Abstract
Pseudo-continuous arterial spin labeling (pCASL) measurements were performed in 20 patients with idiopathic normal pressure hydrocephalus (iNPH) to investigate whether cerebral blood flow (CBF) increases during the first 24 hours after a cerebrospinal fluid tap test (CSF TT). Five pCASL magnetic resonance imaging (MRI) scans were performed. Two scans were performed before removal of 40 mL CSF, and the other three at 30 minutes, 4 hours, and 24 hours, respectively after the CSF TT. Thirteen different regions of interest (ROIs) were manually drawn on coregistered MR images. In patients with increased CBF in lateral and frontal white matter after the CSF TT, gait function improved more than it did in patients with decreased CBF in these regions. However, in the whole sample, there was no significant increase in CBF after CSF removal compared with baseline investigations. The repeatability of CBF measurements at baseline was high, with intraclass correlation coefficients of 0.60 to 0.90 for different ROIs, but the median regional variability was in the range of 5% to 17%. Our results indicate that CBF in white matter close to the lateral ventricles plays a role in the reversibility of symptoms after CSF removal in patients with iNPH.
Keywords: arterial spin labeling, cerebral blood flow, cerebral perfusion, normal pressure hydrocephalus, pCASL, volumetry
Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is a disorder characterized by impairments of balance, gait, cognitive function, and urinary continence.1 The pathophysiology of the condition is not completely understood, but previous studies have shown decreased global and regional cerebral blood flow (CBF) in the periventricular white matter (WM), frontal gray matter and WM, thalamus, and in the basal ganglia.2, 3, 4 The symptoms can be relieved by implantation of a shunt system.
Before patients are selected for shunt insertion, prognostic data based on clinical examination and imaging of the brain are collected, often followed by tests of the cerebrospinal fluid (CSF) system.5 The CSF tap test (CSF TT) with removal of 30 to 50 mL CSF through a lumbar puncture needle is often used.6 Clinical improvement after the CSF removal is considered a positive response, with high positive predictive value for a good outcome after shunt surgery.7
Previous studies have tried to estimate the change in CBF after a CSF TT using inhalation or injection of xenon-133, single photon emission computed tomography, and dynamic susceptibility contrast magnetic resonance imaging (MRI). Increase in CBF after CSF removal has been reported in some of these studies, but the results have not been uniform.8, 9, 10, 11, 12, 13, 14
In a recent study, we showed that clinical improvement after a CSF TT is measurable during the first 24 hours after the drainage.15 The time window for the hypothesized increase in CBF after a CSF TT is, however, unknown.
Pseudo-continuous arterial spin labeling (pCASL) is an MRI technique with the advantage of allowing quantitative measurements of the CBF without injection of any contrast agent.16 The method is completely noninvasive, with a relatively short scan time (~5 minutes), which makes it ideal for repeated measurements, and it has not been used in published iNPH studies.
We measured changes in CBF and their relationship with clinical improvement during the first 24 hours after CSF removal in patients with iNPH. A secondary aim was to measure the repeatability of pCASL in patients with iNPH.
Materials and methods
Patients
Sixty patients were consecutively recruited from the waiting list of patients referred to Uppsala University Hospital for evaluation of suspected NPH. Exclusion criteria were secondary NPH or congenital hydrocephalus, contraindications to MRI, age >84 years, severe cognitive dysfunction with inability to give informed consent, refusal to undergo evaluation or shunt surgery, complete inability to walk, or gait problems that could be explained by other known diseases. Based on these criteria, 23 patients were excluded from the study. In addition, nine patients who were already included in an ongoing study of the CSF TT were not included, one patient had died since the referral had been written, and in one patient the evaluation was postponed because of surgery for an abdominal aortic aneurysm. Finally, 26 patients (15 men and 11 women) were included in the present study.
Out of the 26 patients, 17 were classified as probable iNPH, 6 as possible iNPH, and 3 as unlikely iNPH according the American iNPH guidelines.5 Three patients were excluded from statistical analyses of CBF because of artifacts related to high intravascular signal and apparent hypoperfusion in the brain parenchyma. Clinical data regarding all 26 patients are summarized in Table 1, but only the patients diagnosed with iNPH and without artifacts on perfusion images (n=20) were included in the statistical analyzes of CBF. There were no differences in symptom severity, duration, or any other clinical parameter between the excluded patients with artifacts and the whole sample. However, in the three patients with artifacts, there was significantly lower CBF at baseline in the cerebellum, pons, right and left thalamus, and WM, than in the patients without artifacts. All patients gave written informed consent. The study was approved by the local ethics committee in Uppsala, Sweden, and was conducted according to the Declaration of Helsinki.
Table 1. Demographics.
| Probable iNPH (n=17) | Possible iNPH (n=6) | Unlikely iNPH (n=3) | |
|---|---|---|---|
| Age median (range) | 74 (65–81) | 74.5 (66–81) | 71 (71–79) |
| Antihypertensive drugs, n (%) | 11 (65) | 3 (50) | 1 (33) |
| Diabetes mellitus, n (%) | 4 (24) | 2 (33) | 0 (0) |
| Symptom duration, months, median (range) | 24 (6–72) | 27 (12–48) | 36 (12–80) |
| CSF pressure cmH2O, median (range) | 16.0 (13.0–23.0) | 15.0 (9.0–19.0) | 17.5 (12.5–19.0) |
| Evans index mean (range) | 0.36 (0.30–0.43) | 0.36 (0.30–0.41) | 0.39 (0.36–0.41) |
| DWMHs, median (range) | 2 (0–3) | 2 (1–3) | 2 (2–3) |
| DESH, n (%) | 14 (82) | 2 (33) | 1 (33) |
| Callosal angle, degrees, mean (range) | 69 (53–99) | 64 (40–91) | 52 (37–67) |
| MMSE, median (range) | 25 (19–29) | 26.5 (19–29) | 23 (15–25) |
| mRS, median (range) | 2 (1–4) | 2 (1–3) | 2 (2–3) |
| Gait impairment, n (%) | 17 (100) | 6 (100) | 3 (100) |
| Cognitive impairment, n (%) | 15 (88) | 3 (50) | 3 (100) |
| Incontinence, n (%) | 13 (77) | 4 (67) | 2 (67) |
cmH2O, centimeter of water; CSF, cerebrospinal fluid; DESH, disproportionately enlarged subarachnoid-space hydrocephalus; DWMHs, deep white matter hyperintensities; iNPH, idiopathic normal pressure hydrocephalus; MMSE, mini-mental state examination; mRS, modified Rankin scale.
