strains. The present findings indicate that one simple test of strain differences in post-oral fructose conditioning is to measure preferences for 8% fructose vs. S+S solutions before and after 2-day tests with each sweetener vs. water.
Perspectives and Significance
FVB mice, like B6 mice, acquire strong preferences for glucose-paired flavors, but only FVB mice also learn to prefer fructose-paired flavors in IG conditioning paradigms. Nevertheless, as in B6 mice and SD rats, glucose stimulates greater intakes and preferences than does fructose in FVB mice. The physiological processes by which glucose and fructose condition flavor preferences in rodents are incompletely understood, but the present and prior studies demonstrate that caloric value per se is not the critical factor [4, 29, 31, 32, 43; and (Sclafani A, Zukerman S, and Ackroff K, unpublished data)]. Further study of inbred mouse strains that differ in their conditioning response to these sugars should advance our understanding of post-oral sugar conditioning. Related studies of glucose and fructose conditioning in flies may provide insights into the learning process in mammals (8, 11, 13, 20, 37). Humans learn to prefer the flavor of carbohydrate-rich drinks based on the post-oral actions of glucose (7, 9, 41) but the ability of fructose to support flavor conditioning in humans has not been investigated. Thus, speculations that fructose is more rewarding than glucose to humans may be premature (18, 23).
GRANTS
This research was supported by Grant DK-31135 from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., S.Z., and K.A. conception and design of research; A.S. and S.Z. analyzed data; A.S., S.Z., and K.A. interpreted results of experiments; A.S. prepared figures; A.S. and S.Z. drafted manuscript; A.S., S.Z., and K.A. edited and revised manuscript; A.S., S.Z., and K.A. approved final version of manuscript; S.Z. performed experiments.
ACKNOWLEDGMENTS
The authors thank Kwame McCartney and Martin Zartarian for their expert technical assistance.
REFERENCES
- 1.Ackroff K, Sclafani A. Flavor preferences conditioned by sugars: Rats learn to prefer glucose over fructose. Physiol Behav 50: 815–824, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite 42: 287–297, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning in rats by glucose but not a non-metabolizable glucose analog. Physiol Behav 133: 92–98, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav 72: 691–703, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99: 402–411, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr 27: 389–414, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birch LL, McPhee L, Steinberg L, Sullivan S. Conditioned flavor preferences in young children. Physiol Behav 47: 501–505, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol 21: 746–750, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Curr Biol 23: 878–883, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drozdowski LA, Thomson AB. Intestinal sugar transport. World J Gastroenterol 12: 1657–1670, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dus M, Ai M, Suh GSB. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat Neurosci 16: 526–528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33: 302–305, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol 21: 751–755, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav 51: 515–521, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Glendinning JI, Breinager L, Kyrillou E, Kacuna K, Rocha R, Sclafani A. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiol Behav 101: 331–343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci USA 93: 6631–6634, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman EW. Immediate and delayed reinforcers for flavor preferences in the rat. Learn Motiv 6: 91–100, 1975. [Google Scholar]
- 18.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc 110: 1307–1321, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto T, Amrein H. Diverse roles for the fructose sensor Gr43a. Fly (Austin) 8: 19–25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto T, Wright G, Amrein H. Nutrient sensors. Curr Biol 23: R369–R373, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers KP, Taddeo MS, Richards EK. Sensory-specific appetition: Postingestive detection of glucose rapidly promotes continued consumption of a recently encountered flavor. Physiol Behav 121: 125–133, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PLoS One 6: e24992, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 309: 63–70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, Kakuriev L, Guskova L, Fuzailov I, Touzani K, Sclafani A, Bodnar RJ. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav 105: 451–459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci 24: 938–946, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav 87: 745–756, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite 71: 454–458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiol Behav 56: 399–405, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav 106: 457–461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol 302: R1119–R1133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol 265: R320–R325, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav 67: 227–234, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol 299: R1643–R1650, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav 79: 783–788, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Sclafani A, Glendinning JI. Sugar- and fat-conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712–R720, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J Neurosci 32: 14767–14774, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tordoff MG. Metabolic basis of learned food preferences. In: Appetite and Nutrition, edited by Friedman MI, Tordoff MG, and Kare MR. New York: Marcel Dekker, 1991, p. 239–260. [Google Scholar]
- 39.Tordoff MG, Friedman MI. Hepatic-portal glucose infusions decrease food intake and increase food preference. Am J Physiol Regul Integr Comp Physiol 251: R192–R196, 1986. [DOI] [PubMed] [Google Scholar]
- 40.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99: 2392–2397, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav 93: 798–806, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1647, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol 305: R840–R853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol Behav 109: 33–41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of deleting T1r3 or Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses 38: 421–437, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


