Abstract
Intestinal failure (IF), due to short bowel syndrome (SBS), results from surgical resection of a major portion of the intestine, leading to reduced nutrient absorption and need for parenteral nutrition (PN). The incidence is highest in infants and relates to preterm birth, necrotizing enterocolitis, atresia, gastroschisis, volvulus, and aganglionosis. Patient outcomes have improved, but there is a need to develop new therapies for SBS and to understand intestinal adaptation after different diseases, resection types, and nutritional and pharmacological interventions. Animal studies are needed to carefully evaluate the cellular mechanisms, safety, and translational relevance of new procedures. Distal intestinal resection, without a functioning colon, results in the most severe complications and adaptation may depend on the age at resection (preterm, term, young, adult). Clinically relevant therapies have recently been suggested from studies in preterm and term PN-dependent SBS piglets, with or without a functional colon. Studies in rats and mice have specifically addressed the fundamental physiological processes underlying adaptation at the cellular level, such as regulation of mucosal proliferation, apoptosis, transport, and digestive enzyme expression, and easily allow exogenous or genetic manipulation of growth factors and their receptors (e.g., glucagon-like peptide 2, growth hormone, insulin-like growth factor 1, epidermal growth factor, keratinocyte growth factor). The greater size of rats, and especially young pigs, is an advantage for testing surgical procedures and nutritional interventions (e.g., PN, milk diets, long-/short-chain lipids, pre- and probiotics). Conversely, newborn pigs (preterm or term) and weanling rats provide better insights into the developmental aspects of treatment for SBS in infants owing to their immature intestines. The review shows that a balance among practical, economical, experimental, and ethical constraints will determine the choice of SBS model for each clinical or basic research question.
Keywords: rat, mouse, pig, parenteral and enteral nutrition, glucagon-like peptide 2, newborn, intestine, resection
during the past decades, there has been a dramatic increase in the survival of infants born prematurely, including those with various pathological conditions in the gastrointestinal tract (GIT). The introduction of parenteral nutrition (PN) support by Wilmore and Dudrick (267) has been a pivotal component in this care paradigm. PN allows for the routine survival of neonates suffering a major loss of intestinal length due to surgery, and recent refinements in the provision of PN have further improved these outcomes (214, 227, 255). The clinical problem of inadequate nutrient absorption following intestinal resection has stimulated research into the response of the remnant intestine, a process known as “adaptation.” Intestinal adaptation can be defined as the compensatory physiological process that occurs following loss of mucosal surface area to enhance both the structure and function of the residual bowel to restore the digestive and absorptive capacity of the intestine.
Intestinal adaptation is a complex series of coordinated mucosal, endocrine, and secretory events that ultimately allow for an increase in nutrient absorption, so that the patient can reach nutritional autonomy even when the remnant intestine is less than 15% of the original length (198). In humans, this process may take up to 2 yr (81, 212). It is a significant limitation of existing therapy that the only method to stimulate intestinal adaptation is enteral nutrition (EN), and indeed the use of PN may be detrimental to the adaptive process (263). This clinical situation requires appropriate animal models to better understand the needs and how to optimize care for this patient population.
Short bowel syndrome (SBS) is defined as a clinical condition that results from surgical resection, congenital defect, or disease-associated loss of absorption, leading to an inability to maintain nutrient balance when fed a normal diet (171). SBS has also been defined as a clinical condition requiring prolonged PN, due to intestinal failure (IF) following intestinal resection (227, 267). In principle, a distinction should be made between IF and SBS, since with IF, patients need parenteral support, whereas in SBS the use of defined enteral diets may be sufficient; thus SBS is also referred to as “intestinal insufficiency.” The focus of this review is to describe the feasibility and clinical relevance of various animal models used to model the condition of pediatric SBS.
Pediatric SBS
Within the pediatric population, the definition of IF has now been set as the requirement of PN for longer than 60 days (227). In pediatric patients, the etiologies of SBS and IF are related to the broad categories of congenital causes. Intestinal atresia (15% of SBS infants) is typically caused by a vascular event relatively late in gestation when the remaining small intestine is normal. Gastroschisis (17%) represents a condition in which a defect in the abdominal wall allows a herniation of large parts of the small intestine and colon. The process leads to thickening and scarring of the intestinal wall, resulting in delayed motility, and mechanical torsion can result in further ischemic loss of long segments of small intestine. Meconium ileus related to cystic fibrosis (5%) is caused by a lack of chloride secretion so that intestinal secretions are thick, leading to obstruction, dilation, and often secondary volvulus with necrosis. Aganglionosis (6%, Hirschsprung's disease) represents a lack of enteric nervous system development due to loss of certain neuronal growth factors. Necrotizing enterocolitis (32%; NEC) is an acquired intestinal inflammatory disorder, typically starting in the submucosa of the distal bowel and induced by enteral feeding and bacterial colonization. Volvulus (18% of SBS infants) is a mechanical torsion of the mesentery of the bowel, often due to abnormalities in intestinal development and fixation to the abdominal wall, resulting in ischemic necrosis (206, 214). The incidence of SBS is higher in pediatrics [24.5/100,000 live births or 9/million population (257)] than in adults [5/million population (248)]. Furthermore, the incidence of SBS is ∼100-fold higher in preterm vs. term infants, mainly related to the higher incidence of NEC in preterm infants. In a large prospective study, involving more than 12,000 very low birth weight infants, the incidence of SBS was 0.7% and NEC was the cause of SBS in 96% of these patients (42). It is common that the pathological insult that mandates resection also causes prolonged changes in motility and function, which delay or prevent recovery. The cause of SBS/IF varies among countries, reflecting different ethnicities and health care systems. Mortality from SBS has decreased recently but historically it has been 30–40% in pediatric patients (186, 212, 227) and 25% in adults, and in both populations deaths are often caused by comorbidities. Although pediatric IF cases may adapt better than adult IF cases (188, 248), pediatric patients face the additional challenge of receiving adequate energy and nutrients to sustain normal growth and organ development.
The segment(s) of the intestine remaining after resection vary depending on the primary disease (Table 1). The most common situation is a loss of the distal small intestine, including the ileum and ileocecal valve, which is the most difficult anatomical configuration for remnant intestinal adaptation and nutritional recovery (111, 247, 256). Clinicians have long recognized that the terminal ileum and ileocecal valve have distinct physiological properties, which either provide a stimulus for adaptation or are more sensitive to such signals and therefore have a greater capacity to adapt. The survival of patients with remnant ileum is higher than those without and the speed of adaptation is faster if there is a retained ileum (24, 81). Surgically, the key principle is to preserve the maximal length of intestine. This usually involves creating a proximal stoma, resecting obviously necrotic tissue, and leaving segments that have the potential to recover (59, 190). Thus the most common surgical procedure is distal intestinal resection with the initial creation of a jejunostomy, allowing time for the patient and the remnant intestine to recover and adapt, until a later surgical reanastomosis after 6–8 wk, to establish intestinal continuity. At this time, intestinal adaptation may have increased nutrient absorptive capacity, but adaption may itself also induce new functional deficits. The increase of the remnant intestine diameter may compromise smooth muscle function, reduce peristalsis, and predispose to a potentially dangerous tendency for bacterial overgrowth (1, 123, 212).
Table 1.
Some rodent and pig models of SBS and important considerations
| Species | Type of Surgical Intervention | Feasibility, Technical Challenges, and Clinical Relevance | References |
|---|---|---|---|
| Mice | Proximal: 50% jejunal resection with jejunoileal anastomosis | Adult, 8–12 wk old: The model does not mimic human SBS; tolerates oral feeding with potential for long-term follow-up; PN not reported; genetic models and reporter mice are available | 99, 173 |
| Distal: Ileocecal resection with jejunocolic anastomosis | Adult, 8–12 wk old: Mimics human SBS with distal bowel resection that tolerates EN; potential for long-term follow-up; PN not reported; genetic models and reporter mice are available | 51, 101 | |
| Rats | Proximal: 30–90% jejunoileal resection with jejunoileal anastomosis | Adult, 8–10 wk old: Most common rat resection model; tolerate oral feeding; potential for long-term follow-up after growth factor and EN interventions; does not mimic PN-dependent human SBS | 47, 49, 151 |
| Distal: 50–60% jejunoileal-cecal resection with jejunocolic anastomosis | Adult, 8–10 wk old: EN and PN required; mimics human PN-dependent SBS; weaning from PN with combination treatments of EN + growth factors; technical complexity in maintaining PN | 22, 77, 137 | |
| Weanling, 20 day old: Tolerates oral feeding with diarrhea and hyperphagia; good model to evaluate EN strategies with relevance to human neonatal SBS; PN not feasible | 272, 273 | ||
| Pigs | 75–95% jejunal resection, jejunoileal anastomosis | Young pigs: Clinically stable, long-term follow-up possible; nutrition support with growth factors, PN, EN or combinations; limited translational relevance for SBS in infants | 177, 197, 215 |
| 75% jejunoileal resection, jejunocolic anastomosis | Newborn pigs (<1 wk): Maladaptation, clinical challenges (sepsis, dehydration), short and longer term follow-up with PN, EN, growth factors, mimics term infant SBS with anastomosis | 104, 234, 247 | |
| 50% jejunoileal resection with jejunostomy | Preterm/term newborn pigs: Maladaptation, clinical challenges (sepsis, dehydration, dysmaturation); mimics NEC-sensitive SBS infants on PN; short-term follow-up and balance studies possible | 7, 245, 252 |
SBS, short bowel syndrome; PN, parenteral nutrition; EN, enteral nutrition; NEC, necrotizing enterocolitis.
Clinical management of infant SBS.
The clinical management of infant SBS is key to design animal models with high translational value. This management is a multidisciplinary effort focusing on optimizing intestinal adaptation and minimizing the need for PN via nutritional, pharmacological, or surgical approaches. Although PN is required to support these patients, EN is the most effective stimulus of adaptation (70, 151). A key principle is to continuously challenge the remnant intestine with a slightly higher enteral nutrient load than the remnant intestine can absorb. This will maximally stimulate the endogenous signals to upregulate nutrient transport capacity (211, 263). In this context, intestinal adaptation after resection is a subtype of the physiological regulation of nutrient absorption that occurs in all mammals at times of increased need for nutrient uptake, such as pregnancy and lactation, and in hibernating animals awakening after a long fasting period. Typically, newly resected infants are maintained primarily on PN support, appropriate to maintain growth [100 kcal·kg−1·day−1, with 2–3 g lipid·kg−1·day−1, 2 g protein·kg−1·day−1, and infusion of 8–12% glucose (263)]. Enteral feeds are introduced once evidence of bowel function is seen, typically low volumes of expressed breast milk or an elemental formula, by continuous infusion into the stomach. Feeds are advanced by 10 to 20 ml/kg per day, with tolerance monitored by stoma or stool outputs [e.g., maximum 40 ml·kg−1·day−1 (211)]. Once feeds exceed 5–10 ml/h during day time, two to three oral feeds are introduced to prevent the development of oral aversion with bolus feedings. PN is tapered according to the weight gain, and cycling PN is instituted when feasible. It is often not possible to provide more that 20–30% of calories enterally until the proximal stoma, if present, is closed. Thereafter the speed of tapering of PN is very dependent on the total length of residual intestine and the presence of the ileocecal valve. Because of the relative excess of luminal nutrients, small intestinal bacterial overgrowth (SIBO) is common in the SBS population. Thus rotating antibiotics (e.g., gentamicin and metronidazole) are used in cases of dysmotility with suspected SIBO (56, 212). In cases of severe dysmotility and progressive intestinal dilation, promotility agents (e.g., metoclopramide and erythromycin) are used (56). If the luminal diameter of the remnant intestine increases to greater than 6–8 cm, so that peristalsis becomes ineffective and bacterial overgrowth inevitable, then surgical intervention may be considered (123, 129).
Bianchi and serial transverse enteroplasty procedures.
Several surgical procedures have been developed to improve intestinal function after the development of SBS (255). Both of the commonly used procedures were developed to deal with the problem of excessive dilation of the remnant intestine that may occur in patients after prolonged attempts to induce adaptation by increasing the feeds. In the Bianchi procedure (14), also known as longitudinal intestinal lengthening and tailoring, the mesenteric blood supply is separated into two “leaves” and the dilated segment is subsequently longitudinally divided between the two leaves, leading to a decreased diameter and increased intestinal length. About half of SBS patients show improved nutritional status after this procedure, likely as a result of improved peristalsis and reduced bacterial overgrowth in the previously dilated segment (15, 20, 71). Few studies are available and the outcome of the operation could be influenced by other factors, such as clinical status, especially the degree of parenteral nutrition-associated liver disease (PNALD) (71).
Another intestinal lengthening method is the serial transverse enteroplasty (STEP) procedure that was developed to a large degree based on studies in young pigs (39, 128, 158). By this method, the dilated segment is lengthened by partially transversely dividing, by use of a stapling device, the small intestine in a serial transverse manner from alternating directions, creating a zigzag pattern in the small intestine. Outcomes in pediatric patients have been favorable, with ∼47% of patients coming off PN (123). Use of the STEP procedure may increase the tolerance to enteral feeding in infants with SBS (65), although the process to achieve full intestinal autonomy may take several years (174). A great deal of the translational work to support this intervention as a clinical therapy has been done by use of the pig model (158, 185, 197, 216).
Assessment of intestinal adaptation in human SBS.
The optimal manner to assess digestive adaptation in SBS patients would be to perform a nutrient balance study, with precise measures of enteral nutrient input and corresponding urine and stool outputs. Such studies have been useful to study the effect of various types of resection (109, 114, 116, 117), type of enteral diet (146), and pharmacological agents and growth factors (110, 112, 118, 119) in adult SBS patients. In children, balance studies are complicated by the difficulty in separating stool from urine and the need to use diapers and so are rarely used (5, 43, 132). Biomarkers of nutrient absorptive capacity and intestinal permeability have been used in infants and some of these (lactulose, mannitol, 3-0 methyl glucose) were validated in animal models of SBS (149, 221). In humans, intestinal function is typically evaluated indirectly by standard anthropometric measures, such as height, weight, head circumference, and z-scores, and such growth measures are especially important indicators of intestinal function in infants with SBS (214).
Logistical and ethical constraints limit studies in human SBS patients, especially in the pediatric age group, and access to tissues and cells is difficult. Only minor changes have been observed in intestinal hydrolase activities following resection in humans, indicating that functional adaptation originates mainly from an increased surface area induced by crypt cell proliferation (115). Surrogate markers of enterocyte mass in human SBS patients include the amino acid citrulline, a nonessential amino acid produced by enterocytes. Plasma citrulline levels have helped to identify SBS patients, both adults and children, with permanent IF (45, 46), and to monitor intestinal adaptation (9, 74) and enteral food tolerance (192) in children with SBS. Despite extensive study, we do not know whether the “classical” physiological description of intestinal tissue adaptation, derived mainly from studies in adult rodents, applies to the human infant (153).
Surgical Interventions to Model SBS
The validity of an SBS animal model should be based on the degree to which it simulates and represents the human condition described above, and ideally it should incorporate the possible region- and age-specific responses to intestinal resection. An experimental design that includes appropriate control groups with bowel transection and anastomosis at the same sites as the resected groups and defined methods of nutrition support from EN and/or PN is essential to correctly interpret the etiology of the intestinal adaptive response. Models in rodents have generally used postweaned or adult animals, whereas most studies in pigs have been performed in newborn, suckling, or juvenile pigs. To a large degree, these choices reflect practical limitations in performing the animal studies, e.g., an appropriate size to allow the surgical interventions, catheterizations, and long-term care with PN. For these reasons newborn mice and rats and adult pigs are less desirable and feasible for SBS studies. The dominant physiological processes of intestinal adaptation described in animal models are an increase in the rate of crypt cell proliferation, coupled with a decrease in the rate of apoptosis, which leads to an increase in villus length, mucosal surface area, and the capacity for nutrient absorption (170). There is less evidence describing how epithelial digestive enzymes, transport proteins, and submucosal tissue nerves and muscles are stimulated by intestinal adaptation (218, 237, 264). This adaptation is also influenced by the density and composition of the gut microbiota, but the widespread use of perioperative antibiotic regimens in both animals and infants has limited the interpretation of these effects.