For descriptive purposes, the following imaging features were assessed: Evans index, deep white matter hyperintensities according to the Fazekas scale (ordinal scale from 0 to 3, with 3 representing the most severe deep white matter hyperintensities),17 disproportionately enlarged subarachnoid-space hydrocephalus,18 and the callosal angle;19 Table 1.
Time Scheme
The patients were examined on day 1 by a multidisciplinary NPH team consisting of a neurologist and specially trained physiotherapists and occupational therapists. The first MRI scan (MRI 1) was performed on day 2 between 0800 hours and 1000 hours. A second MRI (MRI 2) was performed 60 minutes after the first scan to assess the repeatability of CBF measurements in iNPH patients. Between MRI 1 and MRI 2, the patients rested in the supine position in a quiet, isolated room next to the scanner. Immediately after MRI 2, a CSF TT was performed using a 20-gauge needle with patients in the lateral recumbent position and 40±2 (mean±s.d.) mL CSF were removed. Routine blood and CSF samples were collected, including CSF biomarkers for dementia (amyloid-beta (1–42), tau, and phosphorylated tau). MRI 3 was performed 30 minutes after the CSF TT was completed; MRI 4 and MRI 5 were performed 4 and 24 hours after the CSF TT, respectively. After MRI 3, MRI 4, and MRI 5, the patients performed the same tests of motor function as on day 1. The tests were: 10 meters walk at maximum pace and the timed ‘up and go' test (TUG). Numbers of steps and time in seconds were recorded. The tests were performed twice at each assessment, and the mean result was used in the statistical analyses.
Patients ate lunch between MRI 3 and MRI 4. They were allowed to take their regular morning medications on days 1 to 3, but anticoagulants were not allowed. To minimize variations in CBF, the patients were not allowed to drink coffee or tea on days 2 and 3, and transportations between investigations were performed with the patient in bed or in a wheelchair. The patients did not leave the MRI department between MRIs 1 and 3 and were not allowed to walk during this time. Three patients missed one MRI investigation and the clinical evaluation, two of them because of technical problems with the MRI scanner and one because the patient suffered from temporary nausea.
Imaging
All patients were examined using a Philips 3T MRI scanner (Philips Achieva, Philips Healthcare, Best, the Netherlands) with a 32-channel head coil. A three-dimensional T1-weighted gradient echo (turbo field echo) sequence was obtained with the following parameters: voxel size 1.0 × 1.0 × 1.0 mm3, repetition time=8.2 ms, echo time=3.7 ms. Perfusion data were acquired using a background-suppressed pCASL sequence, with single-shot echo planar imaging read-out, using the following protocol: field of view=264 × 264 mm2, in-plane resolution=2.75 × 2.75 mm2, slice thickness=5 mm, number of slices=20, repetition time=4100 ms, echo time=14.5 ms, label duration=1650 ms, postlabeling delay (PLD)=1600 ms, and 30 repetitions. To allow for quantitative CBF values, a reference scan was performed based on the pCASL sequence with the following modifications: labeling and background suppression turned off, no repetitions, and repetition time=10000 ms.
Synthetic MRI (SyMRI) data were acquired using the multislice, multiecho, and multisaturation delay sequence QRAPMASTER.20 Because of technical problems, SyMRI data were not acquired in two patients, and in another two patients, data were missing for one investigation time. MRIs 2 to 5 only included pCASL and SyMRI, with total scan time of 10 to 15 minutes, while MRI 1 had a total scan time of 45 to 60 minutes and included several additional sequences, including the three-dimensional T1-weighted gradient echo and a T2 FLAIR (fluid-attenuated inversion recovery) sequence. Headphones were used to dampen scanner noise, and soft music was played during the investigations. Cushions were used inside the head coil to minimize head movements.
Perfusion MRI Post Processing
For perfusion quantification, the compartment model proposed by Alsop and Detre21 was used. It was assumed that the labeled blood remains primarily in the vascular bed rather than exchanging completely with tissue water, which means that the relaxation of arterial blood could be used explicitly. The magnetization difference (control—label) is thus modeled as:
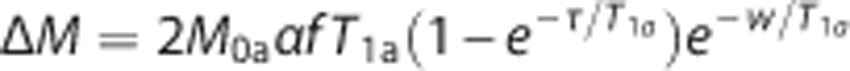 |
where M0a is the equilibrium magnetization of arterial blood, α is the labeling efficiency, f is the tissue perfusion, T1a is the longitudinal relaxation time of arterial blood, τ is the labeling duration, and w is the PLD. Total labeling effiency was set to 70%, T1a was set to 1664 ms, and M0a was estimated by using the intensity of CSF as a calibration standard. For each subject, a region in the lateral ventricles was defined, and the average intensity in the reference image within this region was used as an intensity reference for pure water. The water intensity was corrected for differences (between CSF and arterial blood) in proton density and transverse relaxation effects, and multiplied by the brain density (set to 1.04 g/mL) to yield estimates of M0a. Perfusion values were subsequently estimated from the equation and multiplied by 6000 to obtain values in (mL/100 g/min).
For each patient, perfusion images from all investigation times were coregistered to the perfusion images of MRI 1, to allow for a single delineation per patient for each region of interest (ROI). As an anatomic reference, the FLAIR image from MRI 1 was also coregistered to the perfusion images in MRI 1. The coregistrations were performed with the software ‘elastix' (http://elastix.isi.uu.nl/) using a 12-parameter affine transform, in which the pCASL reference data were used as both the reference and moving image volumes. The CBF parameter maps were spatially smoothed using a Gaussian kernel with a full width at half maximum of 4 mm. Smoothing was applied to the CBF maps to minimize the effect of nonideal image coregistration and to reduce the intrasubject variation caused by varying delineation of regions.
