Abstract
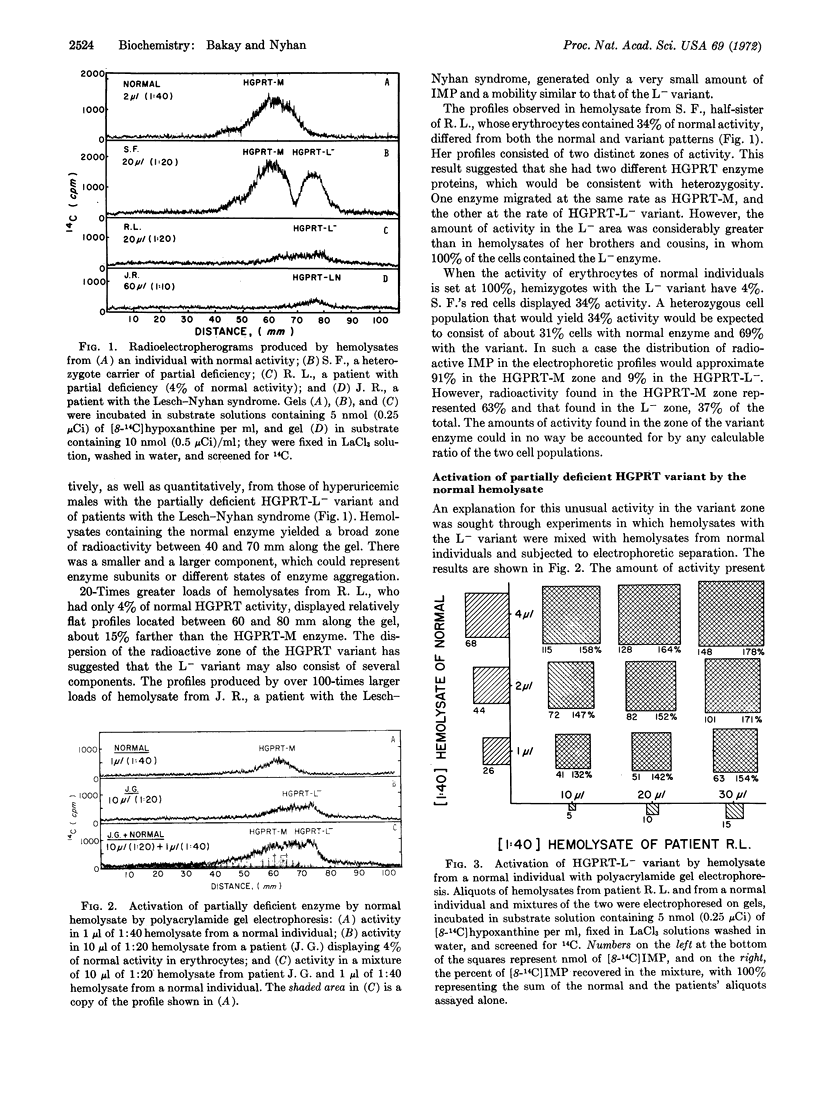
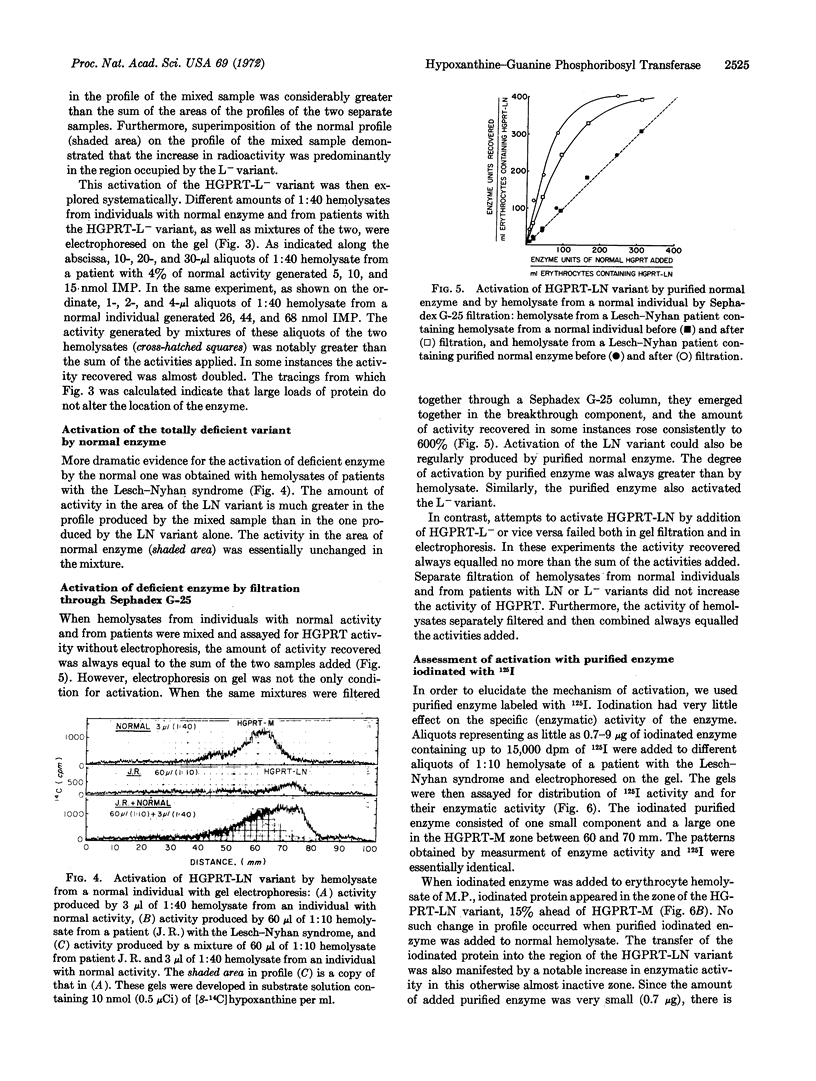
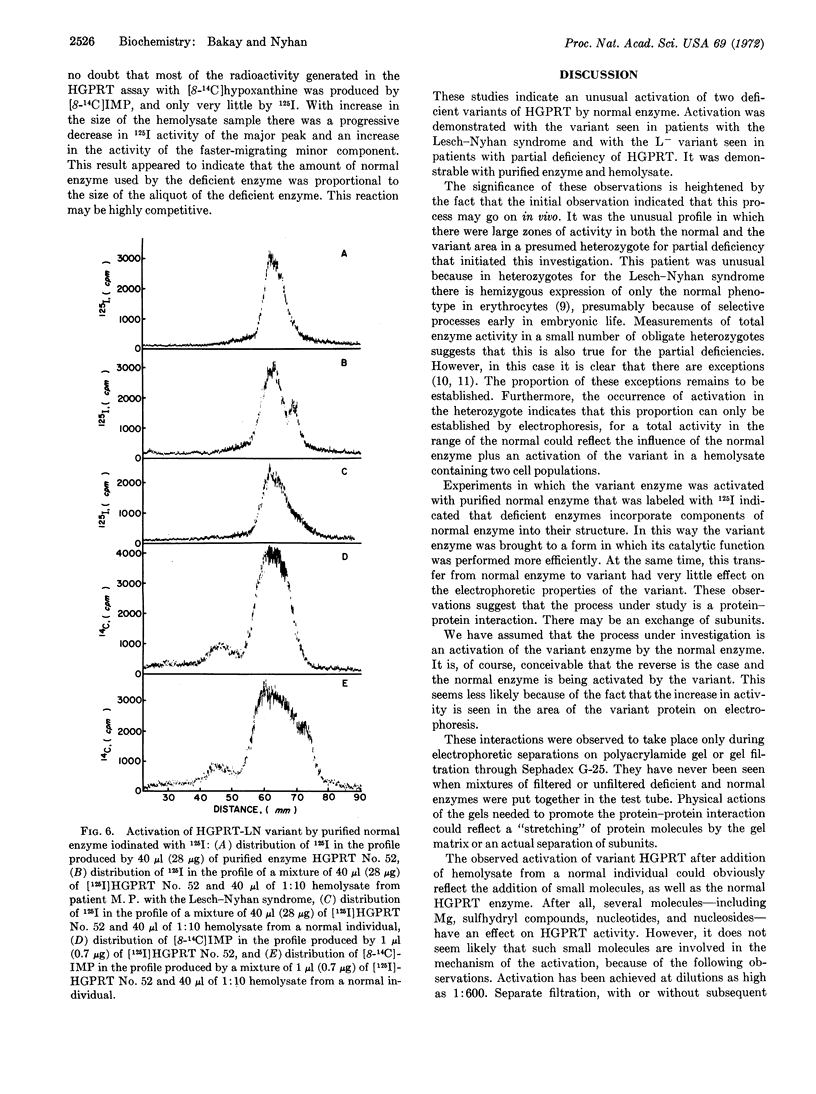
Deficient hypoxanthine-guanine phosphoribosyl transferase (HGPRT; EC 2.4.2.8) enzymes from erythrocytes of patients with hyperuricemia and with the Lesch-Nyhan syndrome migrate 15% faster in polyacrylamide gel disc electrophoresis than the normal enzyme. A half-sister of two males with partial deficiency, who had 34% of normal HGPRT activity in her erythrocytes, yielded profiles containing two distinct zones of activity; one corresponded to the enzyme found in normal individuals and one to the variant of her half-brothers. However, in her profile her variant enzyme showed notably greater activity than that observed in her half-brothers. This increase was due to an activation of the variant by normal enzyme. Electrophoresis of mixtures of normal enzyme with partially deficient enzymes from patients with hyperuricemia and with the Lesch-Nyhan syndrome also led to activation of deficient HGPRT variants by normal enzymes. Deficient variants were also activated by normal enzyme on filtration through Sephadex G-25. Experiments in which deficient variant enzymes were activated with purified normal enzyme labeled with 125I indicated that deficient enzymes incorporate components of the normal enzyme. No such activation of deficient enzymes was ever obtained when mixtures of deficient and normal enzymes were put together in a test tube.