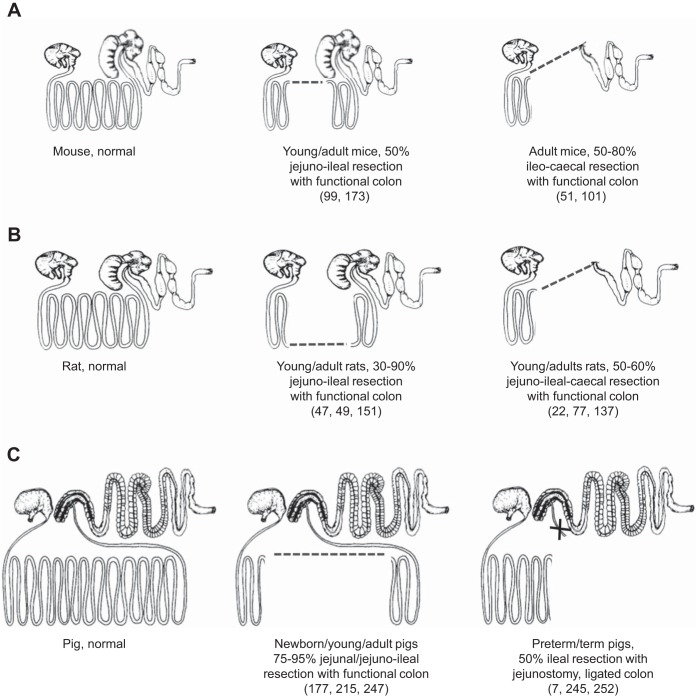
In selecting an animal model of SBS, it is helpful to classify the human SBS condition into three subtypes, based on the segment of the gut that is resected, with the critical points being the length of residual jejunum, ileum, and colon (230). The schematic illustrations in Fig. 1, coupled with the descriptions in Table 1, present some major types of resections performed in mice, rats, and pigs. There is an extensive literature using the first type of resection model, which is well tolerated and includes proximal (jejunal) resection, leaving a remnant ileum and colon. As noted earlier, this is not a condition reflecting the major group of human SBS patients, and, furthermore, these studies have typically been conducted either in adult (rat) or young animals (pigs). Proximal intestinal resection generally leads to an increased secretion of gastrointestinal hormones in both humans and animals (113, 150, 178), which help to explain the pronounced intestinal adaption after this type of resection.
Fig. 1.
Schematic illustration of different types of intestinal resections in mice (A), rats (B), and pigs (C). Adapted with permission from drawings of the gross anatomy of the gastrointestinal tract in different species (230).
The second type is small bowel resection with a partial colon resection and a jejunal-colonic anastomosis with resection of the ileal-cecal valve. Because of the frequent anatomical location of NEC lesions in the distal small intestine and colon, this is a common surgical procedure for infants, especially after an initial period with a jejunostomy to allow the remnant intestine to recover. This type of resection has been investigated in rats, mice, and pig models (Table 1, Fig. 1). Piglet studies showed that a midjejunal resection leads to better nutrient absorption and clinical outcome than distal small intestinal resection (247).
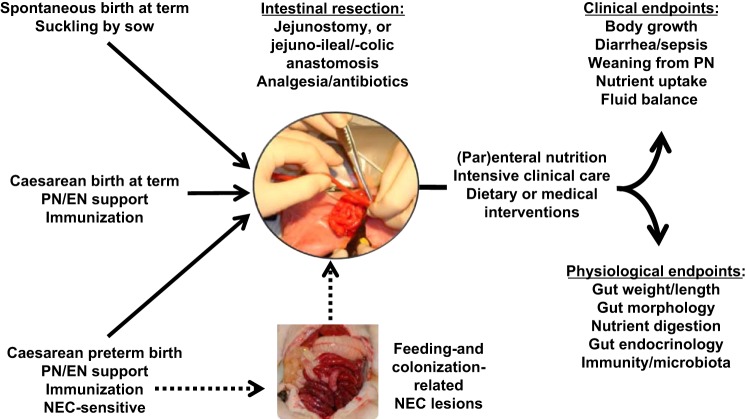
The third type of small bowel resection is removal of distal small intestinal segments, resulting initially in a jejunostomy with a stoma creation until later reanastomosis (e.g., after 6–8 wk). This is the most common surgical procedure for infants with NEC. For this type, the colon is divided and left in the abdomen with full vascularization but separated from luminal nutrients because any remaining digesta is diverted at the jejunostomy (256). Because of the lack of colonic fluid absorption and endocrinological stimulation, such SBS patients are severely affected and have been subject to many studies on intestinal adaptation in humans (111, 167). A functioning colon is believed to support intestinal adaptation (82), although its importance in pediatric SBS remains debated (55). Studies in rats suggest that the colonic secretion of endogenous growth factors (see later section) is not sufficient to induce significant intestinal adaptation (134). Since most studies in animal models have used a proximal or midjejunal resection with an intact colon, these studies generally report dramatic adaptation following resection (16, 51, 150, 172, 180, 221). Recent studies have evaluated distal intestinal resection with an jejunocolic anastomosis in neonatal pigs (234, 247), and some of these have used jejunoileal resection resulting in a jejunostomy without a functional colon, which because of the risk of NEC is a very clinically relevant procedure (7, 245, 252). A schematic presentation of the procedures used for this model is shown in Fig. 2, and a brief record of the advantages and disadvantages of the different models is given in Table 1.
Fig. 2.
Procedures involved in using newborn pigs as models for infant short bowel syndrome (SBS). Both normal newborn pigs (top left; Refs. 104, 234, 247) and caesarean-delivered term or preterm pigs (bottom left; Refs. 7, 245, 252) can be subjected to intestinal resection, with establishment of a jejunostomy or with anastomosis to the remnant ileum or colon. Postsurgical rearing requires intensive care but allows advanced dietary or medical interventions. Artificially reared, colostrum-deprived pigs require intensive care and passive immunization even before resection, especially if born preterm. Immature organ functions and high necrotizing enterocolitis (NEC) sensitivity make preterm pigs similar physiologically to preterm infants, but the increased technical, clinical, and ethical challenges must be weighed against the apparent translational advantage. Thus intestinal resection following NEC outbreak in preterm pigs is in principle possible (broken line) but has not yet been investigated. PN, parenteral nutrition; EN, enteral nutrition.
Rodent Models of SBS
Rodent models of SBS using the rat or mouse provide the most common experimental approach to study the process of intestinal adaptation to massive bowel resection because of their low cost and ease of genetic manipulation. Two adult rat models are widely used and broadly categorized as proximal small bowel resection, where 30–90% of the jejunoileal segments are resected (47, 151), or distal small bowel resection where 50–60% of the distal jejunum, all of the ileum and cecum (approximating the upper colon in humans) are resected (22, 77, 137, 145) (Fig. 1). In addition, Yang and Kock (272) recently developed a weanling rat resection model in which 50% of the distal small bowel, proximal to the ileocecal junction, is resected with cecectomy and a jejunocolic anastomosis. In the following, we review the scientific and technical aspects of rat and mouse models of SBS that have provided important insights regarding the extent and time course of resection-induced adaptation throughout the GIT. The feasibility, limitations, and clinical relevance of the models are summarized in Table 1.
Weanling and adult rat resection models.
Proximal and distal jejunoileal resection studies are often performed in male rats of the Wistar or Sprague-Dawley strains, either at weanling age (e.g., 20 days) or as adults (>6 wk). They are typically subjected to inhalation anesthesia, following transition to a liquid diet for some days, preoperative antibiotic treatment, and a short fasting period prior to surgery. After identification and measurement of the intestinal segment to be resected, the intervening mesenteric arterial blood supply is cauterized to reduce blood loss, and the intestinal segment resected, followed by end-to-end jejunoileal or jejunocolic anastomosis to restore bowel continuity. Following resection and peritoneal fluid resuscitation, the peritoneum and skin incisions are closed. For a few days after surgery, an analgesic drug and antibiotics are administered to reduce postsurgical pain and infections (138).
The proximal small bowel resection or “physiological” model tolerates EN after resection provided by a variety of liquid and solid diets. Adaptation can be assessed over a period of days or weeks, although 7 days postresection is often used. A jejunoileal resection of greater than 50% stimulates dramatic increases in mucosal cellularity (based on mass, protein, and DNA content), increases villus height and crypt depth, with little change in sucrase specific activity in the residual small bowel (94). The ileum demonstrates a large capacity for adaptive growth following proximal bowel resection (278). Increases in the cellularity of residual jejunum and ileum occur rapidly within 12–48 h postresection, probably mediated by the increased gut hormone release (see later section).
In the adult rat model of distal bowel resection with a jejunocolic anastomosis to restore intestinal continuity, EN is not sufficient to meet the nutritional needs and facilitate adequate adaptive growth of the remnant intestine, as observed with proximal small bowel resection. Rats develop prolonged malabsorption with weight loss and high mortality when fed orally after this resection (137, 205), although significant increases in colonic mass, crypt depth, and proglucagon expression occur (77, 79, 134). Jejunum and colon show a differential time course of adaptation with increases in jejunal cellularity that peak at 3–4 days and decline to baseline by 12 days postresection, whereas colon crypt depth steadily increases, peaking at 7–12 days postresection (134). Thus PN in combination with massive gut resection is required to provide the appropriate nutritional support for this stringent distal bowel resection model, which better reflects the condition of SBS with IF seen in human neonates (77, 145). The model introduces the need for an additional surgery to place a catheter in the internal jugular vein for PN and the technical complexity in maintaining rats with PN for up to 22 days postresection. This requires specialized equipment, such as swivel infusion devices attached to a rat harness for mobility, and individual metabolic cages to collect urine and feces for balance studies. The model has the experimental advantage that therapy with intestinal growth factors, either alone or in combination with a mixture of PN and EN, can be tested in an economical manner for their ability to induce intestinal adaptation that does not otherwise occur (79, 133). Moreover, a functional assessment of the intestinal growth can be conducted to determine whether treatments are sufficient to sustain body weight with transition from PN to EN.
The weanling rat model of distal bowel resection may better reflect the human infant SBS condition because of the delayed but rapid structural and functional postnatal development of the rat GIT. The rat GIT is very immature at birth (maybe similar to preterm infants) but matures rapidly postnatally, especially from the time of weaning (18–22 days) to ∼5 wk of age (199). Although weanling rats do not reflect milk-fed infants, nor the specific characteristics of prematurity related to functions beyond the GIT (e.g., immaturity of lungs, liver, kidney, immunity, nutrient metabolism), it is likely that the more immature state of the GIT in weanling rats represent a translational advantage. With regards to the GIT and its maturity at birth and weaning, piglets are intermediate between humans and rodents (199). Thus, in contrast to pigs and infants, the GIT is fully mature when young rats are resected at 6–8 wk of age. A limitation is that models combining resection and PN in weanling rats have not been developed. About 85% survival with EN following ileocecal resection in weanling rats has been reported (272), and the symptoms of diarrhea, hyperphagia, and poor growth observed with EN are similar to the clinical course in human neonates after resection. Thus the weanling rat distal bowel resection model appears useful to evaluate EN support strategies with relevance to human neonatal SBS (Table 1).
Adult mouse resection models.
In mice, jejunoileal intestinal resection is technically difficult and most often conducted by research groups that include pediatric surgeons (51, 101, 173). Warner's research group (243) has addressed concerns about the reproducibility of murine intestinal resection models and concludes that maintenance of mice in pathogen-free conditions and restricting gene expression analysis to specific strains that demonstrate morphological adaptation minimizes experimental variability. It is important to recognize that 50% resection of the proximal small bowel is sufficient to induce adaptive growth, and that models of resection greater than 50% result in increased morbidity and mortality and do not increase the adaptive response to massive small bowel resection in combination with EN (254).
Two murine models of massive intestinal resection are used, 50% proximal small bowel resection (101) and ileocecal resection, which better reflects human neonatal SBS, in which the ileum and colon are often resected (51). Male mice 8–12 wk old (weighing 25–31 g) are typically used, and in the 50% proximal small bowel resection model, ∼15 cm of intestine is removed between 8 cm distal to the ligament of Treitz and 7 cm proximal to the ileocecal junction (99, 173). In the ileocecal resection model, ∼12 cm of ileum, cecum, and proximal right colon are removed between 12 cm proximal to the ileocecal junction and 1 cm distal to the cecum in the ascending colon. Intestinal obstruction is prevented by feeding a rodent liquid diet pre- and postoperatively (101) with return to rodent chow on postoperative day 7 (51). Preoperative antibiotics and postoperative peritoneal fluid rehydration are used. Mice can be maintained for weeks or months postresection to address long-term intestinal adaptation with the functional outcome of change in body weight (Table 1).
A limitation of the mouse models of massive bowel resection is that they do not result in IF that requires PN to sustain body weight, since a mouse model that combines resection with PN has not been developed. However, genetic mouse models in combination with intestinal resection have tremendous advantages in identifying the contribution of specific molecules that mediate the process of intestinal adaptation (195), characterizing the intestinal microbiome (52), and identifying the role of intestinal stem cells via the use of reporter mice (85), and for cell culture of enteroids (225). The use of genetic models and stem cell reporter animals are currently not available in the established rat or pig resection models, although intestinal enteroids have recently been cultured from neonatal pigs (80).
Pig Models of SBS
Models of SBS and intestinal adaptation have been developed in preterm and term newborn pigs, juvenile pigs, and adult pigs. The majority of studies have used young domestic pigs, while only a few studies used minipigs (197). Recently, a comprehensive technical review of the pig models of SBS in juvenile and young pigs has been published (260). The present review has focused on models for infant SBS and therefore the main emphasis will be on the use of neonatal pig models. It is unknown how organ immaturity in preterm infants affects the response to intestinal resection, and there is a need to develop piglet models that allow incorporation of more of the key features of SBS in premature infants, e.g., organ immaturity, high NEC sensitivity, distal intestinal resection without a functioning colon, and long-term use of both PN and EN.
Resection models in young pigs.
An increasing number of studies have induced SBS in pigs younger than 1 wk of age and weighing less than 3–4 kg (12, 97, 236, 245, 247), but the majority of studies have been performed in 3- to 8-wk-old animals weighing 6–15 kg (25, 95, 163, 180, 216, 228). Use of inhalation anesthesia, analgesic drugs, and antibiotics in association with surgery in young growing pigs generally occur without major complications, whereas procedures in newborns, especially preterm newborn pigs, are more challenging.
The proportion of the intestine that has been resected in the different studies in young and juvenile pigs has ranged from 75 to 100% (Table 1). Most investigators have performed a midintestinal, jejunoileal resection in which the remaining small intestine consists of equal parts of jejunum and ileum (57, 96, 216, 236, 247, 261). Models have also been used in which 75% intestine has been resected starting from 0.5–1.0 m beyond the ligament of Treitz, or from 1–2 m proximal to the ileocecal valve distally (163, 178, 228). Distal intestinal resection of part of the jejunum, entire ileum, ileocecal valve, and the first 5 cm of colon has recently been reported in pigs where a jejunocolic anastomosis was made to create intestinal continuity (104, 105, 247). The length of the postresection period has generally been longest in studies on older pigs (260). Only relatively acute responses to resection have been studied in pigs younger than 1 wk of life (7, 12, 245, 252), except the recent studies on PN-fed term pigs (104, 105, 234, 247). When pigs have been older and beyond the weaning age at resection (e.g., 4 wk and onward), an observation period of 4–8 wk has been common, sometimes incorporating several time points of tissue collection to indicate the important temporal GIT response to resection (180). Some studies on bowel lengthening procedures have provided results on follow-up parameters over several months (39, 216).
Body weight gain, food intake, stool output, and clinical chemistry values are important parameters to monitor the clinical adaptation response in SBS pigs, especially in long-term studies (163, 179, 216). Measurements of the intestinal disaccharidase activity (maltase, sucrase, lactase) are commonly used (16, 163, 178, 179, 245, 252) as well as plasma GLP-2 for the endocrine adaptation (12, 105, 177, 252). Only a few studies have attempted to make a direct estimation of nutrient uptake, or apparent nutrient digestibility (25, 216, 245, 252), but more indirect in vivo indexes, such as xylose absorption test (39), intestinal motility (158), portal blood flow, or nutrient influx across the intestine (16, 236, 261) have been used. Among the morphological parameters of adaptation in the different regions of the remnant intestine, villus height and crypt depth are the most commonly reported, along with measurements of intestinal muscle layer thickness, villus diameter and surface area, crypt cell proliferation, and apoptosis (12, 16, 97, 163, 178, 180, 247, 252). Furthermore, mucosal DNA, RNA, and protein concentrations have been used to indicate adaptation (12, 96). The potential for long-term follow-up is an important aspect of translational relevance, but such studies are costly and sometimes also logistically and ethically difficult to justify using newborn or young pigs. The growing SBS piglets must be relatively clinically stable without major challenges related to long-term PN support, systemic infections, or SIBO. The natural weaning change in diet at 3–6 wk (from milk to a dry cereal grain diet) adds further rearing complications.
Resection models in newborn preterm or term pigs.
As noted above, intestinal adaptation after SBS may depend on the physiological state at the time of resection (preterm/term, postnatal age, healthy/NEC-affected) and on the type of resection (e.g., different sites and lengths of intestine, and with/without a functional colon). We have described a short-term model of SBS using either preterm or term newborn pigs, incorporating the considerations of intestinal immaturity, jejunoileal-distal resection with a jejunostomy, and PN and EN support (7, 245, 252) (Fig. 2). Only preterm pigs delivered before 95% gestation are sensitive to develop NEC (199, 201), and key similarities between preterm pigs and infants include similar body size and the immature respiratory, nutritional, immunological, and metabolic conditions after preterm birth (142, 199, 201). All these factors may influence intestinal adaptation after resection. In principle, it is possible in preterm pigs to model surgical resection of a NEC-affected intestine, establish a jejunostomy, and observe postsurgical intestinal adaptation over several weeks. However, NEC develops even more rapidly in preterm pigs, relative to infants, and it is difficult to anesthetize, resect, and maintain the NEC-affected preterm pig in a condition that justifies further studies on intestinal adaptation after resection. Therefore, all the porcine models of SBS established so far, including in preterm pigs, have been done in animals that were in a healthy state at the time of resection (Fig. 2).