Regions of interest
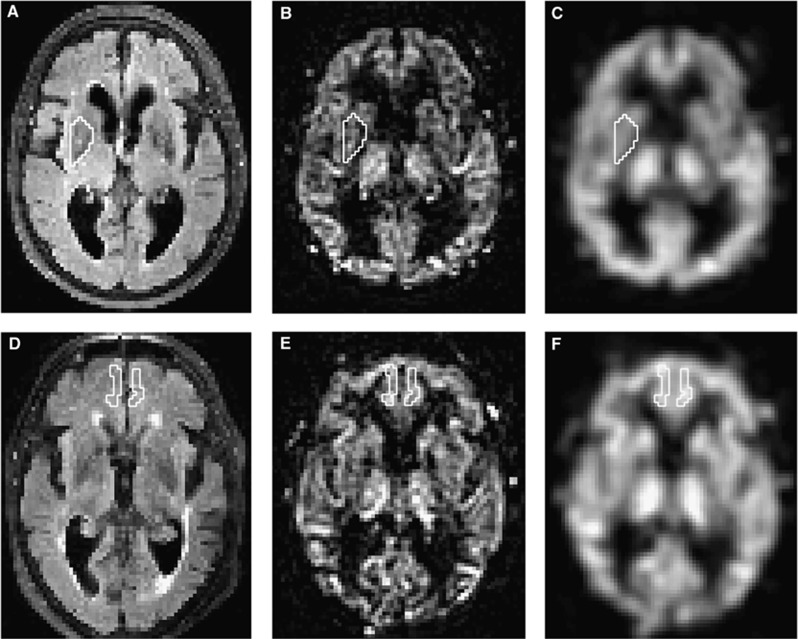
Thirteen different ROIs were drawn manually using the in-house software Eval Gui (developed by Markus Nilsson, Lund University, Lund, Sweden). The following regions were used: cerebellum, medial frontal cortex, left and right lentiform nucleus, medial temporal lobe (MTL), high convexity cortex, pons, supplementary motor area, left and right thalamus, frontal WM, lateral WM, and superior WM. The WM ROI was placed in the WM, 0.5 to 1 cm from the wall of the lateral ventricles, to minimize the impact of partial volume effects (between ventricles and parenchyma) on perfusion values. The ROIs were drawn on morphologic transverse T2 FLAIR images acquired at MRI 1 (Figures 1A and 1D), in each patient, and the coregistration secured that the ROIs had the same anatomic location on the five different scans from the same patient. All slices of all the scans were visually inspected, and care was taken not to include artifacts or large vessels in the ROIs. The ROIs were drawn by the first author (JV) and reviewed by the last author (E-ML). When ROIs were drawn, the investigator was masked to the patients' clinical data. See Table 2 for information about the ROIs and the Supplementary Information for images and anatomic explanations of all ROIs. CBF values were calculated for every voxel, and a mean CBF was provided in each ROI.
Figure 1.
Example of two ROIs in two different patients. Upper row is an example of the right lentiform nucleus ROI in patient 1 and lower row shows the medial frontal cortex ROI in patient 2. (A and D) FLAIR images that were used to draw the ROIs. (B and E) pCASL perfusion images. (C and F) Smoothed pCASL images that were used to calculate CBF. CBF, cerebral blood flow; FLAIR, fluid-attenuated inversion recovery; pCASL, pseudo-continuous arterial spin labeling; ROIs, regions of interests.
Table 2. Mean number of voxels for all ROIs and baseline CBF repeatability.
| ROIs | Slices, no. | Voxels, mean±s.d. | CBF repeatability (ICC)a | Median difference between baseline investigations (%) |
|---|---|---|---|---|
| Cerebellum | 2 | 922±133 | 0.88 | 11 |
| MFC | 3 | 136±29 | 0.89 | 9 |
| Right lentiform | 1 | 56±8 | 0.71 | 7.5 |
| Left lentiform | 1 | 60±8 | 0.80 | 4.7 |
| MTL | 1 | 113±16 | 0.71 | 6.4 |
| HCC | 2 | 1377±463 | 0.65 | 17.1 |
| Pons | 3 | 66±16 | 0.89 | 7.6 |
| SMA | 2 | 129±19 | 0.60 | 8.1 |
| Right thalamus | 2 | 56±10 | 0.85 | 12.6 |
| Left thalamus | 2 | 58±11 | 0.88 | 8.9 |
| Frontal WM | 2 | 41±8 | 0.90 | 11.1 |
| Lateral WM | 2 | 136±32 | 0.87 | 6.4 |
| Superior WM | 2 | 66±8 | 0.84 | 12.1 |
CBF, cerebral blood flow; HCC, high convexity cortex; ICC, intraclass correlation coefficient; MFC, medial frontal cortex; MTL, medial temporal lobe; pCASL, pseudo-continuous arterial spin labeling; ROIs, regions of interest; SMA, supplementary motor area; WM, white matter.
Repeatability of perfusion measurements with pCASL between two baseline investigations.
Volumetric Measurement
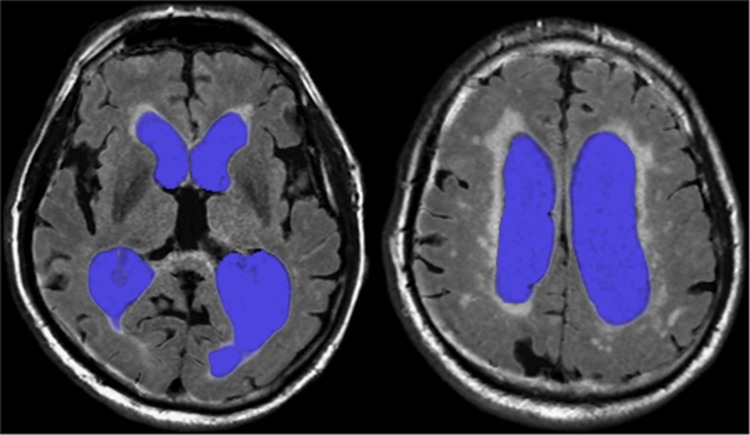
Measurements of CSF volumes were performed using acquired data from the MRI sequence QRAPMASTER, which provides a rapid simultaneous quantification of longitudinal relaxation time (T1), the transverse relaxation time (T2), and proton density.20 The dedicated software SyMRI Brain Studio (SyntheticMR, Linköping, Sweden) uses combinations of T1, T2, and proton density to distinguish between gray matter, WM, and CSF and partial volume combinations of these three types that allows volumetric estimation of these tissues. The total volume of both lateral ventricles was calculated semiautomatically, after manual delineation on each slice for all patients, Figure 2. The software provides the volume in milliliters.
