Abstract
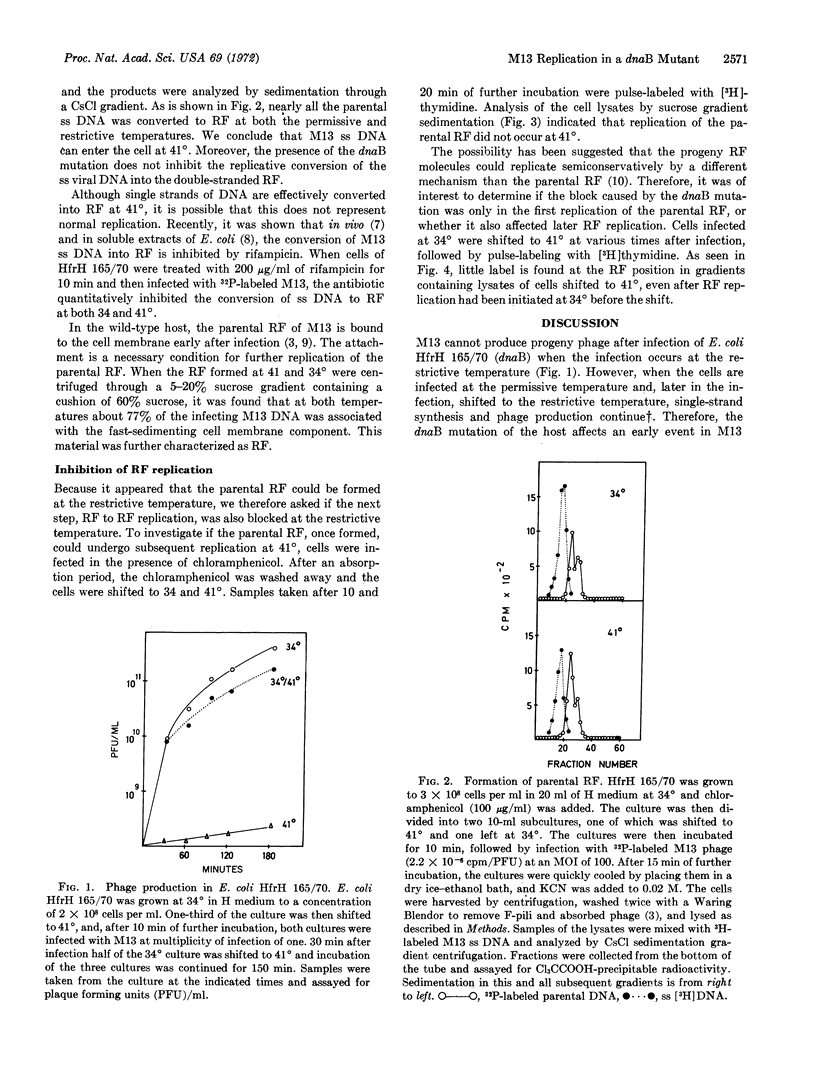
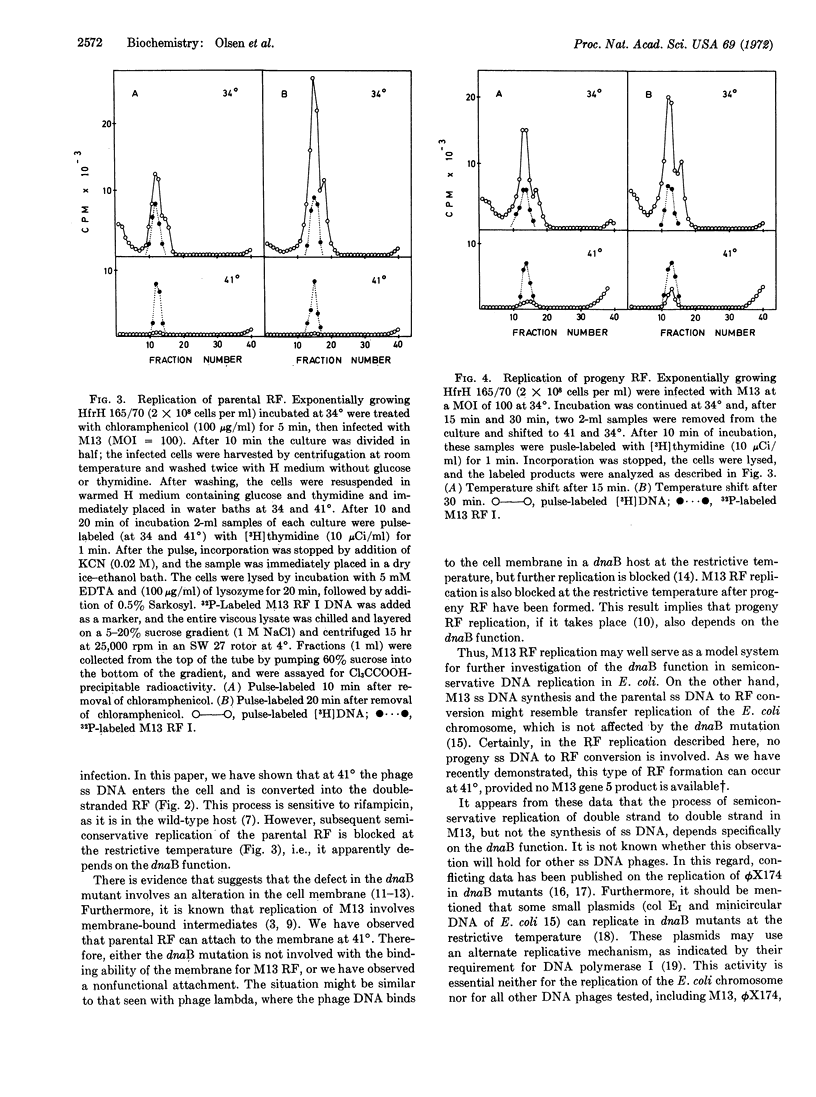
Infection of the temperature-sensitive E. coli mutant HfrH 165/70 (dnaB) with the filamentous single-stranded DNA phage M13 is abortive at the restrictive temperature. Upon infection at 41°, single-stranded phage DNA penetrates the cell and is converted in a rifampicin-sensitive step to the double-stranded replicative form (RF). The parental RF attaches to the cell membrane, but subsequent replication of the RF is blocked. It is concluded that in M13 infection semiconservative RF replication of a double strand to a double strand, in contrast to single-stranded DNA synthesis, depends specifically on the dnaB function.
Keywords: dnaB mutation, DNA synthesis, RF replication
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyersmann D., Schuster H. DNA synthesis in P1 infected E. coli mutants temperature-sensitive in DNA replication. Mol Gen Genet. 1972;114(2):173–176. doi: 10.1007/BF00332788. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsheit A. B., Ray D. S. Replication of bacteriophage M13. VI. Attachment of M13 DNA to a fast-sedimenting host cell component. Virology. 1971 Mar;43(3):647–664. doi: 10.1016/0042-6822(71)90289-3. [DOI] [PubMed] [Google Scholar]
- Goebel W. Replication of the DNA of the colicinogenic factor E 1 (Col E 1 ) at the restrictive temperature in a DNA replication mutant thermosensitive for DNA polymerase. 3. Nat New Biol. 1972 May 17;237(72):67–70. doi: 10.1038/newbio237067a0. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Replication of plasmid DNA in temperature-sensitivity DNA replication mutants of Escherichia coli. Biochim Biophys Acta. 1972 Feb 23;262(1):32–41. doi: 10.1016/0005-2787(72)90216-x. [DOI] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Hohn B., Lechner H., Marvin D. A. Filamentous bacterial viruses. I. DNA synthesis during the early stages of infection with fd. J Mol Biol. 1971 Feb 28;56(1):143–154. doi: 10.1016/0022-2836(71)90090-8. [DOI] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S., Geuther R., Noack D. Membrane attachment of lambda DNA in mutants of Escherichia coli temperature sensitive in DNA replication. Mol Gen Genet. 