In the studies on preterm SBS pigs, catheters and feeding tubes were placed essentially as described for the preterm pig model of NEC (204). Typically, preterm pigs are fed a liquid enteral diet for 1 to 2 days before the resection to adapt the pigs and the GIT to luminal nutrition. In addition, when neonatal pigs are fed diets other than sow's or cow's colostrum, maternal plasma is administered to provide passive immunity, because the pig placenta does not transfer maternal immunoglobulins to the fetus. After 50% distal intestinal resection, a stoma is created by exteriorizing 1 cm of the distal end of the remnant intestine through an incision in the dorsolateral part of the abdomen. The exteriorized portion is everted and secured to the skin. A sterile Silastic tube is inserted into the intestinal lumen through the stoma and fixed to the skin with sutures to allow drainage of fluid. Pigs remain on PN before and after resection and subsequently enteral feeding is gradually introduced during the week after resection (7, 245, 252). In this model, supplemental fluids in excess of PN are required postsurgery to maintain body fluid homeostasis. The animals receive anti-secretory medication (e.g., proton pump inhibitor), and analgesics (e.g., NSAIDS) and broad-spectrum antibiotics for at least 3 days after resection. Besides the translational advantage of distal intestinal resection with a jejunostomy, the stoma bag allows for collection of intestinal contents in nutrient balance studies to evaluate intestinal adaptation (245, 252).
The clinical complications after 50% distal intestine resection in healthy, preterm pigs are similar to those in infants subjected to intestinal resection for reasons other than NEC. Postsurgical adaptation responses to enteral formula feeding are reduced in preterm vs. term SBS pigs, and the clinical complications, such as hemodynamic instability, hypothermia, intestinal dysmotility, dehydration, respiratory distress, and peritonitis, are more severe in preterm vs. term pigs (7). These findings coincide with a lack of a postsurgical increase in intestinal protein synthesis, villus height, crypt depth, and digestive enzyme activities when compared with the corresponding intestinal segment in unresected control preterm pigs (252) and limited short-term effects of providing enteral diets such as infant formula and bovine colostrum (6). The diminished adaptation in preterm pigs contrasts with the structural and functional adaptation of the remnant distal part of the intestine in newborn term pigs (11, 247) and older suckling pigs (163). This has led us to conclude that there is a need to develop interventions that are specifically tailored to support short- and long-term intestinal adaptation of the proximal bowel in preterm newborns. An attractive goal is to combine the SBS model in preterm pigs with NEC, since this is the most common clinical condition that leads to intestinal resection and SBS. The practical and clinical difficulties in performing resection on piglets with NEC may limit the feasibility of a combined NEC+SBS model (Fig. 2), despite its obvious translational relevance.
PN Related to SBS
PN is a vital component of clinical support in patients with SBS and especially IF; thus is it important for animal models to incorporate this feature. Since the introduction of PN over 40 years ago, it has been known that long-term total PN (TPN) is associated with various complications, including both GIT and liver defects. The clinical challenges in infants are particularly important. This patient population has a high need for nutrients to support growth of essential organs, a unique sensitivity to liver disease, and an immature intestine that is sensitive to maldigestion, dysregulated immunity, inappropriate bacterial colonization, and reduced neuroendocrine functions. Although all these effects may be most important for the pediatric population, Buchman and coworkers (23) showed in healthy adults that 2 wk of TPN decreased small intestinal villus heights and increased permeability, although it did not affect intestinal DNA, protein, or disaccharidase activities. These findings have been confirmed by others although the apparent increase in intestinal permeability does not necessarily lead to increased bacterial translocation (208). Intestinal brush border functions such as hydrolase enzyme activities may be affected by TPN in both adults (89, 106, 265) and children (194), interacting with the underlying disease condition (90).
TPN-related gut dysfunctions.
Animal models such as young rats and pigs require a composition and infusion rate of TPN that differs considerably from that in infants, owing to a much higher natural growth rate (leading to higher demands for lipids, amino acids, and certain micronutrients) and inborn differences in metabolism (e.g., differences in indispensable amino acids). Regardless, the general effects of TPN on the GIT and liver may be similar among rats, pigs, and humans, and much information on TPN effects on various organs originates from animal studies. Neonatal pigs have been especially pivotal in understanding how TPN impacts the neonatal GIT, including the effects on intestinal blood flow, gut morphology, protein and DNA contents, digestive and absorptive capacity, secretion of intestinal growth factors, gut barrier function, intestinal permeability, and NEC risk (18, 33, 126, 168). Corresponding studies in adult rats show a dramatic loss of mucosal mass after TPN because of increased enterocyte apoptosis and reduced proliferation (48). In contrast, the mouse may be less sensitive to TPN-induced mucosal atrophy, similar to healthy human adults (23), and greater atrophy of the intestinal muscularis layers compared with the rat (162). The mouse TPN model has also shown how PN impairs mucosal immune function that may predispose to the TPN-related sepsis in humans (90, 102). Mice on PN show reduced amounts of gut-associated lymphoid tissue and secretory IgA from the GIT and lungs, and an increased mortality after bacterial pneumonia, compared with mice on EN (130).
In addition to the nutritional requirements specifically related to SBS, the provision of PN is especially critical for infants born extremely preterm (<28 wk gestation) who have a high endogenous caloric and nutrient requirement (277) and are at increased risk for TPN-related metabolic bone disease (164). We have found a high TPN dependency also in the smallest preterm pigs (40) although these weak newborns also easily develop PN-related hyperglycemia, azotemia, and fluid overload (201, 232). In term newborn pigs, TPN is associated with GIT atrophy, liver damage, and immune and metabolic dysfunctions (107, 168, 231, 232).
Short-chain fatty acids (SCFAs) in the TPN have been tested in several animal models of both TPN-induced intestinal atrophy and SBS. In intact rats, it has been demonstrated that SCFAs inhibit TPN-induced atrophy and increase ileal protein and DNA contents along with glucose transporters (135, 239, 240). Addition of SCFA to TPN induces intestinal adaptation in both rodent and piglet models of SBS using the standard jejunoileal resection (12, 135, 241, 242), and the effects may be mediated in part via intestinal hormones such as glucagon-like peptide 2 (GLP-2) (238).
Glutamine, a preferred fuel for rapidly dividing cells such as enterocytes and lymphocytes, has been proposed to improve mucosal growth, function, and immunity. Glutamine is not included in parenteral amino acid solutions, although glutamate, the precursor of glutamine, is included in pediatric but not adult amino acid solutions. Glutamine is present in high concentrations in umbilical venous blood, amniotic fluid, and breast milk, consistent with a physiological role for glutamine in fetal development. Thus glutamine may be conditionally essential for infants maintained on prolonged glutamine-free PN after SBS or other surgical GIT diseases (175). On the other hand, parenteral glutamine infusion did not prevent mucosal atrophy in TPN-fed neonatal pigs (29), and studies in rodents (169) and surgical infants (155) showed limited or no effects of supplemental glutamine.
TPN-related liver dysfunction.
A serious comorbidity, associated with long-term PN in IF patients, especially children, is PNALD, also referred to as IF-associated liver disease. The association between TPN and liver disease in premature infants was first reported by Rager and Finegold (187), who documented the presence of intrahepatic cholestasis in 9 of 15 children who received PN. The infants who survived without developing cholestasis were the ones that had tolerated EN within a few days after birth. Manifestations of PNALD cover the whole spectrum of hepatic complications from mild fat infiltration, intracellular and intracanalicular cholestasis, steatosis, and periportal fibrosis to extensive fibrosis and cirrhosis (38, 127). Moreover, PNALD is thought to encompass a developmentally related hypersensitivity of the neonatal liver to signals from the intestine.
The neonatal PN-fed piglet displays characteristics of PNALD that are very close to the human condition (249). In piglets, development of cholestasis may be influenced by inflammation, sepsis, and the use of PN lipids, especially those based on saturated and n-6 vegetable oils from soybeans (66, 124, 212). The recent trends of improved survival in infants with IF are largely because of decreased PNALD incidence resulting from reduced overall lipid intake or use of fish oil-based lipids high in n-3 fatty acids and vitamin E (53, 165, 210, 212). The suggested use of a more balanced fatty acid composition for infants (38, 54, 91) has also been tested in piglets, helping to document the mechanistic aspects of these new lipid emulsions in relation to hepatic dysfunction (28, 103, 107, 251, 253). Also in a rat SBS model, did the use of parenteral, but not enteral, elevated n-3 fatty acid levels induce intestinal adaptation with increased mucosal mass and cell proliferation (233)?
Enteral Nutrition Related to SBS
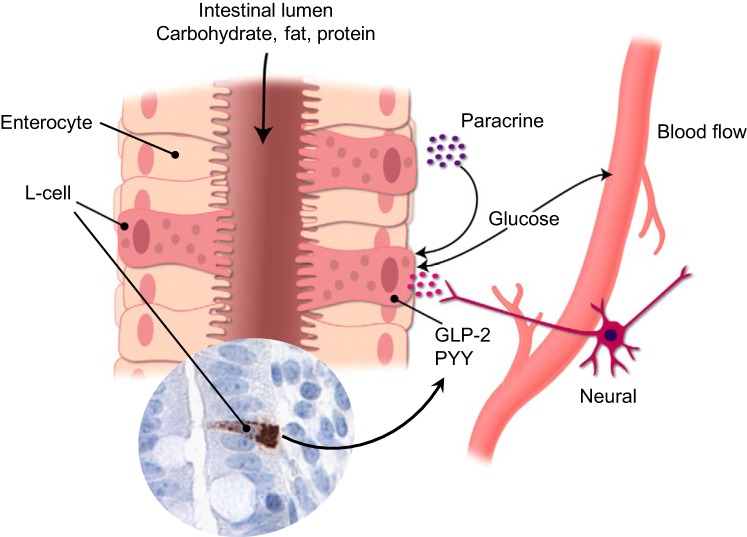
There is universal agreement that enteral feeding is preferred to reduce the detrimental consequences of PN and to meet the high energy and nutrient requirements of preterm infants (84, 277). Enteral nutrients are the primary stimulus for intestinal adaptation by acting directly to provide energy and protein for the enterocytes and to trigger the increase in pancreatic-biliary secretions, neural factors, and intestinal blood flow and release of intestinal hormones (33, 237) (Fig. 3). In recent years, starting enteral feeding from the first day after birth has become a widely accepted method to enhance structural and functional development of the GIT, especially after preterm birth although protocols differ widely (84, 155). In patients with SBS, transition from PN to EN should be made as soon as possible because this will mitigate the risk of PNALD and catheter-related infections (92). However, beyond these initial broad recommendations, there are many areas of controversy in the use of EN in preterm infants and infants following surgical resection. This includes the rate of increase of feeds, the type of EN used, the pattern of feeding (whether as a bolus or continuous drip), the use of specific types of nutrients such as medium- and long-chain fats, polymers, and added pharmacological manipulations. An additional relevant factor is the relationship between all of these potential feeding parameters and the risk of NEC. Typically, preterm infants, both with or without SBS, are fed small volumes of enteral food in addition to PN to induce GIT growth and maturation without overloading the immature and/or resected GIT, an approach known as “minimal enteral nutrition” (MEN). MEN has also been proposed to induce GIT adaptation in SBS preterm infants, but the evidence for a clinical benefit is weak (19, 84). Human milk is the recommended form of EN for all infants, including SBS infants, and this is becoming more widely used with the increasing availability of human milk banks at many hospitals. However, there may be situations in which human milk is not available and formula must be used. The composition of the optimal formula is a matter of controversy and appropriate animal studies will help to clarify this.
Fig. 3.
Schematic overview indicating how nutrient-stimulated release of glucagon-like peptide 2 (GLP-2) from intestinal L cells mediates trophic, anti-inflammatory, and vascular intestinal effects via a combination of endo-, para-, and neurocrine pathways. GLP-2 receptors are present throughout the gut and signal decreased gastric emptying and increased blood flow, cell proliferation, and nutrient uptake.
Models of enteral nutrition for infants.
The preterm pig has been extensively used during the last decade to better define the optimal time (after preterm birth or intestinal resection), amount (minimal or standard amounts), and composition (breast milk, colostrum, or various milk replacers) of MEN and EN diets. Preterm pigs delivered at 90% of gestation require tube feeding because of the immature suckling, swallowing, and breathing abilities (189). The importance of enteral feeding, even for preterm neonates, is illustrated by the marked tropic GIT response to the first enteral food in both preterm and fetal pigs (17, 121, 201), although some functional responses are diminished relative to term pigs [e.g., intestinal motility, immunoglobulin absorption, brush border enzymes, nutrient absorption, permeability (201)]. Natural milk and colostrum products (porcine, bovine, human) exert greater GIT trophic and functional responses in healthy newborn pigs than cow's milk-based formulas (18, 27, 30, 143, 202), whereas the short-term effects of an enteral colostrum diet were limited in newborn SBS pigs (6) (50% distal intestinal resection) or in SBS infants after variable resections (5). Slightly older milk-fed SBS pigs also failed to increase their adaptation after colostrum supplementation (96), whereas 4-wk-old SBS pigs showed a more robust colostrum-induced adaptation response, relative to chow or formula (163). Breast milk has beneficial effects not only to prevent against NEC but also on neonatal SBS, as the number of days SBS infants receive breast milk is inversely correlated with days on PN (4). From studies of piglets with 75% proximal resection and from rats we know that complexity of the diet may improve intestinal adaptation and increase growth (16, 35), but it is difficult to determine whether it is an effect of nutrition per se or an indirect effect (242, 262).
Both animal and human data support careful consideration of EN in neonatal SBS and the effects may be age, diet, and time dependent and depend on the nature of the resection (proximal or distal with or without a functioning colon). Given the complexity of the SBS population in terms of remaining bowel anatomy, pancreatic, liver, and gut endocrine function, each infant will need a unique approach to find the right combination of nutritional and pharmacological strategies to maximize intestinal adaptive capacity. Synergistic effects have been observed for luminal nutrients supplied along with hormonal therapy (GLP-2) to induce intestinal adaptation in a PN-dependent rat model of distal bowel resection (145). In this model, whereas endogenous GLP-2 was not sufficient to promote intestinal growth, exogenous GLP-2 strongly promoted enterocyte proliferation in conjunction with the stimulus provided by luminal nutrients to enhance enterocyte differentiation, as reflected in digestive enzyme maturation. The net effect is increased functional digestive capacity as demonstrated by an 80% reduction in PN with corresponding transition or weaning to EN with an increase in body weight gain of the rats (22).
The ability of EN to promote adaptation may also be indirect and driven by local hormonal release in response to EN and thereby leading to intestinal adaptation. This hypothesis has gained support from rat studies including 0, 50, 75, or 90% proximal resection where meal-stimulated hormonal release correlates closely with the type of resection (150). These findings are in agreement with findings from SBS infants where enteral caloric intake is correlated with weaning from PN (222). Separately, pancreatic and biliary factors may induce intestinal adaptation and be another link between EN and intestinal growth. Bile and pancreatic secretions influence the intestinal morphology in rats (2), and in TPN-fed piglets, enteral bile acids stimulate intestinal growth, probably via endogenous release of intestinal hormones, such as GLP-2 (33, 107).
Optimizing formula composition.
Enteral lipid provides a high-density energy source with low osmolality, which may allow for reduction of PN, and is a potent stimulus for intestinal adaptation after small bowel resection (155). Long-chain triglycerides (LCTs) promote improved bowel adaptation in postsurgical animal models (41, 75, 132, 250), relative to medium-chain triglycerides (MCTs). This is likely because of potent LCT-induced release of enteroendocrine hormones, resulting in delayed proximal bowel transit and stimulation of pancreaticobiliary secretions needed to digest LCT. However, the remnant anatomy impacts the optimal amount and type of fats that are tolerated in human SBS. When there is remnant colon in continuity, MCT is more easily digested than LCT, with improved energy absorption suggesting a colonic absorptive capacity for MCT (114). When there is a remnant colon, provision of a greater intake of complex carbohydrates with subsequent fermentation to SCFAs and lower LCT fat content reduces fat malabsorption and steatorrhea (152). In contrast, higher LCT fat intake improves ostomy output and weight gain in neonates with enterostomies (148). Studies in weanling rats subjected to ileocecal resection demonstrate that n-3 fatty acid-enriched enteral fish oil increases dietary fat absorption, compared with corn oil, without increasing hepatic bile acid synthesis (273).
In addition to the studies on colostrum, studies in SBS rats with jejunal resection suggest that the whey (but not the casein) fraction of normal milk augments the intestinal growth induced by hormonal treatment (144). Both milk and colostrum contain intact proteins, and studies in SBS infants indicate that a semielemental hydrolyzed diet has no advantage over a diet with intact proteins (139). However, in a rat PN model, a semielemental formula containing hydrolyzed whey protein improved mucosal growth in association with reduced intestinal dipeptidyl peptidase IV activity, which prolongs the half-life of bioactive GLP-2 (144). This mechanism of action is supported by the evidence that dipeptidyl peptidase inhibitor administration enhanced intestinal adaptation in a mouse model of SBS (173). These data suggest the potential for hydrolyzed dietary proteins such as whey to reduce the degradation of GLP-2 and thus prolong its ability to stimulate intestinal growth (see also Fig. 3).