Figure 2.
Segmentation of the lateral ventricles in two different slices (using SyMRI), used for CSF volume estimates pre and post CSF TT. CSF, cerebrospinal fluid; CSF TT, CSF tap test; SyMRI, synthetic magnetic resonance imaging.
Statistical Analysis
All analyses were performed using SPSS (IBM SPSS Statistics for Macintosh, Version 21.0; IBM Corp., Armonk, NY, USA). The Friedman test was used to test for differences in CBF and volumes of the lateral ventricles between different investigation times. The Wilcoxon signed-rank test was used as post hoc test. When the investigation time baseline is mentioned, it always refers to the average result of the two baseline investigations. Differences in proportional gait improvement between patients with increased CBF versus decreased CBF were analyzed with the Mann–Whitney U-test. When this difference was analyzed, to ascertain consistent results, only ROIs with a significant difference for at least two investigation times were reported as significant results. To avoid type II errors, no correction for multiple analyses was made in the analyses described above because of a limited sample of patients. The level of significance was set to P<0.05 for all statistical tests except for correlation analyses. Correlations were assessed with Spearman's correlation, and the level of significance was set to P<0.01. An intraclass correlation coefficient (ICC; two-way mixed, single measures) was used to calculate repeatability of CBF and volumetric measurements between baseline investigations. The proportional difference between baseline investigations was calculated as: (absolute difference of MRI 1 and MRI 2/average of MRI 1 and MRI 2) × 100.
Results
Cerebral Blood Flow
Cerebral blood flow values (median with interquartile range) in all ROIs and investigation times are presented in Table 3. In the MTL, CBF increased between 30 minutes post CSF TT (28.3 (25.6 to 33.0) mL/100 g/min) and 4 hours post CSF TT (29.8 (26.0 to 35.3) mL/100 g/min), P<0.001. This increase was preceded by a reduced CBF in MTL 30 minutes post CSF TT compared with baseline (29.7 (26.6 to 32.9) mL/100 g/min), P<0.05. However, at group level there was no significant increase in CBF in any of the ROIs at any investigation time after the CSF TT as compared with baseline.
Table 3. Perfusion values in all ROIs at different investigation times.
| ROIs | Baselinea perfusion, mL/100 g/min, median (IQR), n=20 | 30 Minutes post CSF TT, mL/100 g/min, median (IQR), n=20 | 4 Hours post CSF TT, mL/100 g/min, median (IQR), n=19 | 24 Hours post CSF TT, mL/100 g/min, median (IQR), n=18 | P valueb |
|---|---|---|---|---|---|
| Cerebellum | 23.1 (17.9–27.8) | 24.0 (17.4–27.0) | 19.6 (16.7–26.2) | 22.4 (19.0–31.5) | ns |
| MFC | 24.2 (21.0–29.0) | 22.9 (21.6–26.9) | 22.8 (18.9–26.5) | 24.9 (18.7–30.2) | ns |
| Right lentiform | 19.7 (17.4–22.6) | 20.2 (17.8–23.9) | 18.6 (16.3–23.2) | 20.1 (16.6–22.6) | ns |
| Left lentiform | 21.4 (18.6–23.5) | 21.6 (18.5–23.6) | 19.1 (16.6–23.1) | 20.2 (18.4–22.8) | ns |
| MTL | 29.7 (26.6–32.9) | 28.3 (25.6–33.0)* | 29.8 (26.0–35.3)*** | 30.6 (25.1–34.6) | <0.05 |
| HCC | 23.0 (19.2–27.3) | 21.5 (20.1–23.9) | 19.1 (16.8–23.9) | 21.5 (16.8–28.3) | ns |
| Pons | 24.7 (19.2–26.6) | 23.3 (17.0–26.8) | 21.5 (15.7–26.2) | 21.5 (18.8–28.2) | ns |
| SMA | 25.5 (22.5–27.3) | 24.2 (22.0–25.9) | 23.7 (21.3–26.6) | 24.8 (20.4–31.1) | ns |
| Right thalamus | 30.1 (25.7–33.2) | 27.8 (21.5–33.5) | 26.9 (20.7–32.7) | 28.5 (21.1–35.4) | ns |
| Left thalamus | 30.9 (24.9–32.3) | 27.2 (21.8–33.9) | 25.0 (17.9–33.8) | 27.0 (20.4–34.0) | ns |
| Frontal WM | 13.3 (8.5–15.4) | 12.5 (8.8–16.2) | 11.9 (6.9–16.0) | 11.3 (6.0–15.0) | ns |
| Lateral WM | 13.2 (10.7–15.4) | 12.8 (11.1–14.4) | 11.8 (9.9–15.3) | 12.9 (10.3–15.0) | ns |
| Superior WM | 15.2 (12.2–18.0) | 14.7 (12.4–18.2) | 13.3 (12.0–17.5) | 14.8 (11.3–18.5) | ns |
CSF TT, cerebrospinal fluid tap test; HCC, high convexity cortex; IQR, interquartile range; MFC, medial frontal cortex; MTL, medial temporal lobe; ns, not significant; ROIs, regions of interest; SMA, supplementary motor area; WM, white matter.
Average of the two baseline investigations.
Friedman test with Wilcoxon signed-rank test as post hoc test.
Significant decrease compared with baseline, P<0.05.
Significant increase between 30 minutes post CSF TT and 4 hours post CSF TT, P<0.001.
There was a significant difference in total median symptom duration between patients with increased and reduced CBF in the left lentiform nucleus, 24 months (12 to 30) versus 42 months (24 to 72), P<0.05. There was a difference in median CSF levels of amyloid-beta (1–42) between patients with increased and reduced CBF in the supplementary motor area, 644 ng/L (498 to 883) and 424 ng/L (383 to 527), P<0.05, respectively.