1972;115(1):97–100. doi: 10.1007/BF00272223. [DOI] [PubMed] [Google Scholar]
- Lanka E., Schuster H. Replication of bacteriophages in Escherichia coli mutants thermosensitive in DNA synthesis. Mol Gen Genet. 1970;106(3):274–285. doi: 10.1007/BF00340386. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Primrose S. B., Brown L. R., Dowell C. E. Host cell participation in small virus replication. I. Replication of M-13 in a strain of Escherichia coli with a temperature-sensitive lesion in deoxyribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1308–1314. doi: 10.1128/jvi.2.11.1308-1314.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose S. B., Landers W. M., Dowell C. E. Replication of bacteriophage phiX174 in a temperature-sensitive deoxyribonucleic acid host cell. J Virol. 1971 Oct;8(4):594–596. doi: 10.1128/jvi.8.4.594-596.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siccardi A. G., Shapiro B. M. On the process of cellular division in Escherichia coli. IV. Altered protein composition and turnover of the membranes of thermosensitive mutants defective in chromosomal replication. J Mol Biol. 1971 Mar 28;56(3):475–490. doi: 10.1016/0022-2836(71)90395-0. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Membrane attachment of replicating parental DNA molecules of bacteriophage M13. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1035–1041. doi: 10.1016/0006-291x(71)90008-8. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz A., Hofschneider P. H. Replication of the single stranded DNA bacteriophage M 13. Intracellular flow of parental DNA and transfer to progeny particles. Eur J Biochem. 1970 Nov;17(1):141–150. doi: 10.1111/j.1432-1033.1970.tb01146.x. [DOI] [PubMed] [Google Scholar]