Adult SBS patients with a residual colon have the capacity to salvage energy from malabsorbed complex carbohydrates, acting as prebiotics to stimulate microbial fermentation, SCFA production, and GLP-2 secretion. The importance for pediatric SBS patients may be less due to the less-developed state of the colon. Recent studies suggest that an intact ileum is important for GLP-2 secretion, likely contributing to the intestinal adaptation by allowing for a faster transition to EN from PN, as shown by comparison of jejunoileal with jejunocolic anastomosis in piglets (104, 105, 247). Benefits of colonic SCFA in SBS have been indicated from studies in pigs and rats, and the effects include provision of additional energy, increased colonic sodium and water absorption, and stimulation of proglucagon expression with release of GLP-2 (11, 136). Supplementation of prebiotic short-chain fructooligosaccharides into the EN improved intestinal adaptation in piglets as shown by higher ileal mucosa weight, villus height, and epithelial proliferation (11). Soluble fiber such as pectin provides a prebiotic substrate for colonic fermentation and may also improve diarrhea by bulking stool or ostomy effluent consistency. Since prebiotics also promote bacterial proliferation, supplementation may be contraindicated for infants without an intact ileocecal valve because of the increased risk for SIBO (191).
Infants with SBS often have disruption of the normal bacterial colonization of the GIT due to disturbed EN flow and antibiotic therapy and may instead show colonization with a pathogenic microbiota that may increase risk for septicemia due to bacterial translocation. Probiotic supplementation with the aim of directing intestinal colonization toward a healthier colonization, favoring bifidobacteria, lactobacilli, and saccharomyces species offers promise for preterm infants with SBS, as supported by case reports (37, 125). Animal studies show that probiotics decrease bacterial translocation and stimulate mucosal adaptation after SBS (246), and some evidence for changes in the microbiota in the mouse ileocecal resection model suggests that the microbiota is a potential regulator of postsurgical adaptive growth or function (52). Moreover, the ability of inflammation to enhance resection-induced intestinal adaptive growth in IL-10-null mice is dependent on colonization with normal fecal microbiota (224). Given this evidence that both the microbiota and intestinal inflammation augment adaptation of the small bowel after ileocecal resection, additional research is needed to optimize clinical management with respect to antibiotics, immunosuppression drugs, and probiotics.
Growth Factors and SBS
The variability of the adaptive response following different patterns of bowel resection suggests that growth and regulatory factors, especially those produced in the distal gut (ileum and colon), are important in regulating intestinal adaptation. Multiple hormones and trophic agents have been studied and reviewed including GLP-2, growth hormone, insulin-like growth factor, epidermal growth factor, cholecystokinin, gastrin, insulin, neurotensin, and peptide YY. These studies have been done almost exclusively in animal models, so a complete understanding of the effects on the corresponding human condition is lacking. An important research priority is to establish whether the rodent and porcine responses to endogenous release or exogenous supply of hormones after resection also occur in human infants with SBS. This section will focus on some major regulatory and growth factors that have been linked to adaptation in animal models, as shown schematically in Fig. 4. The discovery of the link between intestinal adaptation and secreted gut hormones and local growth factors has prompted investigation into their use therapeutically to promote intestinal adaptation. Numerous studies in animal models have documented beneficial effects of various hormone therapies, yet it has been disappointing that none of these have become routine clinical practice for either adult or pediatric SBS patients. However, the recent approval of the GLP-2 analog teduglutide for treatment of adult SBS in the US and Europe is promising and may pave the way for the pediatric indication as well as additional pharmacological options. This paucity of translation into clinical practice is related to a number of scientific unknowns about short- and long-term physiological effects of hormonal therapies and regulatory issues as well as safety concerns. Thus there continues to be a need to establish the mechanisms, efficacy, and safety of various therapies in animal models.
Fig. 4.
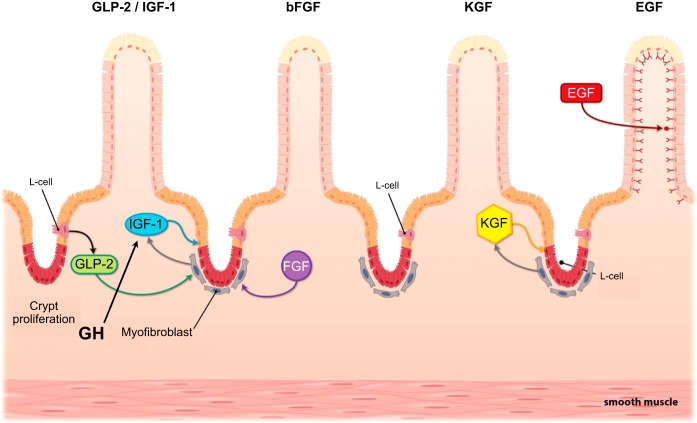
Spontaneous intestinal adaptation following resection depends on the action of intestinal growth factors, and exogenous supply of growth factors may enhance adaptation during SBS. GLP-2 from L cells and growth hormone (GH) from the pituitary may both work via local intestinal release of insulin-like growth factor 1 (IGF-1). The interacting effects of other growth factors, such as keratinocyte growth factor (KGF), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) may exert trophic effects via pathways that are similar to those of IGF-1 and GLP-2.
Glucagon-Like Peptide 2
As indicated above, the enteroendocrine hormone GLP-2 appears to be a key factor in signaling intestinal adaptation. GLP-2 is a 33-amino-acid peptide produced and released together with GLP-1 from the enteroendocrine L cells that are located mainly in the distal small intestine and colon (Fig. 4). Secretion from L cells is stimulated by direct contact with luminal nutrients, especially long-chain fatty acids in the terminal ileum (Fig. 3) (61). There is also evidence for an acute first peak of GLP-2 secretion in association with a proximal-distal neuroendocrine loop involving the enteric nervous system and the afferent and efferent vagus nerves in the rat (62), although evidence in vagotomized pigs does not support this (93). GLP-2 exerts a variety of functions ranging from suppressing gastric emptying and secretion and improving intestinal barrier function to stimulating bowel growth and nutrient absorption (58, 64, 69, 274). This information is based mainly on studies in rodents and pigs where the GLP-2 receptor (GLP-2R) has been described to be located on enteric neurons, vagal afferents, enteroendocrine cells, and subepithelial myofibroblasts (87, 141, 166, 276). The multiple cell types reported for the GLP-2R localization suggest that several signaling pathways are involved in the intestinal trophic actions. One proposed mechanism is that GLP-2 mediates its intestinotrophic effects through the induction of ErbB ligands mimicking the effects of epidermal growth factor (EGF), since ErbB ligands have been shown to be required for the GLP-2 effects and ErbB ligands are linked to the GLP-2-mediated intestinal adaptation following feeding (8, 50, 274, 275). Another mechanism has implicated the generation of local insulin-like growth factor-1 (IGF-1) secretion from the subepithelial myofibroblasts (63, 141) that induces proliferation through interaction with β-catenin (64) (Fig. 4). In support of this theory it has been shown that functional epithelial IGF-1 receptors (IGF-1R) are essential for the proliferative actions of GLP-2 (196).
In animals and human patients subjected to proximal intestinal resection, circulating GLP-2 levels increase and are highly correlated with spontaneous adaptation, suggesting that GLP-2 is important in initiating and maintaining spontaneous adaptation (113, 151, 178, 217, 247). Exogenous GLP-2 to animals increases nutrient absorption by 20–100%, depending on the length of residual bowel (145, 203, 207, 252). In adult human studies, GLP-2 or its analogs have been shown to have modest effects on nutrient and fluid absorption, with a mean 20% reduction in parenteral fluid dependence (108); however, the effects are dependent on continued administration of the hormone. It is not clear what the effects of exogenous GLP-2, even for short periods, may have on the long-term function of the intestine in the neonate. Given the high levels of endogenous production during the neonatal period, it is likely that GLP-2 may act to induce long-term changes in intestinal function during this developmental phase. The GLP-2 secretion response to feeding seems to be more robust in infants than adults. Basal levels are relatively high in preterm infants without meal stimulation, suggesting an elevated trophic tone in inducing gut growth in the final weeks of gestation (3). In intact enterally fed premature infants, plasma GLP-2 levels are high (up to 450 pM/l), and in SBS infants levels correlate positively with residual small intestinal length and markers of intestinal absorption (217, 220). Importantly, infants without a remnant ileum had very low levels of GLP-2, even if the colon was intact, and infants with GLP levels <15 pM/l and <40 kcal/kg intake of enteral diet died of their SBS complications (217). Also in infants with gastroschisis there is a clear association between GLP-2 levels and tolerance to EN (223), and this may partly explain the prolonged intestinal dysfunction seen in this patient population.
In relation to PN and SBS, GLP-2 induces intestinal growth in rats and also increases glucose transporters in the residual intestine (151). Recently it has been seen in mice that intestinal resection can induce intestinal stem cell expansion, and this expansion can be dramatically augmented after immediate postsurgical GLP-2 treatment (51, 76). In a recent study in preterm pigs, 50% distal intestinal resection combined with a jejunostomy resulted in low GLP-2 levels (252) (see Fig. 2) equivalent to the levels seen in infants without a remnant ileum (217). Daily infusions of GLP-2 with PN markedly increased GLP-2 levels (from 15 to 3,000 pM) triggered a dramatic adaptation of both structural and functional intestinal indexes with increased nutrient absorption within the first week of resection. In a similar study in term neonatal SBS pigs, using once daily injections of the GLP-2 analog teduglutide, the short-term adaptation was more moderate (245), probably because of lower overall exposure of GLP-2. Similarly, exogenous GLP-2 to newborn term pigs, subjected to 75% distal resection with anastomosis to the colon, showed improved clinical outcomes, as reflected by fewer days on PN and a higher level of calories tolerated enterally (234).
From intact neonatal pigs, it is indicated that the early perinatal GIT growth and maturation are mediated by GLP-2 (184) and GLP-2 increases intestinal growth in a dose-dependent manner, likely by decreasing apoptosis and proteolysis (26, 31, 32, 34, 245). GLP-2 is also known to stimulate functional indexes in neonates, where GLP-2 is known to induce brush border enzymes (183, 252) and nutrient absorption (44, 203), although the response may depend on gestational age at birth (182, 200). Furthermore, GLP-2 has been shown to increase intestinal blood flow in piglets through nitric oxide synthase (87, 88) and these effects are also supported by data from adult SBS patients (21). Stimulating endogenous GLP-2 secretion through the use of parenteral SCFA has shown promising potential to stimulate mucosal growth in neonatal pigs (12). In pigs, GLP-2 responsiveness may decrease during the postnatal period, considering the contradictory reports of GLP-2 administration to juvenile pigs (105, 178, 213, 244).
Growth hormone and insulin-like growth factor.
Growth hormone is an anabolic hormone responsible for growth through activation of local IGF-1 production in several tissues, including the intestine (Fig. 4). Exogenous GH induces mucosal growth and improves weight gain in rodent SBS models with jejunoileal resection (86, 259), although it fails to improve weight gain in pigs with SBS (13). Exogenous GH increases body weight and plasma IGF-I but fails to prevent TPN-induced mucosal atrophy in rats in association with GH stimulating a suppressor of cytokine signaling, SOCS-2, which acts to inhibit intestinal epithelial cell proliferation and intestinal growth (156). GH has also been tested in human SBS patients and some initial data are promising for both infants (140) and adults (209), leading to recent FDA approval (154). Regardless, some studies on GH administration to adult SBS patients have been disappointing and have shown a high incidence of adverse effects (120, 235). Thus it remains to be seen how continued administration of GH (and/or of other growth factors) can be best used to support weaning from TPN in both humans and animal models (83, 181).
IGF-1 has long been known to have trophic effects throughout the body via both local (paracrine) and systemic (endocrine) actions, combined with the regulation of IGF-1 receptors and IGF-1 binding proteins (147, 159). Although the intestinal mucosa is highly responsive to exogenous IGF-1 treatment, this is not likely to be a suitable therapy for SBS patients because of the widespread actions of systemic therapy, especially in the growing infant. Another concern is that IGF-1 has been linked to cancer development. On the other hand, there may be instances in which a patient is growth restricted because of a primary or secondary reduction in GH or IGF-1, in addition to the nutritional restriction of SBS, so that exogenous supplementation of either GH or IGF-1 would be therapeutic for both problems. Systemic GH administration has modest effect on fluid absorption (and probably no effect on energy absorption) in adult SBS patients and a specific formulation is licensed for this population (36). The likely trophic mechanism of GH action in the intestine is via increased circulating IGF-1 (from hepatic secretion) and local paracrine production. Small studies on GH administration to PN-dependent pediatric SBS patients indicated that 3 mo of treatment enhanced weaning from PN and increased plasma citrulline levels (a marker of mucosal mass), but a year later only 25% of the patients remained off PN (83). Another study indicated that the modest benefits obtained during GH treatment were lost after GH discontinuation and that treatment effects might be caused by GH-induced hyperphagia (181).
In mice experiencing TPN-induced intestinal atrophy, exogenous IGF-1 increases intestinal mass, DNA, and protein contents (161, 162), partly dependent on the level of IGF-binding proteins (160). In rodent SBS models, IGF-1 induced intestinal growth through an increase in proliferation in the remnant jejunum (49, 268). This finding is supported by findings in transgenic mice that overexpress IGF-1, which show a significant postresection increase in mucosal surface area (131). Despite that IGF-1 does not show a positive effect on digestive enzyme activity, the overall effects to increase jejunal adaptive growth may aid in the transition from PN to EN (77, 78). No human studies have used IGF-1 for treating SBS in infants, likely because of the rather small effects reported in most of the above mentioned animal studies.
Epidermal growth factor.
EGF is a member of the EGF family of ligands in which EGF and TGF-α are the active ligands in vivo (10). EGF is produced by the salivary and Brunner's glands and has been shown to be important in maintaining epithelial tissues, and inducing mucosal trophic actions. The EGF receptor appears to be localized to the basolateral side of the epithelial mucosa (Fig. 4), thus access (and activity) of the ligand may be increased in the states of mucosal damage or the increased permeability that occurs in SBS (229). There is a limited experience with the use of exogenous enteral EGF clinically in SBS. A pilot study in human SBS infants indicated that EGF had modest benefits in improving active glucose absorption and enteral food tolerance, and it possibly reduced bacterial translocation, without any adverse effects (219).
Mouse studies with knockout animals have shown convincing evidence that EGF is involved in the normal, postresection adaptive response (229, 258), and exogenous EGF treatment improves ileal mucosal morphology and upregulates proliferation and reduces apoptosis. An EGF receptor (EGFR, ErbB1) appears to be crucial for this response, since the response is diminished in mice with a reduced EGFR protein tyrosine kinase activity (98, 100). In addition, mice subjected to a partial enterectomy and overexpressing EGF show increased mucosal protein and DNA concentration and improved morphology (68). As noted above, GLP-2 increases the production of some of the important EGF ligands and receptors; thus the EGF and GLP-2 pathways may be closely related (274).
Keratinocyte growth factor.
Keratinocyte growth factor (KGF), or basic fibroblast growth factor 7, is a 163-amino-acid peptide secreted primarily by fibroblast-like stromal cells, and it functions as a potent epithelial cell growth factor and likely has a mechanistic pathway that is similar to IGF-1 (Fig. 4). KGF is produced by the pericryptal myofibroblast and acts to stimulate cell division within the crypt epithelium (73). KGF may also act to alter the differentiation of dividing cells and so alter the phenotype of the mucosal cells (269). In mice, KGF prevents TPN-induced mucosal atrophy by decreasing apoptosis and increasing proliferation (270), and also in rodent SBS models, KGF stimulates mucosal growth and epithelial function (122, 266, 271). KGF also has the ability to induce goblet cell expansion and upregulate trefoil peptides (259), which are implicated in repair of intestinal damage. Comparative studies of KGF, GH, and GLP-2 in SBS rats showed that KGF was less potent than GLP-2 and was primarily active within the colon, where it specifically increased the production of goblet cells and mucosal protective factors (259). There may be an overlap with the upstream effects of GLP-2, which has been shown to stimulate the production of KGF within the colon (176). Although KGF is a potential useful component for gut protection for conditions such as chemotherapy-induced mucositis, it is less likely to find use in the therapy of SBS patients (72).
Optimal SBS Models Considering Biological and Technical Constraints
The choice of the most appropriate animal model for the study of SBS depends on the research question and the clinical context being addressed (see Table 1). A major advantage of the mouse model of SBS is the ability to use genetic models involving transgenic and targeted global and tissue-specific knockout mice to establish the mechanistic role of specific genes and signaling pathways (195). On the other hand, the technical challenges related to surgical procedures in mice are an important limitation to the mouse SBS model. The adult rat model of SBS allows PN support and most of the anatomical surgical preparations are feasible, except the jejunostomy (Table 1). The use of PN support in weanling rats is likely also possible given reports of TPN in mice of similar body weight (67, 157). The weanling rat reflects an immature stage of intestinal development but it probably does not model the human preterm infant as well as the newborn preterm or term pig. Rats and mice have the advantages of easy handling, relatively low cost and space requirements, and fewer ethical constraints compared with pigs.