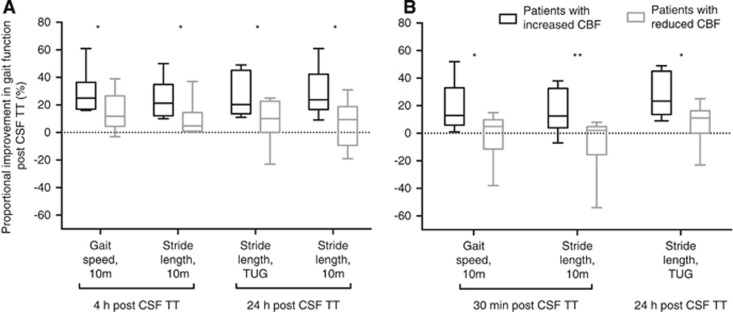
Patients with an increased CBF in lateral WM improved more in gait speed and stride length 30 minutes post CSF TT and in stride length 24 hours after the CSF TT compared with patients with decreased CBF in lateral WM. Patients with an increased CBF in frontal WM improved more in both gait speed and stride length at 4 and 24 hours after the CSF TT; Figure 3.
Figure 3.
Differences in proportional gait improvement between patients with an increased versus reduced CBF at different investigation times. The whiskers in the box-and-whiskers graph represent maximum/minimum. (A) Frontal white matter. (B) Lateral white matter. CSF TT, cerebrospinal fluid tap test; 10 m, 10 m walking test at maximum pace; TUG, timed ‘up and go'. Significant difference: *P<0.05; **P<0.01.
Still, at a group level, improvements in gait function did not correlate with proportional changes in CBF. No correlation was seen between severity of clinical symptoms at baseline and CBF at baseline in any of the ROIs. There were negative correlations between symptom duration and proportional CBF change in the left lentiform nucleus 30 minutes post CSF TT (r=−0.60, P<0.01), the left lentiform nucleus 4 hours post CSF TT (r=−0.59, P<0.01), and in the right thalamus 4 hours post CSF TT (r=−0.65, P<0.01). At baseline, there was no correlation between severity of deep white matter hyperintensities and CBF in the WM ROIs.
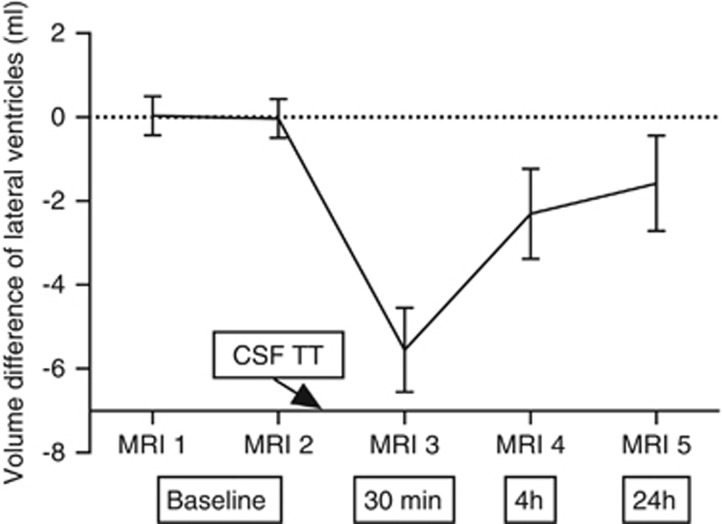
Ventricular Volume
The mean total lateral ventricular volume at baseline was 125 mL (95% CI: 116 to 134), and it was significantly reduced 30 minutes after the CSF TT. At 24 hours after the CSF TT, the volume was no longer significantly different compared with baseline, Figure 4. There was a correlation at baseline between ventricular size and CBF in superior WM (r=0.70, P<0.01). There were no correlations between changes in ventricular size and changes in CBF or between reduced ventricular volume and improvement in gait function.
Figure 4.
The mean value of the measured volumes at MRI 1 and MRI 2 was taken as reference volume. This was subtracted from the volumes at all five investigation times to obtain the volume difference per subject. The error bars indicate the 95% CI of the volume difference, obtained from the complete group. Cerebrospinal fluid tap test (CSF TT) was performed after MRI 2. The boxes: 30 minutes, 4 hours, and 24 hours, refer to time after the CSF TT. A significant difference was found between baseline (average of MRI 1 and MRI 2) and 30 minutes (P<0.001); baseline and 4 hours (P<0.01); 30 minutes and 4 hours (P<0.001); and 30 minutes and 24 hours (P<0.01), using the Wilcoxon signed-rank test.
Repeatability
Test–retest reliability of CBF for different ROIs, analyzed with ICC, was 0.60 to 0.90, and the median difference between baseline investigations was 4.7% to 17.1% Table 2. For volumetric measurement of lateral ventricles, ICC was 1.0, and the median difference between baseline investigations was 1%.
Discussion
To our knowledge this is the first published study using pCASL to investigate CBF in patients with iNPH. Our main findings were that patients with increased CBF in lateral and frontal WM after the CSF TT improved more in gait function than did patients with decreased CBF in these regions. However, in the whole sample, there was no significant increase in CBF after CSF removal compared with baseline investigations. We found an acceptable repeatability for pCASL, which strengthens the reliability of our results.
White matter surrounding the lateral ventricles, especially in the frontal lobes, seems to be of importance for gait function both in elderly people in general and in patients with iNPH.22,23 Fibers in these areas connect brain regions likely involved in iNPH, such as the supplementary motor cortex, basal ganglia, and thalamus, and in elderly people without iNPH, WM hyperintensities in the frontal lobe and periventricular WM have the strongest relationship with impairments in balance and gait.24 Our findings suggest that there may be an important relationship between the reversibility of iNPH symptoms and CBF in the deep WM.
In accordance with our result, Kushner et al,12 Kristensen et al,10 and Dumarey et al25 reported no consistent increase in CBF after the CSF TT. Too short or too long time interval between CSF removal and the perfusion imaging has been proposed as an explanation for these negative results.14 In these studies, only one perfusion investigation was performed after the CSF TT, whereas we repeated the perfusion MRI scans three times during the first 24 hours after the CSF TT. Still, we did not see any general increases in CBF, although a small increase in MTL between 30 minutes and 4 hours post CSF TT was observed. In contrast, several other studies have reported an increased CBF after drainage of CSF.8,9,11,13,14,26, 27, 28 The techniques used in these studies were diverse, few used a tomographic method, and none of them adjusted for normal variation in CBF. All but two of these studies investigated a mixed sample of both patients with iNPH and secondary NPH, and the other two described an atypical picture in some patients. Walter et al11 included patients with cognitive dysfunction without gait impairment and Hertel et al14 patients as young as 39 years.