Keywords: isoenzymes, Lesch-Nyhan syndrome, hyperuricemia, electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakay B. Detection of radioactive components in polyacrylamide gel disc electropherograms by automated mechanical fractionation. Anal Biochem. 1971 Apr;40(2):429–439. doi: 10.1016/0003-2697(71)90403-9. [DOI] [PubMed] [Google Scholar]
- Bakay B., Nyhan W. L. Electrophoretic properties of hypoxanthine-guanine phosphoribosyl transferase in erythrocytes of subjects with Lesch-Nyhan syndrome. Biochem Genet. 1972 Apr;6(2):139–146. doi: 10.1007/BF00486398. [DOI] [PubMed] [Google Scholar]
- Bakay B., Nyhan W. L., Fawcett N., Kogut M. D. Isoenzymes of hypoxanthine-guanine-phosphoribosyl transferase in a family with partial deficiency of the enzyme. Biochem Genet. 1972 Aug;7(1):73–85. doi: 10.1007/BF00487011. [DOI] [PubMed] [Google Scholar]
- Bakay B., Nyhan W. L. The separation of adenine and hypoxanthine-guanine phosphoribosyl transferases isoenzymes by disc gel electrophoresis. Biochem Genet. 1971 Feb;5(1):81–90. doi: 10.1007/BF00485733. [DOI] [PubMed] [Google Scholar]
- Emmerson B. T., Wyngaarden J. B. Purine metabolism in heterozygous carriers of hypoxanthine-guanine phosphoribosyltransferase deficiency. Science. 1969 Dec 19;166(3912):1533–1535. doi: 10.1126/science.166.3912.1533. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Gaucher's disease: deficiency of 'acid' -glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kogut M. D., Donnell G. N., Nyhan W. L., Sweetman L. Disorder of purine metabolism due to partial deficiency of hypoxanthine-guanine phosphoribosyltransferase. A study of a family. Am J Med. 1970 Feb;48(2):148–161. doi: 10.1016/0002-9343(70)90111-7. [DOI] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S. A specific apoprotein activator for lipoprotein lipase. Biochem Biophys Res Commun. 1970 Oct 9;41(1):57–62. doi: 10.1016/0006-291x(70)90468-7. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Melchers F., Messer W. Enhanced stability against heat denaturation of E. coli wild type and mutant beta-galactosidase in the presence of specific antibodies. Biochem Biophys Res Commun. 1970 Aug 11;40(3):570–575. doi: 10.1016/0006-291x(70)90940-x. [DOI] [PubMed] [Google Scholar]
- Messerw, Melchers F. Genetic analysis of mutants producing defective beta-galactosidase which can be activated by specific antibodie. Mol Gen Genet. 1970;109(2):152–161. doi: 10.1007/BF00269651. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Der Kaloustian V. M., Nyhan W. L., Yough W. J., Childs B. X-linked hypoxanthine-guanine phosphoribosyl transferase deficiency: heterozygote has two clonal populations. Science. 1968 Apr 26;160(3826):425–427. doi: 10.1126/science.160.3826.425. [DOI] [PubMed] [Google Scholar]
- Nyhan W. L., Bakay B., Connor J. D., Marks J. F., Keele D. K. Hemizygous expression of glucose-6-phosphate dehydrogenase in erythrocytes of heterozygotes for the Lesch-Nyhan syndrome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):214–218. doi: 10.1073/pnas.65.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann J., DeMars R., Benke P. Single-allele expression at an X-linked hyperuricemia locus in heterozygous human cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):545–552. doi: 10.1073/pnas.60.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]