The main advantage of the pig is the translational relevance for human SBS conditions. The piglet is likely a better model than young or adult rodents, especially for neonates, which represent the largest population of the human SBS patients. The translational relevance relates to the body size and the GIT anatomy and physiology in piglets. The available evidence shows that these aspects, even after resection, more closely resemble the human condition than other animal models. The pig more easily allows study of different anatomical situations that approximate the most difficult clinical cases, e.g., those with either a high jejunal stoma or a jejunocolonic anastomosis (7, 247, 252). In these models, piglets enable the provision of PN and EN support and allow study of the responses in newborn, or even preterm animals, for a period after resection, using body growth and intestinal absorptive function as markers of adaptation. The experimental procedures remain technically difficult to manage with newborn pigs, and as yet no research groups have studied intestinal adaptation in preterm or newborn SBS pigs for longer time periods (>2 wk). It has also been difficult to combine the induced SBS condition with a relevant underlying disease, such as NEC, or with ancillary therapies, such as the STEP procedure, after a period of small bowel dilatation. In principle, such combined clinically relevant disease-surgery-therapy procedures are possible in piglets, but they will require a large additional effort to develop, and experimental difficulties may limit the added translational value.
The main disadvantage of the piglet models is the high cost and large animal facility requirements for housing and surgery, especially when pregnant sows are used for caesarean section delivery of preterm and newborn pigs and when PN and EN are required for clinical support. The use of conventional-production pigs is less expensive than various breeds of miniature pigs (e.g., Gottingen, Sinclair, Yucatan, Hanford), but the larger body size at adolescent ages (between weaning and puberty) can offset this cost advantage. Whereas the new studies in neonatal SBS pigs reflect the initial postresection intestinal response to SBS, the earlier studies in weanling pigs and young rats have often documented long-term adaptation responses. It is important to relate the intestinal adaptation response after SBS to the time after intestinal resection since it is known that the physiological characteristics of short-term adaptation may differ widely from the long-term responses. Thus studies in young pigs demonstrated that intestinal proliferation 2 wk after resection was not maintained 6 wk after resection (180). The differential adaptation responses after different ages at resection (preterm, term, juvenile, adult) and different lengths of the postresection period require further study. The inability to make serial sampling of intestinal tissue from the same animals is an important limitation to clarify the temporal events and mechanisms of the adaptation process. In principle, temporal collection of tissue by endoscopic biopsy from SBS piglets fitted with a jejunostomy is feasible but technically demanding.
Future Opportunities and Challenges
The last two decades have seen demonstrable progress in our understanding of the underlying mechanisms of intestinal adaptation, clinical support, and treatment of SBS. Many of these advancements have been achieved through the use of animal models, mainly rodents and pigs. One of the most promising developments for SBS and IF patients is the approval of the GLP-2 analog teduglutide for treatment in adult SBS patients. This milestone culminates 20 years of research and development since the original report on the effects of this peptide by Drucker, Brubaker, and colleagues (60). Other important advances include a more detailed understanding of the cellular growth factor pathways and signaling mechanisms that mediate the dominant trophic effect of EN and endogenous gastrointestinal secretions on the intestinal adaptive response after resection. Although most of the mechanistic findings were derived from rodents, the use of mouse genetics to address fundamental questions of intestinal adaptation and functional outcomes has only just begun and has been confined to laboratories with the requisite surgical expertise to perform these challenging procedures. Thus the rapid advancements in mouse genetic models, such as transgenic overexpression, global knockout, and conditional tissue-specific and inducible knockout mice represent a substantial opportunity for future research on mechanisms of SBS. The technical capacity to generate transgenic rat and pig models is rapidly emerging and holds promise to gain mechanistic insights regarding the physiological regulation of the underlying processes that globally result in intestinal adaptation. The use of targeted genetic models of rats and pigs will enable researchers to probe the underlying mechanisms that explain the unique features of adaptation related to presence of colon, PN, and prematurity that are challenging, if not impossible in mice.
With the recent development of the preterm and term piglet models of SBS, another important question and challenge for the future is to test whether the intestinal adaptive response after bowel resection is affected by postnatal age and time after resection. This is a key priority given that most SBS and IF cases are pediatric patients and that normal organ development will likely affect spontaneous intestinal adaptation and the response to dietary and medical interventions. In an infant SBS model, normal somatic growth and functional development is an important clinical outcome, and the piglet SBS model provides an opportunity to connect intestinal adaptation with indexes of growth and longer term development. The pig model of resection with jejunostomy suggests evidence for deficiency in GLP-2, even with EN support, which should allow for studies to test the type of trophic stimulus early after surgical resection, and how and when later reanastomosis impacts long-term adaptation and growth. The ability to maintain preterm and term pigs on PN with jejunostomy allows more detailed investigation of how the absence of luminal nutrients in continuity with the distal intestine impacts adaptation. It is possible that such intestinal adaptation is affected by the nature of the disease leading to resection, and here the high NEC sensitivity of the preterm pig opens another unexplored path of experimental studies, combining NEC and SBS studies in preterm pigs and studying both short- and long-term outcomes.
Two emerging areas of biology that promise to be important for future SBS research are stem cell biology and the role of the gut microbiome (51, 76, 193). Intestinal adaptation following surgical resection leads to an upregulation of stem cell populations, and the possible time and microbe dependency of this response may have implications for the optimal timing of nutritional and other therapeutic interventions. The gut microbiome responses may be important for intestinal adaptation in SBS patients from a fundamental host-microbiome perspective, but also because gut bacterial overgrowth, with or without previous antibiotic therapy, is a common clinical observation in human SBS patients. Stem cell research is likely to impact SBS research and facilitate regenerative medicine approaches and autologous replacement of cells and tissue to expand the functional capacity of the intestine (226). An important element in this area is the development of intestinal organoids and enteroid models. The use of these models is rapidly expanding to characterize the underlying biology of how stem cell genetics, extracellular matrix, and microenvironment influence cell growth and tissue architecture. There is progress in this area already in both rodent and pig models; thus combination of this approach with surgical resection may lead to a regenerative therapy of SBS in the future.
In conclusion, rodent models will continue to provide important basic knowledge about intestinal adaptation whereas results from piglets could help to identify more clinically relevant therapies to support infants in their difficult recovery from SBS. Until now, few experimental approaches have reached clinical practice, and there is great need to better define the age, time, and disease specificity of nutritional, medical, and surgical interventions in both patients and animal models. This is a complex task that requires close collaboration between clinical practice and experimental model research to ask the most relevant questions. This scientific interaction will be key to continuously refining the existing animal models of SBS with the aim to improve the clinical care for pediatric patients with SBS.
GRANTS
The work was supported in part by the UNIK program at University of Copenhagen, NEOMUNE grant from the Danish Research Councils, federal funds from the USDA Agricultural Research Service under Cooperative Agreement Number 58-6250-6-001, National Institutes of Health Grants DK-094616 and DK-042835, the Texas Medical Center Digestive Diseases Center (National Institutes of Health Grant P30 DK-56338), Alberta Children's Hospital Research Foundation, and Sidra Medical and Research Centre Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.T.S., D.M.N., D.L.S., A.V., and D.G.B. interpreted results of experiments; P.T.S., D.M.N., D.L.S., A.V., and D.G.B. prepared figures; P.T.S., D.M.N., D.L.S., A.V., and D.G.B. drafted manuscript; P.T.S., D.M.N., D.L.S., A.V., and D.G.B. edited and revised manuscript; P.T.S., D.M.N., D.L.S., A.V., and D.G.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our many colleagues that have been central to perform the studies cited in this review.
REFERENCES
- 1.Almond SL, Haveliwala Z, Khalil B, Morabito A. Autologous intestinal reconstructive surgery to reduce bowel dilatation improves intestinal adaptation in children with short bowel syndrome. J Pediatr Gastroenterol Nutr 56: 631–634, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Altmann GG. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. Am J Anat 132: 167–177, 1971. [DOI] [PubMed] [Google Scholar]
- 3.Amin H, Holst JJ, Hartmann B, Wallace L, Wright J, Sigalet DL. Functional ontogeny of the proglucagon-derived peptide axis in the premature human neonate. Pediatrics 121: e180–e186, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Andorsky DJ, Lund DP, Lillehei CW, Jaksic T, DiCanzio J, Richardson DS, Collier SB, Lo C, Duggan C. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr 139: 27–33, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Aunsholt L, Jeppesen PB, Lund P, Sangild PT, Ifaoui IB, Qvist N, Husby S. Bovine colostrum to children with short bowel syndrome: a randomized, double-blind, crossover pilot study. JPEN J Parenter Enteral Nutr 38: 99–106, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Aunsholt L, Stoll B, Vegge A, Qvist N, Eriksen T, Burrin DG, Sangild PT, Thymann T. Effect of bovine colostrum on adaptation after intestinal resection in newborn pigs and human infants. Proc 41st of Eur Soc Pediatr Gastroent Hep Nutr London 2013, p. 1577P. [Google Scholar]
- 7.Aunsholt L, Thymann T, Qvist N, Sigalet DL, Husby S, Sangild PT. Prematurity reduces adaptation to intestinal resection in piglets. JPEN J Parenter Enteral Nutr. 2014. March 31 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138: 2447–2456, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Bailly-Botuha C, Colomb V, Thioulouse E, Berthe MC, Garcette K, Dubern B, Goulet O, Couderc R, Girardet JP. Plasma citrulline concentration reflects enterocyte mass in children with short bowel syndrome. Pediatr Res 65: 559–563, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology 108: 564–580, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Barnes JL, Hartmann B, Holst JJ, Tappenden KA. Intestinal adaptation is stimulated by partial enteral nutrition supplemented with the prebiotic short-chain fructooligosaccharide in a neonatal intestinal failure piglet model. JPEN J Parenter Enteral Nutr 36: 524–537, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr 28: 210–222; discussion 222–213, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Benhamou PH, Canarelli JP, Richard S, Cordonnier C, Postel JP, Grenier E, Leke A, Dupont C. Human recombinant growth hormone increases small bowel lengthening after massive small bowel resection in piglets. J Pediatr Surg 32: 1332–1336, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi A. Intestinal loop lengthening—a technique for increasing small intestinal length. J Pediatr Surg 15: 145–151, 1980. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. J R Soc Med 90: 429–432, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bines JE, Taylor RG, Justice F, Paris MC, Sourial M, Nagy E, Emselle S, Catto-Smith AG, Fuller PJ. Influence of diet complexity on intestinal adaptation following massive small bowel resection in a preclinical model. J Gastroenterol Hepatol 17: 1170–1179, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Bjornvad CR, Schmidt M, Petersen YM, Jensen SK, Offenberg H, Elnif J, Sangild PT. Preterm birth makes the immature intestine sensitive to feeding-induced intestinal atrophy. Am J Physiol Regul Integr Comp Physiol 289: R1212–R1222, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Bjornvad CR, Thymann T, Deutz NE, Burrin DG, Jensen SK, Jensen BB, Molbak L, Boye M, Larsson LI, Schmidt M, Michaelsen KF, Sangild PT. Enteral feeding induces diet-dependent mucosal dysfunction, bacterial proliferation, and necrotizing enterocolitis in preterm pigs on parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 295: G1092–G1103, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Bombell S, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev: CD001970, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Bonnard A, Staub G, Segura JF, Malbezin S, Dorgeret S, Aigrain Y, de Lagausie P. Evaluation of intestinal absorption after longitudinal intestinal lengthening for short bowel syndrome. J Pediatr Surg 40: 1587–1591, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Bremholm L, Hornum M, Andersen UB, Hartmann B, Holst JJ, Jeppesen PB. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept 168: 32–38, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman AS, Murali SG, Hitt S, Solverson PM, Holst JJ, Ney DM. Enteral nutrients potentiate glucagon-like peptide-2 action and reduce dependence on parenteral nutrition in a rat model of human intestinal failure. Am J Physiol Gastrointest Liver Physiol 303: G610–G622, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr 19: 453–460, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 124: 1111–1134, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Buie WD, Thurston OG, vanAerde JE, Aherne FX, Thomson AB, Fedorak RN. Jejunum is preferable for construction of a Bianchi bowel-lengthening procedure in swine short bowel. J Pediatr Surg 28: 102–109, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Burrin D, Guan X, Stoll B, Petersen YM, Sangild PT. Glucagon-like peptide 2: a key link between nutrition and intestinal adaptation in neonates? J Nutr 133: 3712–3716, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37: 593–599, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Burrin DG, Ng K, Stoll B, De Pipaon MS. Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr 5: 82–91, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burrin DG, Shulman RJ, Langston C, Storm MC. Supplemental alanylglutamine, organ growth, and nitrogen metabolism in neonatal pigs fed by total parenteral nutrition. JPEN J Parenter Enteral Nutr 18: 313–319, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Burrin DG, Shulman RJ, Reeds PJ, Davis TA, Gravitt KR. Porcine colostrum and milk stimulate visceral organ and skeletal muscle protein synthesis in neonatal piglets. J Nutr 122: 1205–1213, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab 292: E281–E291, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH, Jr, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr 71: 1603–1610, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Burrin DG, Stoll B, Jiang R, Petersen Y, Elnif J, Buddington RK, Schmidt M, Holst JJ, Hartmann B, Sangild PT. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol 279: G1249–G1256, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Buts JP, Morin CL, Ling V. Influence of dietary components on intestinal adaptation after small bowel resection in rats. Clin Invest Med 2: 59–66, 1979. [PubMed] [Google Scholar]