The pCASL technique used in the present study has several advantages compared with other perfusion methods. The test–retest reliability in healthy controls and in patients with Alzheimer's disease (AD) is high, and CBF values have been validated against positron emission tomography.29 Similar to positron emission tomography, the pCASL technique can provide quantitative perfusion data without contrast agent injection. Dynamic susceptibility contrast MRI is an alternative perfusion MRI technique that requires injection of a gadolinium-based contrast agent, and for this reason it is not used repeatedly to evaluate potential perfusion changes over time. In addition, dynamic susceptibility contrast MRI does not allow absolute quantification of CBF in the clinical setting. Since pCASL is noninvasive, without ionizing radiation, and has a rather short scan time, the method is ideal for repeated measurements.
Measurements of perfusion in iNPH have been performed during the past 40 years, but to our knowledge this is the first time the repeatability between two baseline investigations has been systematically estimated in this patient category. Excellent repeatability was seen in 9/13 ROIs (ICC>0.74) and good repeatability was seen in 4/13 ROIs (ICC 0.6 to 0.74). However, the variation at baseline was not negligible, which must be taken into account in perfusion studies in which differences after CSF TT and shunting are analyzed.
An unexpected result was a small decrease in CBF in MTL at 30 minutes after CSF TT. In patients with AD or undefined dementia, it has been reported that CBF decreases after removal of CSF.9,28 Actually, we found low levels of amyloid-beta in CSF in patients with reduced CBF in supplementary motor area, compared with patients with increased CBF after the CSF TT. A reduced level of amyloid-beta in CSF is a well-known finding in AD,30 and in studies of brain biopsies in patients with iNPH, a deposit of amyloid-beta in the cortex is not an uncommon finding.31 Based on the clinical evaluation, we doubt that there were patients with manifest AD in this sample, and the imaging did not reveal pronounced atrophy in any of the patients. Potential MTL atrophy can be difficult to evaluate in iNPH owing to the dilated temporal horns. However, some comorbidity cannot be ruled out, and iNPH and AD can sometimes coexist in the same patient.32 Finally, the decrease in CBF in MTL 30 minutes after CSF TT must be interpreted in the light of normal variation between baseline investigations of 6.4%. Also, the MTL region is often affected by susceptibility effects from air in the sphenoid sinus, which leads to artifacts.
It has been proposed that mechanical stretching of blood vessels by the dilated ventricles may cause reduction of perfusion in NPH, and when CSF is removed the stretching ceases and perfusion is restored.28 This was not confirmed in our study, since there were no negative correlations between ventricular volume and CBF, or correlations between changes in ventricular volume and CBF. Other mechanisms are probably involved in the reduction of perfusion and reversibility after CSF removal in some patients. The periventricular hyperintensities seen in iNPH, which are reported to decrease after shunting,33 are believed to represent increased water content mainly in the extracellular space.34 It is possible that this increased water content in WM leads to a reduced elimination of toxic substances and disturbances in metabolism and regional CBF.35
We hypothesize that when CSF is removed from the subarachnoid space, the edema is partly resorbed back to the ventricles because of a temporary pressure gradient. Disturbances in the WM caused by edema may become temporarily reversed and CBF increases. In half of the patients in this sample, no increase in CBF in WM followed the CSF removal, which may be the reason for less clinical improvement, possibly because of a pathologic process that had become irreversible. Contradicting the theory of stretched arteries, we found a positive correlation between ventricular volume and CBF in the superior WM at baseline, possibly explained by an increased density of brain parenchyma in this region because of compression from the dilated ventricles.36
No correlation was seen between severity of clinical symptoms at baseline and CBF at baseline in any ROI. However, the level of significance was set at P<0.01 for correlation analyses to avoid a type I error caused by multiple correlations. The lack of correlations suggests that the etiology of symptoms in iNPH is multifactorial and influenced by factors other than CBF. This is further supported by a study that compared symptomatic and asymptomatic patients, both with iNPH morphology on MRI, and found no difference in CBF between the two groups.37
In the present study, patients with an increase in CBF in the left lentiform nucleus had a shorter duration of iNPH symptoms than those with no increase, and there was a correlation between symptom duration and decrease in CBF in both the lentiform nucleus and the thalamus. This may partly explain inconsistent results in previous studies investigating CBF change after CSF removal, i.e., it is possible that increase in CBF may be greater in patients with a short duration of symptoms compared with those with a prolonged course.
The volume of the lateral ventricles was determined using SyMRI. Ventricular volume was reduced by 4.4% 30 minutes after CSF TT, and the day after the drainage the volume was restored. Since calculation of regional CBF was made using manually drawn ROIs, and since all images were visually evaluated to exclude artifacts and morphologic changes from any of the ROIs, the change in ventricular volume is not likely to cause partial volume effects on the CBF results in this study.
The CBF values in our patients were low compared with those reported by others. For example, in the thalamus and cerebellum, CBF was 14% and 34%, respectively, lower than found in a positron emission tomography study by Owler et al.3 However, this did not have any impact on the results in our study because the patients were their own controls.
Limitations
Three patients were excluded because of artifacts related to high intravascular signal with low signal intensity in the brain parenchyma. This occurs when the PLD of the ASL sequence is shorter than the arterial transit time, the time needed for the labeled blood to move from the labeling plane to the tissue of interest in the brain. Arterial transit time seems to be longer in the aging brain and in pathologic cerebrovascular systems.38 We used a standard clinical protocol for pCASL, with a PLD of 1600 ms. Because of artifacts related to prolonged arterial transit time, no analysis could be performed for watershed areas in parietal/occipital regions of the brain. A longer PLD produces a lower signal-to-noise ratio, but to avoid the problems we had in this study it may be beneficial to use a PLD of at least 2000 ms in patients with iNPH, which was the recommended PLD for elderly clinical patients in a recently published ASL white paper.39 It should also be noted that pCASL-based WM perfusion estimates ought to be treated with some caution owing to longer arterial transit time and low signal-to-noise ratio, compared with gray matter, and it has been suggested that many repetitions, and possibly longer PLDs, are needed for reliable results.40 However, the statistical analysis in our study was based on 20 patients, scanned multiple times each, and only ROIs with a significant difference at two investigation times were reported, which strengthens the findings. Moreover, a recent study by Wu et al38 found that the optimal PLD to detect WM perfusion with pCASL was 1500 to 1800 ms (i.e., similar to the PLD used in this study), based on simulated and experimental data from healthy volunteers. This means that, even when WM perfusion is of interest, the PLD should probably only be modified to account for age and pathology. No control group was included in the present study, which is a limitation since comparisons of baseline CBF between iNPH patients and healthy controls has not been performed using pCASL.
The ROIs were drawn manually slice-by-slice on coregistered FLAIR images, a time consuming but usually anatomically correct method. The disadvantage is that it is, to some degree, operator dependent. The more commonly used method is to normalize the images to a standard brain template and use automatically drawn ROIs to calculate mean CBF in different groups. These methods assume an intact morphology, which is seldom the case in iNPH patients with wide ventricles, effaced sulci at the convexity, and focally dilated sulci in other regions.
In the evaluation of clinical improvement after the CSF TT, we only used tests of gait function because of the rigid time scheme and because cognitive tests take more time. Impairment of cognition is an important symptom in iNPH, and a thorough investigation of the relationships between cognition and CBF, after CSF removal, would be of great interest.
The level of significance was adjusted for correlations because of large correlation matrices. To avoid Type II errors, no correction for multiple analyses were made to the other analyses, given the limited sample of patients.
The strength of this work was the consecutive inclusion in such a complicated study with a rigid time scheme and the repeated CBF measurements with clinical evaluations in connection with every MRI scan.
Future studies will focus on comparing iNPH with healthy controls using pCASL and investigating perfusion changes after shunting.
Conclusions
In this study, we measured regional brain perfusion in iNPH patients before and several times after CSF removal to investigate the relationship between CBF changes and improvement of gait function. In the whole sample of 20 investigated iNPH patients, CBF did not increase during 24 hours after CSF TT compared with baseline. However, gait function improved more in patients with increased CBF in WM compared with those with decreased CBF. The variation of CBF was low but must be accounted for in studies of perfusion in iNPH.
Acknowledgements
The authors thank Markus Nilsson for the software Eval Gui, Marcel Warntjes for assistance with the software SyMRI, Lisa Wernroth for statistical consultation and Markus Fahlström for technical assistance. The authors also thank our NPH team and the MRI staff, especially Britt-Mari Bolinder. Finally, the authors thank the European COST action BM1103 on arterial spin labelling initiative in dementia (AID) for promoting networking and collaborative work between research centers and Selanders Stiftelse for their support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with ‘normal' cerebrospinal-fluid pressure—a treatable syndrome. N Engl J Med. 1965;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- Owler BK, Pickard JD. Normal pressure hydrocephalus and cerebral blood flow: a review. Acta Neurol Scand. 2001;104:325–342. doi: 10.1034/j.1600-0404.2001.00092.x. [DOI] [PubMed] [Google Scholar]
- Owler BK, Momjian S, Czosnyka Z, Czosnyka M, Pena A, Harris NG, et al. Normal pressure hydrocephalus and cerebral blood flow: a PET study of baseline values. J Cereb Blood Flow Metab. 2004;24:17–23. doi: 10.1097/01.WCB.0000093326.88757.49. [DOI] [PubMed] [Google Scholar]
- Ziegelitz D, Starck G, Kristiansen D, Jakobsson M, Hultenmo M, Mikkelsen IK, et al. Cerebral perfusion measured by dynamic susceptibility contrast MRI is reduced in patients with idiopathic normal pressure hydrocephalus. J Magn Reson Imaging. 2013;39:1533–1542. doi: 10.1002/jmri.24292. [DOI] [PubMed] [Google Scholar]
- Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S4–S16. doi: 10.1227/01.neu.0000168185.29659.c5. [DOI] [PubMed] [Google Scholar]
- Wikkelso C, Andersson H, Blomstrand C, Lindqvist G. The clinical effect of lumbar puncture in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1982;45:64–69. doi: 10.1136/jnnp.45.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikkelso C, Hellstrom P, Klinge PM, Tans JT, European i NPHMSG The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF Tap Test in patients with idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2013;84:562–568. doi: 10.1136/jnnp-2012-303314. [DOI] [PubMed] [Google Scholar]
- Mathew NT, Meyer JS, Hartmann A, Ott EO. Abnormal cerebrospinal fluid-blood flow dynamics. Implications in diagnosis, treatment, and prognosis in normal pressure hydrocephalus. Arch Neurol. 1975;32:657–664. doi: 10.1001/archneur.1975.00490520027003. [DOI] [PubMed] [Google Scholar]
- Mamo HL, Meric PC, Ponsin JC, Rey AC, Luft AG, Seylaz JA. Cerebral blood flow in normal pressure hydrocephalus. Stroke. 1987;18:1074–1080. doi: 10.1161/01.str.18.6.1074. [DOI] [PubMed] [Google Scholar]