- 36.Byrne TA, Persinger RL, Young LS, Ziegler TR, Wilmore DW. A new treatment for patients with short-bowel syndrome. Growth hormone, glutamine, and a modified diet. Ann Surg 222: 243–254; discussion 254–245, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candy DC, Densham L, Lamont LS, Greig M, Lewis J, Bennett H, Griffiths M. Effect of administration of Lactobacillus casei shirota on sodium balance in an infant with short bowel syndrome. J Pediatr Gastroenterol Nutr 32: 506–508, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol 4: 277–287, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Chang RW, Javid PJ, Oh JT, Andreoli S, Kim HB, Fauza D, Jaksic T. Serial transverse enteroplasty enhances intestinal function in a model of short bowel syndrome. Ann Surg 243: 223–228, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Che L, Thymann T, Bering SB, Le Huërou-Luron I, D'Inca R, Zhang K, Sangild PT. IUGR does not predispose to necrotizing enterocolitis or compromise postnatal intestinal adaptation in preterm pigs. Pediatr Res 67: 54–59, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Chen WJ, Yang CL, Lai HS, Chen KM. Effects of lipids on intestinal adaptation following 60% resection in rats. J Surg Res 58: 253–259, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ; Eunice Kennedy Shriver NICHD Neonatal Research Network. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 122: e573–e582, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooke RJ, Paule C, Ruckman K. Nutrient balance in the preterm infant. 3. Effect of balance duration on outcome measurements. J Pediatr Gastroenterol Nutr 8: 355–358, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Cottrell JJ, Stoll B, Buddington RK, Stephens JE, Cui L, Chang X, Burrin DG. Glucagon-like peptide-2 protects against TPN-induced intestinal hexose malabsorption in enterally refed piglets. Am J Physiol Gastrointest Liver Physiol 290: G293–G300, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 27: 328–339, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124: 1210–1219, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, Ney DM. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol 284: G670–G682, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr 132: 2010–2014, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol 285: R800–R808, 2003. [DOI] [PubMed] [Google Scholar]
- 50.de Heuvel E, Wallace L, Sharkey KA, Sigalet DL. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-γ signaling. Am J Physiol Endocrinol Metab 303: E994–E1005, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol 293: G1013–G1022, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Devine AA, Gonzalez A, Speck KE, Knight R, Helmrath M, Lund PK, Azcarate-Peril MA. Impact of ileocecal resection and concomitant antibiotics on the microbiome of the murine jejunum and colon. PLoS One 8: e73140, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diamond IR, de Silva NT, Tomlinson GA, Pencharz PB, Feldman BM, Moore AM, Ling SC, Wales PW. The role of parenteral lipids in the development of advanced intestinal failure-associated liver disease in infants: a multiple-variable analysis. JPEN J Parenter Enteral Nutr 35: 596–602, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr 48: 209–215, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Diamond IR, Struijs MC, de Silva NT, Wales PW. Does the colon play a role in intestinal adaptation in infants with short bowel syndrome? A multiple variable analysis. J Pediatr Surg 45: 975–979, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Dicken BJ, Sergi C, Rescorla FJ, Breckler F, Sigalet D. Medical management of motility disorders in patients with intestinal failure: a focus on necrotizing enterocolitis, gastroschisis, and intestinal atresia. J Pediatr Surg 46: 1618–1630, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Digalakis M, Papamichail M, Glava C, Grammatoglou X, Sergentanis TN, Papalois A, Bramis J. Interposition of a reversed jejunal segment enhances intestinal adaptation in short bowel syndrome: an experimental study on pigs. J Surg Res 171: 551–557, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Dong CX, Zhao W, Solomon C, Rowland KJ, Ackerley C, Robine S, Holzenberger M, Gonska T, Brubaker PL. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology 155: 370–379, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Downard CD, Renaud E, St Peter SD, Abdullah F, Islam S, Saito JM, Blakely ML, Huang EY, Arca MJ, Cassidy L, Aspelund G; 2002 American Pediatric Surgical Association Outcomes Clinical Trials Committee. Treatment of necrotizing enterocolitis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg 47: 2111–2122, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 93: 7911–7916, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like Peptide-2. Annu Rev Physiol 76: 561–583, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Dube PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm Metab Res 36: 755–760, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131: 589–605, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Dube PE, Rowland KJ, Brubaker PL. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149: 291–301, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Duggan C, Piper H, Javid PJ, Valim C, Collier S, Kim HB, Jaksic T. Growth and nutritional status in infants with short-bowel syndrome after the serial transverse enteroplasty procedure. Clin Gastroenterol Hepatol 4: 1237–1241, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Duro D, Mitchell PD, Kalish LA, Martin C, McCarthy M, Jaksic T, Dunn J, Brandt ML, Nobuhara KK, Sylvester KG, Moss RL, Duggan C. Risk factors for parenteral nutrition-associated liver disease following surgical therapy for necrotizing enterocolitis: a Glaser Pediatric Research Network Study [corrected]. J Pediatr Gastroenterol Nutr 52: 595–600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Kasmi KC, Anderson AL, Devereaux MW, Fillon SA, Harris JK, Lovell MA, Finegold MJ, Sokol RJ. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 55: 1518–1528, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erwin CR, Helmrath MA, Shin CE, Falcone RA, Jr, Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol Gastrointest Liver Physiol 277: G533–G540, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr 26: 391–411, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Feldman EJ, Dowling RH, McNaughton J, Peters TJ. Effects of oral versus intravenous nutrition on intestinal adaptation after small bowel resection in the dog. Gastroenterology 70: 712–719, 1976. [PubMed] [Google Scholar]
- 71.Figueroa-Colon R, Harris PR, Birdsong E, Franklin FA, Georgeson KE. Impact of intestinal lengthening on the nutritional outcome for children with short bowel syndrome. J Pediatr Surg 31: 912–916, 1996. [DOI] [PubMed] [Google Scholar]
- 72.Finch PW, Rubin JS. Keratinocyte growth factor expression and activity in cancer: implications for use in patients with solid tumors. J Natl Cancer Inst 98: 812–824, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res 91: 69–136, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Fitzgibbons S, Ching YA, Valim C, Zhou J, Iglesias J, Duggan C, Jaksic T. Relationship between serum citrulline levels and progression to parenteral nutrition independence in children with short bowel syndrome. J Pediatr Surg 44: 928–932, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galluser M, Czernichow B, Dreyfus H, Gosse F, Guerold B, Kachelhoffer J, Doffoel M, Raul F. Comparison of different lipid substrates on intestinal adaptation in the rat. Gut 34: 1069–1074, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garrison AP, Dekaney CM, von Allmen DC, Lund PK, Henning SJ, Helmrath MA. Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. Am J Physiol Gastrointest Liver Physiol 296: G643–G650, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillingham MB, Dahly EM, Carey HV, Clark MD, Kritsch KR, Ney DM. Differential jejunal and colonic adaptation due to resection and IGF-I in parenterally fed rats. Am J Physiol Gastrointest Liver Physiol 278: G700–G709, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Gillingham MB, Dahly EM, Murali SG, Ney DM. IGF-I treatment facilitates transition from parenteral to enteral nutrition in rats with short bowel syndrome. Am J Physiol Regul Integr Comp Physiol 284: R363–R371, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Gillingham MB, Kritsch KR, Murali SG, Lund PK, Ney DM. Resection upregulates the IGF-I system of parenterally fed rats with jejunocolic anastomosis. Am J Physiol Gastrointest Liver Physiol 281: G1158–G1168, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, Magness ST. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One 8: e66465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goulet O, Baglin-Gobet S, Talbotec C, Fourcade L, Colomb V, Sauvat F, Jais JP, Michel JL, Jan D, Ricour C. Outcome and long-term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg 15: 95–101, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Goulet O, Colomb-Jung V, Joly F. Role of the colon in short bowel syndrome and intestinal transplantation. J Pediatr Gastroenterol Nutr 48, Suppl 2: S66–S71, 2009. [DOI] [PubMed] [Google Scholar]
- 83.Goulet O, Dabbas-Tyan M, Talbotec C, Kapel N, Rosilio M, Souberbielle JC, Corriol O, Ricour C, Colomb V. Effect of recombinant human growth hormone on intestinal absorption and body composition in children with short bowel syndrome. JPEN J Parenter Enteral Nutr 34: 513–520, 2010. [DOI] [PubMed] [Google Scholar]
- 84.Goulet O, Olieman J, Ksiazyk J, Spolidoro J, Tibboe D, Kohler H, Yagci RV, Falconer J, Grimble G, Beattie RM. Neonatal short bowel syndrome as a model of intestinal failure: physiological background for enteral feeding. Clin Nutr 32: 162–171, 2013. [DOI] [PubMed] [Google Scholar]
- 85.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol 298: G590–G600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu Y, Wu ZH, Xie JX, Jin DY, Zhuo HC. Effects of growth hormone (rhGH) and glutamine supplemented parenteral nutrition on intestinal adaptation in short bowel rats. Clin Nutr 20: 159–166, 2001. [DOI] [PubMed] [Google Scholar]
- 87.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150–164, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology 125: 136–147, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Guedon C, Schmitz J, Lerebours E, Metayer J, Audran E, Hemet J, Colin R. Decreased brush border hydrolase activities without gross morphologic changes in human intestinal mucosa after prolonged total parenteral nutrition of adults. Gastroenterology 90: 373–378, 1986. [DOI] [PubMed] [Google Scholar]
- 90.Guglielmi FW, Boggio-Bertinet D, Federico A, Forte GB, Guglielmi A, Loguercio C, Mazzuoli S, Merli M, Palmo A, Panella C, Pironi L, Francavilla A. Total parenteral nutrition-related gastroenterological complications. Dig Liver Dis 38: 623–642, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, Puder M. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics 118: e197–e201, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Gutierrez IM, Kang KH, Jaksic T. Neonatal short bowel syndrome. Semin Fetal Neonatal Med 16: 157–163, 2011. [DOI] [PubMed] [Google Scholar]
- 93.Hansen L, Lampert S, Mineo H, Holst JJ. Neural regulation of glucagon-like peptide-1 secretion in pigs. Am J Physiol Endocrinol Metab 287: E939–E947, 2004. [DOI] [PubMed] [Google Scholar]
- 94.Hanson WR, Osborne JW, Sharp JG. Compensation by the residual intestine after intestinal resection in the rat. I. Influence of amount of tissue removed. Gastroenterology 72: 692–700, 1977. [PubMed] [Google Scholar]
- 95.Healey KL, Bines JE, Thomas SL, Wilson G, Taylor RG, Sourial M, Pereira-Fantini PM. Morphological and functional changes in the colon after massive small bowel resection. J Pediatr Surg 45: 1581–1590, 2010. [DOI] [PubMed] [Google Scholar]
- 96.Heemskerk VH, van Heurn LW, Farla P, Buurman WA, Piersma F, ter Riet G, Heineman E. Effect of IGF-rich colostrum on bowel adaptation in neonatal piglets with short bowel syndrome. J Pediatr Gastroenterol Nutr 34: 47–51, 2002. [DOI] [PubMed] [Google Scholar]
- 97.Heemskerk VH, van Heurn LW, Farla P, Buurman WA, Piersma F, ter Riet G, Heineman E. A successful short-bowel syndrome model in neonatal piglets. J Pediatr Gastroenterol Nutr 29: 457–461, 1999. [DOI] [PubMed] [Google Scholar]
- 98.Helmrath MA, Erwin CR, Warner BW. A defective EGF-receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res 69: 76–80, 1997. [DOI] [PubMed] [Google Scholar]
- 99.Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol 292: G215–G222, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Helmrath MA, Shin CE, Erwin CR, Warner BW. The EGF\EGF-receptor axis modulates enterocyte apoptosis during intestinal adaptation. J Surg Res 77: 17–22, 1998. [DOI] [PubMed] [Google Scholar]
- 101.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 183: 441–449, 1996. [PubMed] [Google Scholar]
- 102.Hermsen JL, Sano Y, Kudsk KA. Food fight! Parenteral nutrition, enteral stimulation and gut-derived mucosal immunity. Langenbecks Arch Surg 394: 17–30, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hua Z, Sergi C, Nation PN, Wizzard PR, Ball RO, Pencharz PB, Turner JM, Wales PW. Hepatic ultrastructure in a neonatal piglet model of intestinal failure-associated liver disease (IFALD). J Electron Microsc (Tokyo) 61: 179–186, 2012. [DOI] [PubMed] [Google Scholar]
- 104.Hua Z, Turner JM, Mager DR, Sigalet DL, Wizzard PR, Nation PN, Ball RO, Pencharz PB, Wales PW. Effects of polymeric formula vs elemental formula in neonatal piglets with short bowel syndrome. JPEN J Parenter Enteral Nutr 38: 498–506, 2013. [DOI] [PubMed] [Google Scholar]
- 105.Hua Z, Turner JM, Sigalet DL, Wizzard PR, Nation PN, Mager DR, Ball RO, Pencharz PB, Wales PW. Role of glucagon-like peptide-2 deficiency in neonatal short-bowel syndrome using neonatal piglets. Pediatr Res 73: 742–749, 2013. [DOI] [PubMed] [Google Scholar]
- 106.Inoue Y, Espat NJ, Frohnapple DJ, Epstein H, Copeland EM, Souba WW. Effect of total parenteral nutrition on amino acid and glucose transport by the human small intestine. Ann Surg 217: 604–612; discussion 612–604, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol 302: G218–G224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeppesen PB. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap Adv Gastroenterol 5: 159–171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jeppesen PB, Christensen MS, Hoy CE, Mortensen PB. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr 65: 837–843, 1997. [DOI] [PubMed] [Google Scholar]
- 110.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60: 902–914, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut 45: 559–563, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Jeppesen PB, Hartmann B, Thulesen J, Hansen BS, Holst JJ, Poulsen SS, Mortensen PB. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut 47: 370–376, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jeppesen PB, Mortensen PB. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J Parenter Enteral Nutr 23: S101–S105, 1999. [DOI] [PubMed] [Google Scholar]
- 115.Jeppesen PB, Mortensen PB. Enhancing bowel adaptation in short bowel syndrome. Curr Gastroenterol Rep 4: 338–347, 2002. [DOI] [PubMed] [Google Scholar]
- 116.Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut 43: 478–483, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeppesen PB, Mortensen PB. Significance of a preserved colon for parenteral energy requirements in patients receiving home parenteral nutrition. Scand J Gastroenterol 33: 1175–1179, 1998. [DOI] [PubMed] [Google Scholar]
- 118.Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'Keefe SJ, Forbes A, Heinze H, Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 143: 1473–1481.e3, 2012. [DOI] [PubMed] [Google Scholar]
- 119.Jeppesen PB, Staun M, Tjellesen L, Mortensen PB. Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut 43: 763–769, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeppesen PB, Szkudlarek J, Hoy CE, Mortensen PB. Effect of high-dose growth hormone and glutamine on body composition, urine creatinine excretion, fatty acid absorption, and essential fatty acids status in short bowel patients: a randomized, double-blind, crossover, placebo-controlled study. Scand J Gastroenterol 36: 48–54, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Jiang P, Wan JM, Sit WH, Lee CL, Schmidt M, Sangild PT. Enteral feeding in utero induces marked intestinal structural and functional proteome changes in pig fetuses. Pediatr Res 69: 123–128, 2011. [DOI] [PubMed] [Google Scholar]
- 122.Johnson WF, DiPalma CR, Ziegler TR, Scully S, Farrell CL. Keratinocyte growth factor enhances early gut adaptation in a rat model of short bowel syndrome. Vet Surg 29: 17–27, 2000. [DOI] [PubMed] [Google Scholar]
- 123.Jones BA, Hull MA, Potanos KM, Zurakowski D, Fitzgibbons SC, Ching YA, Duggan C, Jaksic T, Kim HB; International STEP Data Registry. Report of 111 consecutive patients enrolled in the International Serial Transverse Enteroplasty (STEP) Data Registry: a retrospective observational study. J Am Coll Surg 216: 438–446, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kalish BT, Le HD, Fitzgerald JM, Wang S, Seamon K, Gura KM, Gronert K, Puder M. Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am J Physiol Gastrointest Liver Physiol 305: G818–G828, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kanamori Y, Iwanaka T, Sugiyama M, Komura M, Takahashi T, Yuki N, Morotomi M, Tanaka R. Early use of probiotics is important therapy in infants with severe congenital anomaly. Pediatr Int 52: 362–367, 2010. [DOI] [PubMed] [Google Scholar]
- 126.Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 285: G1162–G1170, 2003. [DOI] [PubMed] [Google Scholar]
- 127.Kaufman SS, Gondolesi GE, Fishbein TM. Parenteral nutrition associated liver disease. Semin Neonatol 8: 375–381, 2003. [DOI] [PubMed] [Google Scholar]
- 128.Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 38: 425–429, 2003. [DOI] [PubMed] [Google Scholar]
- 129.King B, Carlson G, Khalil BA, Morabito A. Intestinal bowel lengthening in children with short bowel syndrome: systematic review of the Bianchi and STEP procedures. World J Surg 37: 694–704, 2013. [DOI] [PubMed] [Google Scholar]
- 130.King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg 229: 272–278, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knott AW, Juno RJ, Jarboe MD, Profitt SA, Erwin CR, Smith EP, Fagin JA, Warner BW. Smooth muscle overexpression of IGF-I induces a novel adaptive response to small bowel resection. Am J Physiol Gastrointest Liver Physiol 287: G562–G570, 2004. [DOI] [PubMed] [Google Scholar]
- 132.Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr 28: 41–45, 1999. [DOI] [PubMed] [Google Scholar]
- 133.Koopmann MC, Chen X, Holst JJ, Ney DM. Sustained glucagon-like peptide-2 infusion is required for intestinal adaptation, and cessation reverses increased cellularity in rats with intestinal failure. Am J Physiol Gastrointest Liver Physiol 299: G1222–G1230, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koopmann MC, Liu X, Boehler CJ, Murali SG, Holst JJ, Ney DM. Colonic GLP-2 is not sufficient to promote jejunal adaptation in a PN-dependent rat model of human short bowel syndrome. JPEN J Parenter Enteral Nutr 33: 629–638; discussion 638–629, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koruda MJ, Rolandelli RH, Bliss DZ, Hastings J, Rombeau JL, Settle RG. Parenteral nutrition supplemented with short-chain fatty acids: effect on the small-bowel mucosa in normal rats. Am J Clin Nutr 51: 685–689, 1990. [DOI] [PubMed] [Google Scholar]
- 136.Koruda MJ, Rolandelli RH, Settle RG, Zimmaro DM, Rombeau JL. Effect of parenteral nutrition supplemented with short-chain fatty acids on adaptation to massive small bowel resection. Gastroenterology 95: 715–720, 1988. [DOI] [PubMed] [Google Scholar]
- 137.Kripke SA, De Paula JA, Berman JM, Fox AD, Rombeau JL, Settle RG. Experimental short-bowel syndrome: effect of an elemental diet supplemented with short-chain triglycerides. Am J Clin Nutr 53: 954–962, 1991. [DOI] [PubMed] [Google Scholar]
- 138.Krugner-Higby L, Smith L, Clark M, Heath TD, Dahly E, Schiffman B, Hubbard-VanStelle S, Ney D, Wendland A. Liposome-encapsulated oxymorphone hydrochloride provides prolonged relief of postsurgical visceral pain in rats. Comp Med 53: 270–279, 2003. [PubMed] [Google Scholar]
- 139.Ksiazyk J, Piena M, Kierkus J, Lyszkowska M. Hydrolyzed versus nonhydrolyzed protein diet in short bowel syndrome in children. J Pediatr Gastroenterol Nutr 35: 615–618, 2002. [DOI] [PubMed] [Google Scholar]
- 140.Ladd AP, Grosfeld JL, Pescovitz OH, Johnson NB. The effect of growth hormone supplementation on late nutritional independence in pediatric patients with short bowel syndrome. J Pediatr Surg 40: 442–445, 2005. [DOI] [PubMed] [Google Scholar]
- 141.Leen JL, Izzo A, Upadhyay C, Rowland KJ, Dube PE, Gu S, Heximer SP, Rhodes CJ, Storm DR, Lund PK, Brubaker PL. Mechanism of action of glucagon-like peptide-2 to increase IGF-I mRNA in intestinal subepithelial fibroblasts. Endocrinology 152: 436–446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lennon D, Zanganeh T, Borum PR. Development of the piglet neonatal intensive care unit for translational research. Lab Anim (NY) 40: 253–258, 2011. [DOI] [PubMed] [Google Scholar]
- 143.Li Y, Jensen ML, Chatterton DE, Jensen BB, Thymann T, Kvistgaard AS, Sangild PT. Raw bovine milk improves gut responses to feeding relative to infant formula in preterm piglets. Am J Physiol Gastrointest Liver Physiol 306: G81–G90, 2014. [DOI] [PubMed] [Google Scholar]
- 144.Liu X, Murali SG, Holst JJ, Ney DM. Whey protein potentiates the intestinotrophic action of glucagon-like peptide-2 in parenterally fed rats. Am J Physiol Regul Integr Comp Physiol 297: R1554–R1562, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr 84: 1142–1150, 2006. [DOI] [PubMed] [Google Scholar]
- 146.Lund P, Sangild PT, Aunsholt L, Hartmann B, Holst JJ, Mortensen J, Mortensen PB, Jeppesen PB. Randomised controlled trial of colostrum to improve intestinal function in patients with short bowel syndrome. Eur J Clin Nutr 66: 1059–1065, 2012. [DOI] [PubMed] [Google Scholar]
- 147.Lund PK. Molecular basis of intestinal adaptation: the role of the insulin-like growth factor system. Ann NY Acad Sci 859: 18–36, 1998. [DOI] [PubMed] [Google Scholar]
- 148.Malcolm WF, Lenfestey RW, Rice HE, Rach E, Goldberg RN, Cotten CM. Dietary fat for infants with enterostomies. J Pediatr Surg 42: 1811–1815, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Martin GR, Meddings JB, Sigalet DL. 3-0 methylglucose absorption in vivo correlates with nutrient absorption and intestinal surface area in experimental short bowel syndrome. JPEN J Parenter Enteral Nutr 27: 65–70, 2003. [DOI] [PubMed] [Google Scholar]
- 150.Martin GR, Wallace LE, Hartmann B, Holst JJ, Demchyshyn L, Toney K, Sigalet DL. Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 288: G431–G438, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 286: G964–G972, 2004. [DOI] [PubMed] [Google Scholar]
- 152.Matarese LE. Nutrition and fluid optimization for patients with short bowel syndrome. JPEN J Parenter Enteral Nutr 37: 161–170, 2013. [DOI] [PubMed] [Google Scholar]
- 153.McDuffie LA, Bucher BT, Erwin CR, Wakeman D, White FV, Warner BW. Intestinal adaptation after small bowel resection in human infants. J Pediatr Surg 46: 1045–1051, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Messing B, Blethen S, Dibaise JK, Matarese LE, Steiger E. Treatment of adult short bowel syndrome with recombinant human growth hormone: a review of clinical studies. J Clin Gastroenterol 40, Suppl 2: S75–S84, 2006. [DOI] [PubMed] [Google Scholar]
- 155.Miller M, Burjonrappa S. A review of enteral strategies in infant short bowel syndrome: evidence-based or NICU culture? J Pediatr Surg 48: 1099–1112, 2013. [DOI] [PubMed] [Google Scholar]
- 156.Miller ME, Michaylira CZ, Simmons JG, Ney DM, Dahly EM, Heath JK, Lund PK. Suppressor of cytokine signaling-2: a growth hormone-inducible inhibitor of intestinal epithelial cell proliferation. Gastroenterology 127: 570–581, 2004. [DOI] [PubMed] [Google Scholar]
- 157.Miyasaka EA, Feng Y, Poroyko V, Falkowski NR, Erb-Downward J, Gillilland MG, 3rd, Mason KL, Huffnagle GB, Teitelbaum DH. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol 190: 6607–6615, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Modi BP, Ching YA, Langer M, Donovan K, Fauza DO, Kim HB, Jaksic T, Nurko S. Preservation of intestinal motility after the serial transverse enteroplasty procedure in a large animal model of short bowel syndrome. J Pediatr Surg 44: 229–235; discussion 235, 2009. [DOI] [PubMed] [Google Scholar]
- 159.Monzavi R, Cohen P. IGFs and IGFBPs: role in health and disease. Best Pract Res Clin Endocrinol Metab 16: 433–447, 2002. [DOI] [PubMed] [Google Scholar]
- 160.Murali SG, Brinkman AS, Solverson P, Pun W, Pintar JE, Ney DM. Exogenous GLP-2 and IGF-I induce a differential intestinal response in IGF binding protein-3 and -5 double knockout mice. Am J Physiol Gastrointest Liver Physiol 302: G794–G804, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murali SG, Liu X, Nelson DW, Hull AK, Grahn M, Clayton MK, Pintar JE, Ney DM. Intestinotrophic effects of exogenous IGF-I are not diminished in IGF binding protein-5 knockout mice. Am J Physiol Regul Integr Comp Physiol 292: R2144–R2150, 2007. [DOI] [PubMed] [Google Scholar]
- 162.Murali SG, Nelson DW, Draxler AK, Liu X, Ney DM. Insulin-like growth factor-I (IGF-I) attenuates jejunal atrophy in association with increased expression of IGF-I binding protein-5 in parenterally fed mice. J Nutr 135: 2553–2559, 2005. [DOI] [PubMed] [Google Scholar]
- 163.Nagy ES, Paris MC, Taylor RG, Fuller PJ, Sourial M, Justice F, Bines JE. Colostrum protein concentrate enhances intestinal adaptation after massive small bowel resection in juvenile pigs. J Pediatr Gastroenterol Nutr 39: 487–492, 2004. [DOI] [PubMed] [Google Scholar]
- 164.Nehra D, Carlson SJ, Fallon EM, Kalish B, Potemkin AK, Gura KM, Simpser E, Compher C, Puder M; American Society for Parenteral and Enteral Nutrition. A.S.P.E.N. clinical guidelines: nutrition support of neonatal patients at risk for metabolic bone disease. JPEN J Parenter Enteral Nutr 37: 570–598, 2013. [DOI] [PubMed] [Google Scholar]
- 165.Nehra D, Fallon EM, Carlson SJ, Potemkin AK, Hevelone ND, Mitchell PD, Gura KM, Puder M. Provision of a soy-based intravenous lipid emulsion at 1 g/kg/d does not prevent cholestasis in neonates. JPEN J Parenter Enteral Nutr 37: 498–505, 2013. [DOI] [PubMed] [Google Scholar]
- 166.Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 148: 1954–1962, 2007. [DOI] [PubMed] [Google Scholar]
- 167.Nightingale JM, Lennard-Jones JE. The short bowel syndrome: what's new and old? Dig Dis 11: 12–31, 1993. [DOI] [PubMed] [Google Scholar]
- 168.Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr 134: 1467–1474, 2004. [DOI] [PubMed] [Google Scholar]
- 169.Nose K, Yang H, Sun X, Nose S, Koga H, Feng Y, Miyasaka E, Teitelbaum DH. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res 30: 67–80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.O'Brien DP, Nelson LA, Huang FS, Warner BW. Intestinal adaptation: structure, function, and regulation. Semin Pediatr Surg 10: 56–64, 2001. [DOI] [PubMed] [Google Scholar]
- 171.O'Keefe SJ, Buchman AL, Fishbein TM, Jeejeebhoy KN, Jeppesen PB, Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 4: 6–10, 2006. [DOI] [PubMed] [Google Scholar]
- 172.Obertop H, Nundy S, Malamud D, Malt RA. Onset of cell proliferation in the shortened gut. Rapid hyperplasia after jejunal resection. Gastroenterology 72: 267–270, 1977. [PubMed] [Google Scholar]
- 173.Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery 150: 217–223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oliveira C, de Silva N, Wales PW. Five-year outcomes after serial transverse enteroplasty in children with short bowel syndrome. J Pediatr Surg 47: 931–937, 2012. [DOI] [PubMed] [Google Scholar]
- 175.Ong EG, Eaton S, Wade AM, Horn V, Losty PD, Curry JI, Sugarman ID, Klein NJ, Pierro A; SIGN Trial Group. Randomized clinical trial of glutamine-supplemented versus standard parenteral nutrition in infants with surgical gastrointestinal disease. Br J Surg 99: 929–938, 2012. [DOI] [PubMed] [Google Scholar]
- 176.Orskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124: 105–112, 2005. [DOI] [PubMed] [Google Scholar]
- 177.Paris MC, Fuller PJ, Carstensen B, Nagy E, Taylor RG, Sourial M, Holst JJ, Hartmann B, Binesm JE. Plasma GLP-2 levels and intestinal markers in the juvenile pig during intestinal adaptation: effects of different diet regimens. Dig Dis Sci 49: 1688–1695, 2004. [DOI] [PubMed] [Google Scholar]
- 178.Pereira-Fantini PM, Nagy ES, Thomas SL, Taylor RG, Sourial M, Paris MC, Holst JJ, Fuller PJ, Bines JE. GLP-2 administration results in increased proliferation but paradoxically an adverse outcome in a juvenile piglet model of short bowel syndrome. J Pediatr Gastroenterol Nutr 46: 20–28, 2008. [DOI] [PubMed] [Google Scholar]
- 179.Pereira-Fantini PM, Thomas SL, Taylor RG, Nagy E, Sourial M, Fuller PJ, Bines JE. Colostrum supplementation restores insulin-like growth factor -1 levels and alters muscle morphology following massive small bowel resection. JPEN J Parenter Enteral Nutr 32: 266–275, 2008. [DOI] [PubMed] [Google Scholar]
- 180.Pereira-Fantini PM, Thomas SL, Wilson G, Taylor RG, Sourial M, Bines JE. Short- and long-term effects of small bowel resection: a unique histological study in a piglet model of short bowel syndrome. Histochem Cell Biol 135: 195–202, 2011. [DOI] [PubMed] [Google Scholar]
- 181.Peretti N, Loras-Duclaux I, Kassai B, Restier-Miron L, Guimber D, Gottrand F, Coopman S, Michaud L, Marinier E, Yantren H, Michalski MC, Aubert F, Mercier C, Pelosse M, Lopez M, Chatelain P, Lachaux A. Growth hormone to improve short bowel syndrome intestinal autonomy: a pediatric randomized open-label clinical trial. JPEN J Parenter Enteral Nutr 35: 723–731, 2011. [DOI] [PubMed] [Google Scholar]
- 182.Petersen YM, Burrin DG, Sangild PT. GLP-2 has differential effects on small intestine growth and function in fetal and neonatal pigs. Am J Physiol Regul Integr Comp Physiol 281: R1986–R1993, 2001. [DOI] [PubMed] [Google Scholar]
- 183.Petersen YM, Elnif J, Schmidt M, Sangild PT. Glucagon-like peptide 2 enhances maltase-glucoamylase and sucrase-isomaltase gene expression and activity in parenterally fed premature neonatal piglets. Pediatr Res 52: 498–503, 2002. [DOI] [PubMed] [Google Scholar]
- 184.Petersen YM, Hartmann B, Holst JJ, Le Huerou-Luron I., Bjornvad CR, Sangild PT. Introduction of enteral food increases plasma GLP-2 and decreases GLP-2 receptor mRNA abundance during pig development. J Nutr 133: 1781–1786, 2003. [DOI] [PubMed] [Google Scholar]
- 185.Piper H, Modi BP, Kim HB, Fauza D, Glickman J, Jaksic T. The second STEP: the feasibility of repeat serial transverse enteroplasty. J Pediatr Surg 41: 1951–1956, 2006. [DOI] [PubMed] [Google Scholar]
- 186.Pironi L, Goulet O, Buchman A, Messing B, Gabe S, Candusso M, Bond G, Gupte G, Pertkiewicz M, Steiger E, Forbes A, Van Gossum A, Pinna AD; Home Artificial Nutrition and Chronic Intestinal failure Working Group of ESPEN. Outcome on home parenteral nutrition for benign intestinal failure: a review of the literature and benchmarking with the European prospective survey of ESPEN. Clin Nutr 31: 831–845, 2012. [DOI] [PubMed] [Google Scholar]
- 187.Rager R, Finegold MJ. Cholestasis in immature newborn infants: is parenteral alimentation responsible? J Pediatr 86: 264–269, 1975. [DOI] [PubMed] [Google Scholar]
- 188.Raman M, Gramlich L, Whittaker S, Allard JP. Canadian home total parenteral nutrition registry: preliminary data on the patient population. Can J Gastroenterol 21: 643–648, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet — a model in the study of oesophageal development in preterm neonates. Acta Paediatr 99: 201–208, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Raval MV, Moss RL. Current concepts in the surgical approach to necrotizing enterocolitis. Pathophysiology 21: 105–110, 2014. [DOI] [PubMed] [Google Scholar]
- 191.Reddy VS, Patole SK, Rao S. Role of probiotics in short bowel syndrome in infants and children—a systematic review. Nutrients 5: 679–699, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rhoads JM, Plunkett E, Galanko J, Lichtman S, Taylor L, Maynor A, Weiner T, Freeman K, Guarisco JL, Wu GY. Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. J Pediatr 146: 542–547, 2005. [DOI] [PubMed] [Google Scholar]
- 193.Rigby RJ, Hunt MR, Scull BP, Simmons JG, Speck KE, Helmrath MA, Lund PK. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut 58: 1104–1112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rossi TM, Lee PC, Young C, Tjota A. Small intestinal mucosa changes, including epithelial cell proliferative activity, of children receiving total parenteral nutrition (TPN). Dig Dis Sci 38: 1608–1613, 1993. [DOI] [PubMed] [Google Scholar]
- 195.Rowland KJ, McMellen ME, Wakeman D, Wandu WS, Erwin CR, Warner BW. Enterocyte expression of epidermal growth factor receptor is not required for intestinal adaptation in response to massive small bowel resection. J Pediatr Surg 47: 1748–1753, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rowland KJ, Trivedi S, Lee D, Wan K, Kulkarni RN, Holzenberger M, Brubaker PL. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology 141: 2166–2175.e7, 2011. [DOI] [PubMed] [Google Scholar]
- 197.Sacher P, Stauffer UG. An animal model for short-bowel syndrome in piglets to assess the efficiency of bowel-lengthening procedures. Eur J Pediatr Surg 7: 207–211, 1997. [DOI] [PubMed] [Google Scholar]
- 198.Sala D, Chomto S, Hill S. Long-term outcomes of short bowel syndrome requiring long-term/home intravenous nutrition compared in children with gastroschisis and those with volvulus. Transplant Proc 42: 5–8, 2010. [DOI] [PubMed] [Google Scholar]
- 199.Sangild PT. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 231: 1695–1711, 2006. [DOI] [PubMed] [Google Scholar]
- 200.Sangild PT, Malo C, Schmidt M, Petersen YM, Elnif J, Holst JJ, Buddington RK. Glucagon-like peptide 2 has limited efficacy to increase nutrient absorption in fetal and preterm pigs. Am J Physiol Regul Integr Comp Physiol 293: R2179–R2184, 2007. [DOI] [PubMed] [Google Scholar]