- Kristensen B, Malm J, Fagerland M, Hietala SO, Johansson B, Ekstedt J, et al. Regional cerebral blood flow, white matter abnormalities, and cerebrospinal fluid hydrodynamics in patients with idiopathic adult hydrocephalus syndrome. J Neurol Neurosurg Psychiatry. 1996;60:282–288. doi: 10.1136/jnnp.60.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter C, Hertel F, Naumann E, Morsdorf M. Alteration of cerebral perfusion in patients with idiopathic normal pressure hydrocephalus measured by 3D perfusion weighted magnetic resonance imaging. J Neurol. 2005;252:1465–1471. doi: 10.1007/s00415-005-0891-z. [DOI] [PubMed] [Google Scholar]
- Kushner M, Younkin D, Weinberger J, Hurtig H, Goldberg H, Reivich M. Cerebral hemodynamics in the diagnosis of normal pressure hydrocephalus. Neurology. 1984;34:96–99. doi: 10.1212/wnl.34.1.96. [DOI] [PubMed] [Google Scholar]
- Mori K, Maeda M, Asegawa S, Iwata J. Quantitative local cerebral blood flow change after cerebrospinal fluid removal in patients with normal pressure hydrocephalus measured by a double injection method with N-isopropyl-p-[(123)I] iodoamphetamine. Acta Neurochir (Wien) 2002;144:255–262. doi: 10.1007/s007010200033. [DOI] [PubMed] [Google Scholar]
- Hertel F, Walter C, Schmitt M, Morsdorf M, Jammers W, Busch HP, et al. Is a combination of Tc-SPECT or perfusion weighted magnetic resonance imaging with spinal tap test helpful in the diagnosis of normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2003;74:479–484. doi: 10.1136/jnnp.74.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J, Cesarini KG, Laurell K. The CSF tap test in normal pressure hydrocephalus: evaluation time, reliability and the influence of pain. Eur J Neurol. 2012;19:271–276. doi: 10.1111/j.1468-1331.2011.03486.x. [DOI] [PubMed] [Google Scholar]
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ishikawa M, Mori E, Kuwana N, Study of Ioni Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res. 2010;7:18. doi: 10.1186/1743-8454-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kanda T, Harada A, Miyamoto N, Kawaguchi T, Shimada K, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol. 2008;18:2678–2683. doi: 10.1007/s00330-008-1044-4. [DOI] [PubMed] [Google Scholar]
- Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med. 2008;60:320–329. doi: 10.1002/mrm.21635. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- Jurcoane A, Keil F, Szelenyi A, Pfeilschifter W, Singer OC, Hattingen E. Directional diffusion of corticospinal tract supports therapy decisions in idiopathic normal-pressure hydrocephalus. Neuroradiology. 2013;56:5–13. doi: 10.1007/s00234-013-1289-8. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Delbaere K, Close JC, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011;42:2086–2090. doi: 10.1161/STROKEAHA.110.610360. [DOI] [PubMed] [Google Scholar]
- Dumarey NE, Massager N, Laureys S, Goldman S. Voxel-based assessment of spinal tap test-induced regional cerebral blood flow changes in normal pressure hydrocephalus. Nucl Med Commun. 2005;26:757–763. doi: 10.1097/01.mnm.0000170937.90958.22. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Jr., Raichle ME, Gado MH, Eichling JO, Hughes CP. Cerebral blood flow, oxygen utilization, and blood volume in dementia. Neurology. 1977;27:905–910. doi: 10.1212/wnl.27.10.905. [DOI] [PubMed] [Google Scholar]
- Moretti JL, Sergent A, Louarn F, Rancurel G, le Percq M, Flavigny R, et al. Cortical perfusion assessment with 123I-isopropyl amphetamine (123I-IAMP) in normal pressure hydrocepha lus (NPH) Eur J Nucl Med. 1988;14:73–79. doi: 10.1007/BF00253445. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Tachibana H, Hardenberg JP, Dowell RE, Jr., Kitagawa Y, Mortel KF. Normal pressure hydrocephalus. Influences on cerebral hemodynamic and cerebrospinal fluid pressure—chemical autoregulation. Surg Neurol. 1984;21:195–203. doi: 10.1016/0090-3019(84)90342-2. [DOI] [PubMed] [Google Scholar]
- Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR Biomed. 2010;23:286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C, Hansson O, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer's disease—current concepts. Mol Neurodegener. 2013;8:20. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen V, Rinne JO, Virtanen KA, Eskola O, Rummukainen J, Huttunen J, et al. Positron emission tomography with [18F]flutemetamol and [11C]PiB for in vivo detection of cerebral cortical amyloid in normal pressure hydrocephalus patients. Eur J Neurol. 2013;20:1043–1052. doi: 10.1111/ene.12102. [DOI] [PubMed] [Google Scholar]
- Malm J, Graff-Radford NR, Ishikawa M, Kristensen B, Leinonen V, Mori E, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS. 2013;10:22. doi: 10.1186/2045-8118-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullberg M, Hultin L, Ekholm S, Mansson JE, Fredman P, Wikkelso C. White matter changes in normal pressure hydrocephalus and Binswanger disease: specificity, predictive value and correlations to axonal degeneration and demyelination. Acta Neurol Scand. 2002;105:417–426. doi: 10.1034/j.1600-0404.2002.01189.x. [DOI] [PubMed] [Google Scholar]
- Aygok G, Marmarou A, Fatouros P, Young H. Brain tissue water content in patients with idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl. 2006;96:348–351. doi: 10.1007/3-211-30714-1_72. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Sonnewald U, Tullberg M, Wikkelso C. Brain metabolism in adult chronic hydrocephalus. J Neurochem. 2008;106:1515–1524. doi: 10.1111/j.1471-4159.2008.05422.x. [DOI] [PubMed] [Google Scholar]
- Ishii K, Hashimoto M, Hayashida K, Hashikawa K, Chang CC, Nakagawara J, et al. A multicenter brain perfusion SPECT study evaluating idiopathic normal-pressure hydrocephalus on neurological improvement. Dement Geriatr Cogn Disord. 2011;32:1–10. doi: 10.1159/000328972. [DOI] [PubMed] [Google Scholar]
- Takaya M, Kazui H, Tokunaga H, Yoshida T, Kito Y, Wada T, et al. Global cerebral hypoperfusion in preclinical stage of idiopathic normal pressure hydrocephalus. J Neurol Sci. 2010;298:35–41. doi: 10.1016/j.jns.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Wu WC, Lin SC, Wang DJ, Chen KL, Li YD. Measurement of cerebral white matter perfusion using pseudocontinuous arterial spin labeling 3T magnetic resonance imaging—an experimental and theoretical investigation of feasibility. PLoS One. 2013;8:e82679. doi: 10.1371/journal.pone.0082679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia Magn Reson Med 2014. doi: 10.1002/mrm.25197(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- van Osch MJ, Teeuwisse WM, van Walderveen MA, Hendrikse J, Kies DA, van Buchem MA. Can arterial spin labeling detect white matter perfusion signal. Magn Reson Med. 2009;62:165–173. doi: 10.1002/mrm.22002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.