- 201.Sangild PT, Petersen YM, Schmidt M, Elnif J, Petersen TK, Buddington RK, Greisen G, Michaelsen KF, Burrin DG. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr 132: 3786–3794, 2002. [DOI] [PubMed] [Google Scholar]
- 202.Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M, Moelbak L, Buddington RK, Westrom BR, Holst JJ, Burrin DG. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130: 1776–1792, 2006. [DOI] [PubMed] [Google Scholar]
- 203.Sangild PT, Tappenden KA, Malo C, Petersen YM, Elnif J, Bartholome AL, Buddington RK. Glucagon-like peptide 2 stimulates intestinal nutrient absorption in parenterally fed newborn pigs. J Pediatr Gastroenterol Nutr 43: 160–167, 2006. [DOI] [PubMed] [Google Scholar]
- 204.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: The preterm pig as a model in pediatric gastroenterology. J Anim Sci 91: 4713–4729, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Scarpello JH, Cary BA, Sladen GE. Effects of ileal and caecal resection on the colon of the rat. Clin Sci Mol Med 54: 241–249, 1978. [DOI] [PubMed] [Google Scholar]
- 206.Schwartz MZ. Novel therapies for the management of short bowel syndrome in children. Pediatr Surg Int 29: 967–974, 2013. [DOI] [PubMed] [Google Scholar]
- 207.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol Gastrointest Liver Physiol 275: G911–G921, 1998. [DOI] [PubMed] [Google Scholar]
- 208.Sedman PC, MacFie J, Palmer MD, Mitchell CJ, Sagar PM. Preoperative total parenteral nutrition is not associated with mucosal atrophy or bacterial translocation in humans. Br J Surg 82: 1663–1667, 1995. [DOI] [PubMed] [Google Scholar]
- 209.Seguy D, Vahedi K, Kapel N, Souberbielle JC, Messing B. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: a positive study. Gastroenterology 124: 293–302, 2003. [DOI] [PubMed] [Google Scholar]
- 210.Shin JI, Namgung R, Park MS, Lee C. Could lipid infusion be a risk for parenteral nutrition-associated cholestasis in low birth weight neonates? Eur J Pediatr 167: 197–202, 2008. [DOI] [PubMed] [Google Scholar]
- 211.Sigalet D, Boctor D. Assessment of mucosal mass and hormonal therapy. In: Clinical Management of Intestinal failure, edited by Duggan C, Gura KM, Jaksic T. Boca Raton, FL: CRC, 2012. [Google Scholar]
- 212.Sigalet D, Boctor D, Brindle M, Lam V, Robertson M. Elements of successful intestinal rehabilitation. J Pediatr Surg 46: 150–156, 2011. [DOI] [PubMed] [Google Scholar]
- 213.Sigalet DL, de Heuvel E, Wallace L, Bulloch E, Turner J, Wales PW, Nation P, Wizzard PR, Hartmann B, Assad M, Holst JJ. Effects of chronic glucagon-like peptide-2 therapy during weaning in neonatal pigs. Regul Pept 188: 70–80, 2014. [DOI] [PubMed] [Google Scholar]
- 214.Sigalet DL, Lam V, Boctor D. The assessment, and glucagon-like peptide-2 modulation, of intestinal absorption and function. Semin Pediatr Surg 19: 44–49, 2010. [DOI] [PubMed] [Google Scholar]
- 215.Sigalet DL, Lees GM, Aherne F, Van Aerde JE, Fedorak RN, Keelan M, Thomson AB. The physiology of adaptation to small bowel resection in the pig: an integrated study of morphological and functional changes. J Pediatr Surg 25: 650–657, 1990. [DOI] [PubMed] [Google Scholar]
- 216.Sigalet DL, Lees GM, Aherne FX, Fedorak R, Keelan M, Thomson AB, van Aerde J. Nutritional effects of surgical and medical treatment for short bowel syndrome. JPEN J Parenter Enteral Nutr 25: 330–336, 2001. [DOI] [PubMed] [Google Scholar]
- 217.Sigalet DL, Martin G, Meddings J, Hartman B, Holst JJ. GLP-2 levels in infants with intestinal dysfunction. Pediatr Res 56: 371–376, 2004. [DOI] [PubMed] [Google Scholar]
- 218.Sigalet DL, Martin GR. Mechanisms underlying intestinal adaptation after massive intestinal resection in the rat. J Pediatr Surg 33: 889–892, 1998. [DOI] [PubMed] [Google Scholar]
- 219.Sigalet DL, Martin GR, Butzner JD, Buret A, Meddings JB. A pilot study of the use of epidermal growth factor in pediatric short bowel syndrome. J Pediatr Surg 40: 763–768, 2005. [DOI] [PubMed] [Google Scholar]
- 220.Sigalet DL, Martin GR, Meddings JB. 3-0 Methylglucose uptake as a marker of nutrient absorption and bowel length in pediatric patients. JPEN J Parenter Enteral Nutr 28: 158–162, 2004. [DOI] [PubMed] [Google Scholar]
- 221.Sigalet DL, Martin GR, Poole A. Differential sugar absorption as a marker for adaptation in short bowel syndrome. J Pediatr Surg 35: 661–664, 2000. [DOI] [PubMed] [Google Scholar]
- 222.Sondheimer JM, Cadnapaphornchai M, Sontag M, Zerbe GO. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr 132: 80–84, 1998. [DOI] [PubMed] [Google Scholar]
- 223.Soon IS, Boctor D, Holst JJ, Wallace LE, Lam V, Sigalet DL. T1121 altered development of the glucagon like peptide-2 response in infants with gastroschisis. Gastroenterology 136: A-504, 2009. [Google Scholar]
- 224.Speck KE, Garrison AP, Rigby RJ, von Allmen DC, Lund PK, Helmrath MA. Inflammation enhances resection-induced intestinal adaptive growth in IL-10 null mice. J Surg Res 168: 62–69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Spurrier RG, Grikscheit TC. Tissue engineering the small intestine. Clin Gastroenterol Hepatol 11: 354–358, 2013. [DOI] [PubMed] [Google Scholar]
- 227.Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, Rhee S, Sudan D, Mercer D, Martinez JA, Carter BA, Soden J, Horslen S, Rudolph JA, Kocoshis S, Superina R, Lawlor S, Haller T, Kurs-Lasky M, Belle SH; Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal failure Consortium. J Pediatr 161: 723–728.e2, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stephens AN, Pereira-Fantini PM, Wilson G, Taylor RG, Rainczuk A, Meehan KL, Sourial M, Fuller PJ, Stanton PG, Robertson DM, Bines JE. Proteomic analysis of the intestinal adaptation response reveals altered expression of fatty acid binding proteins following massive small bowel resection. J Proteome Res 9: 1437–1449, 2010. [DOI] [PubMed] [Google Scholar]
- 229.Stern LE, Falcone RA, Jr, Huang F, Kemp CJ, Erwin CR, Warner BW. Epidermal growth factor alters the bax:bcl-w ratio following massive small bowel resection. J Surg Res 91: 38–42, 2000. [DOI] [PubMed] [Google Scholar]
- 230.Stevens CE. Comparative Physiology of the Vertebrate Digestive System. Cambridge, UK: Cambridge University Press, 1988. [Google Scholar]
- 231.Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, Burrin DG. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr 140: 2193–2200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. JPEN J Parenter Enteral Nutr 36: 538–550, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sukhotnik I, Shany A, Bashenko Y, Hayari L, Chemodanov E, Mogilner J, Coran AG, Shaoul R. Parenteral but not enteral omega-3 fatty acids (Omegaven) modulate intestinal regrowth after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr 34: 503–512, 2010. [DOI] [PubMed] [Google Scholar]
- 234.Suri M, Turner JM, Sigalet DL, Wizzard PR, Nation PN, Ball RO, Pencharz PB, Brubaker PL, Wales PW. Exogenous glucagon-like peptide-2 improves outcomes of intestinal adaptation in a distal-intestinal resection neonatal piglet model of short bowel syndrome. Pediatr Res 76: 370–377, 2014. [DOI] [PubMed] [Google Scholar]
- 235.Szkudlarek J, Jeppesen PB, Mortensen PB. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: a randomised, double blind, crossover, placebo controlled study. Gut 47: 199–205, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Taguchi S, Masumoto K, Yamanouchi T, Suita S. Decrease in hepatic circulation induces hepatic fibrosis in a neonatal piglet model with short bowel syndrome. J Pediatr Surg 40: 1592–1597, 2005. [DOI] [PubMed] [Google Scholar]
- 237.Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology 130: S93–S99, 2006. [DOI] [PubMed] [Google Scholar]
- 238.Tappenden KA, Albin DM, Bartholome AL, Mangian HF. Glucagon-like peptide-2 and short-chain fatty acids: a new twist to an old story. J Nutr 133: 3717–3720, 2003. [DOI] [PubMed] [Google Scholar]
- 239.Tappenden KA, Drozdowski LA, Thomson AB, McBurney MI. Short-chain fatty acid-supplemented total parenteral nutrition alters intestinal structure, glucose transporter 2 (GLUT2) mRNA and protein, and proglucagon mRNA abundance in normal rats. Am J Clin Nutr 68: 118–125, 1998. [DOI] [PubMed] [Google Scholar]
- 240.Tappenden KA, McBurney MI. Systemic short-chain fatty acids rapidly alter gastrointestinal structure, function, and expression of early response genes. Dig Dis Sci 43: 1526–1536, 1998. [DOI] [PubMed] [Google Scholar]
- 241.Tappenden KA, Thomson AB, Wild GE, McBurney MI. Short-chain fatty acid-supplemented total parenteral nutrition enhances functional adaptation to intestinal resection in rats. Gastroenterology 112: 792–802, 1997. [DOI] [PubMed] [Google Scholar]
- 242.Tappenden KA, Thomson AB, Wild GE, McBurney MI. Short-chain fatty acids increase proglucagon and ornithine decarboxylase messenger RNAs after intestinal resection in rats. JPEN J Parenter Enteral Nutr 20: 357–362, 1996. [DOI] [PubMed] [Google Scholar]
- 243.Taylor JA, Martin CA, Nair R, Guo J, Erwin CR, Warner BW. Lessons learned: optimization of a murine small bowel resection model. J Pediatr Surg 43: 1018–1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thymann T, Le Huërou-Luron I, Petersen YM, Hedemann MS, Elnif J, Jensen BB, Holst JJ, Hartmann B, Sangild PT. Glucagon-like peptide 2 (GLP-2) treatment improves intestinal adaptation during weaning. J Anim Sci 92: 2070–2079, 2014. [DOI] [PubMed] [Google Scholar]
- 245.Thymann T, Stoll B, Mecklenburg L, Burrin DG, Vegge A, Qvist N, Eriksen T, Jeppesen PB, Sangild PT. Acute effects of the glucagon-like peptide 2 analogue, teduglutide, on intestinal adaptation in newborn pigs with short bowel syndrome. J Pediatr Gastroenterol Nutr 58: 694–702, 2014. [DOI] [PubMed] [Google Scholar]
- 246.Tolga Muftuoglu MA, Civak T, Cetin S, Civak L, Gungor O, Saglam A. Effects of probiotics on experimental short-bowel syndrome. Am J Surg 202: 461–468, 2011. [DOI] [PubMed] [Google Scholar]
- 247.Turner JM, Wales PW, Nation PN, Wizzard P, Pendlebury C, Sergi C, Ball RO, Pencharz PB. Novel neonatal piglet models of surgical short bowel syndrome with intestinal failure. J Pediatr Gastroenterol Nutr 52: 9–16, 2011. [DOI] [PubMed] [Google Scholar]
- 248.Ugur A, Marashdeh BH, Gottschalck I, Brobech Mortensen P, Staun M, Bekker Jeppesen P. Home parenteral nutrition in Denmark in the period from 1996 to 2001. Scand J Gastroenterol 41: 401–407, 2006. [DOI] [PubMed] [Google Scholar]
- 249.Van Aerde JE, Duerksen DR, Gramlich L, Meddings JB, Chan G, Thomson ABR, Clandinin MT. Intravenous fish oil emulsion attenuates total parenteral nutrition-induced cholestasis in newborn piglets. Pediatr Res 45: 202–208, 1999. [DOI] [PubMed] [Google Scholar]
- 250.Vanderhoof JA, Grandjean CJ, Kaufman SS, Burkley KT, Antonson DL. Effect of high percentage medium-chain triglyceride diet on mucosal adaptation following massive bowel resection in rats. JPEN J Parenter Enteral Nutr 8: 685–689, 1984. [DOI] [PubMed] [Google Scholar]
- 251.Vegge A, Thymann T, Lauritzen L, Bering SB, Wiinberg B, Sangild PT. Parenteral lipids and partial enteral nutrition affect hepatic lipid composition but have limited short term effects on formula-induced necrotizing enterocolitis in preterm piglets. Clin Nutr 2014. March 19 pii: S0261-5614(14)00077-6. 10.1016/j.clnu.2014.03.004 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 252.Vegge A, Thymann T, Lund P, Stoll B, Bering SB, Hartmann B, Jelsing J, Qvist N, Burrin DG, Jeppesen PB, Holst JJ, Sangild PT. Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates. Am J Physiol Gastrointest Liver Physiol 305: G277–G285, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vlaardingerbroek H, Ng K, Stoll B, Benight N, Chacko S, Kluijtmans LA, Kulik W, Squires EJ, Olutoye O, Schady D, Finegold ML, van Goudoever JB, Burrin DG. New generation lipid emulsions prevent PNALD in chronic parenterally-fed preterm pigs. J Lipid Res 55: 466–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wakeman D, Longshore SW, McMellen ME, Santos JA, Guo J, Erwin CR, Warner BW. Extent of small bowel resection does not influence the magnitude of intestinal adaptation in the mouse. J Pediatr Surg 45: 1274–1279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wales PW. Surgical therapy for short bowel syndrome. Pediatr Surg Int 20: 647–657, 2004. [DOI] [PubMed] [Google Scholar]
- 256.Wales PW, Christison-Lagay ER. Short bowel syndrome: epidemiology and etiology. Semin Pediatr Surg 19: 3–9, 2010. [DOI] [PubMed] [Google Scholar]
- 257.Wales PW, de Silva N, Kim J, Lecce L, To T, Moore A. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg 39: 690–695, 2004. [DOI] [PubMed] [Google Scholar]
- 258.Warner BW, Erwin CR. Critical roles for EGF receptor signaling during resection-induced intestinal adaptation. J Pediatr Gastroenterol Nutr 43, Suppl 1: S68–S73, 2006. [DOI] [PubMed] [Google Scholar]
- 259.Washizawa N, Gu LH, Gu L, Openo KP, Jones DP, Ziegler TR. Comparative effects of glucagon-like peptide-2 (GLP-2), growth hormone (GH), and keratinocyte growth factor (KGF) on markers of gut adaptation after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr 28: 399–409, 2004. [DOI] [PubMed] [Google Scholar]
- 260.Weih S, Nickkholgh A, Kessler M, Frongia G, Hafezi M, Golriz M, Fard N, Holland-Cunz S, Mehrabi A. Models of short bowel syndrome in pigs: a technical review. Eur Surg Res 51: 66–78, 2013. [DOI] [PubMed] [Google Scholar]
- 261.Welters CF, Deutz NE, Dejong CH, Soeters PB, Heineman E. Supplementation of enteral nutrition with butyrate leads to increased portal efflux of amino acids in growing pigs with short bowel syndrome. J Pediatr Surg 31: 526–529, 1996. [DOI] [PubMed] [Google Scholar]
- 262.Weser E, Babbitt J, Hoban M, Vandeventer A. Intestinal adaptation. Different growth responses to disaccharides compared with monosaccharides in rat small bowel. Gastroenterology 91: 1521–1527, 1986. [PubMed] [Google Scholar]
- 263.Wessel JJ, Kocoshis SA. Nutritional management of infants with short bowel syndrome. Semin Perinatol 31: 104–111, 2007. [DOI] [PubMed] [Google Scholar]
- 264.Whang EE, Dunn JC, Joffe H, Mahanty H, Zinner MJ, McFadden DW, Ashley SW. Enterocyte functional adaptation following intestinal resection. J Surg Res 60: 370–374, 1996. [DOI] [PubMed] [Google Scholar]
- 265.Wicks C, Somasundaram S, Bjarnason I, Menzies IS, Routley D, Potter D, Tan KC, Williams R. Comparison of enteral feeding and total parenteral nutrition after liver transplantation. Lancet 344: 837–840, 1994. [DOI] [PubMed] [Google Scholar]
- 266.Wildhaber BE, Yang H, Teitelbaum DH. Keratinocyte growth factor decreases total parenteral nutrition-induced apoptosis in mouse intestinal epithelium via Bcl-2. J Pediatr Surg 38: 92–96; discussion 92–96, 2003. [DOI] [PubMed] [Google Scholar]
- 267.Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA 203: 860–864, 1968. [PubMed] [Google Scholar]
- 268.Yamaguchi M, Asakawa K, Kuzume M, Nemoto H, Sanada Y, Kumada K. Effects of insulin-like growth factor-1 on short bowel syndrome without ileocecal valve in rats. Eur Surg Res 33: 291–296, 2001. [DOI] [PubMed] [Google Scholar]
- 269.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 172: 4151–4158, 2004. [DOI] [PubMed] [Google Scholar]
- 270.Yang H, Wildhaber B, Tazuke Y, Teitelbaum DH. 2002 Harry M. Vars Research Award. Keratinocyte growth factor stimulates the recovery of epithelial structure and function in a mouse model of total parenteral nutrition. JPEN J Parenter Enteral Nutr 26: 333–340; discussion 340–331, 2002. [DOI] [PubMed] [Google Scholar]
- 271.Yang H, Wildhaber BE, Teitelbaum DH. 2003 Harry M Vars Research Award. Keratinocyte growth factor improves epithelial function after massive small bowel resection. JPEN J Parenter Enteral Nutr 27: 198–206; discussion 206–197, 2003. [DOI] [PubMed] [Google Scholar]
- 272.Yang Q, Kock ND. Intestinal adaptation following massive ileocecal resection in 20-day-old weanling rats. J Pediatr Gastroenterol Nutr 50: 16–21, 2010. [DOI] [PubMed] [Google Scholar]
- 273.Yang Q, Lan T, Chen Y, Dawson PA. Dietary fish oil increases fat absorption and fecal bile acid content without altering bile acid synthesis in 20-d-old weanling rats following massive ileocecal resection. Pediatr Res 72: 38–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 137: 986–996, 2009. [DOI] [PubMed] [Google Scholar]
- 275.Yusta B, Holland D, Waschek JA, Drucker DJ. Intestinotrophic glucagon-like peptide-2 (GLP-2) activates intestinal gene expression and growth factor-dependent pathways independent of the vasoactive intestinal peptide gene in mice. Endocrinology 153: 2623–2632, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744–755, 2000. [DOI] [PubMed] [Google Scholar]
- 277.Ziegler EE. Meeting the nutritional needs of the low-birth-weight infant. Ann Nutr Metab 58, Suppl 1: 8–18, 2011. [DOI] [PubMed] [Google Scholar]
- 278.Ziegler TR, Mantell MP, Chow JC, Rombeau JL, Smith RJ. Intestinal adaptation after extensive small bowel resection: differential changes in growth and insulin-like growth factor system messenger ribonucleic acids in jejunum and ileum. Endocrinology 139: 3119–3126, 1998. [DOI] [PubMed] [Google Scholar]