Abstract
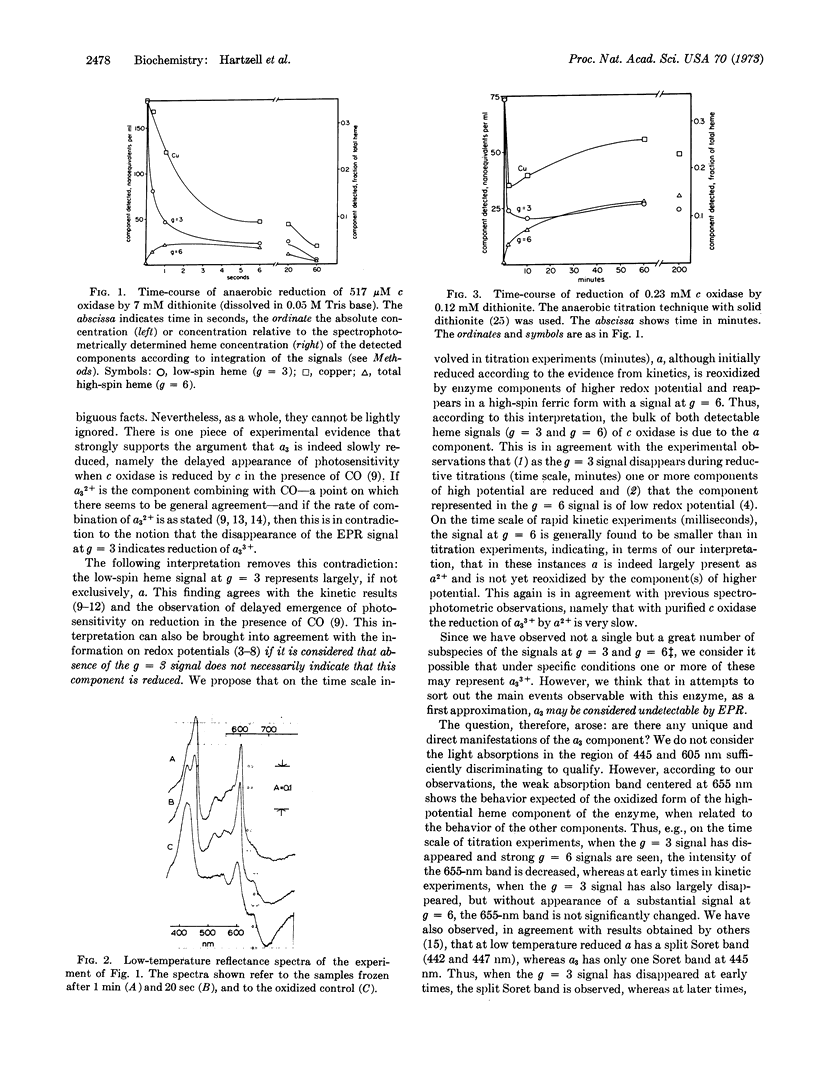
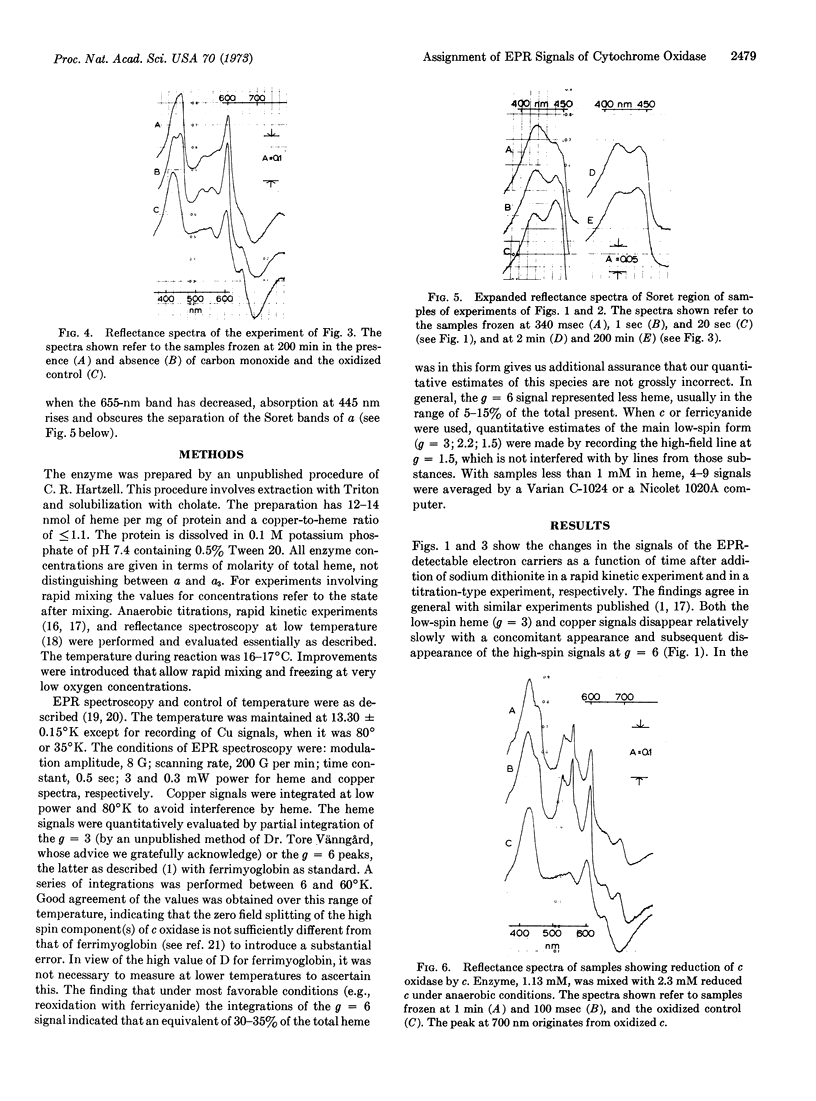
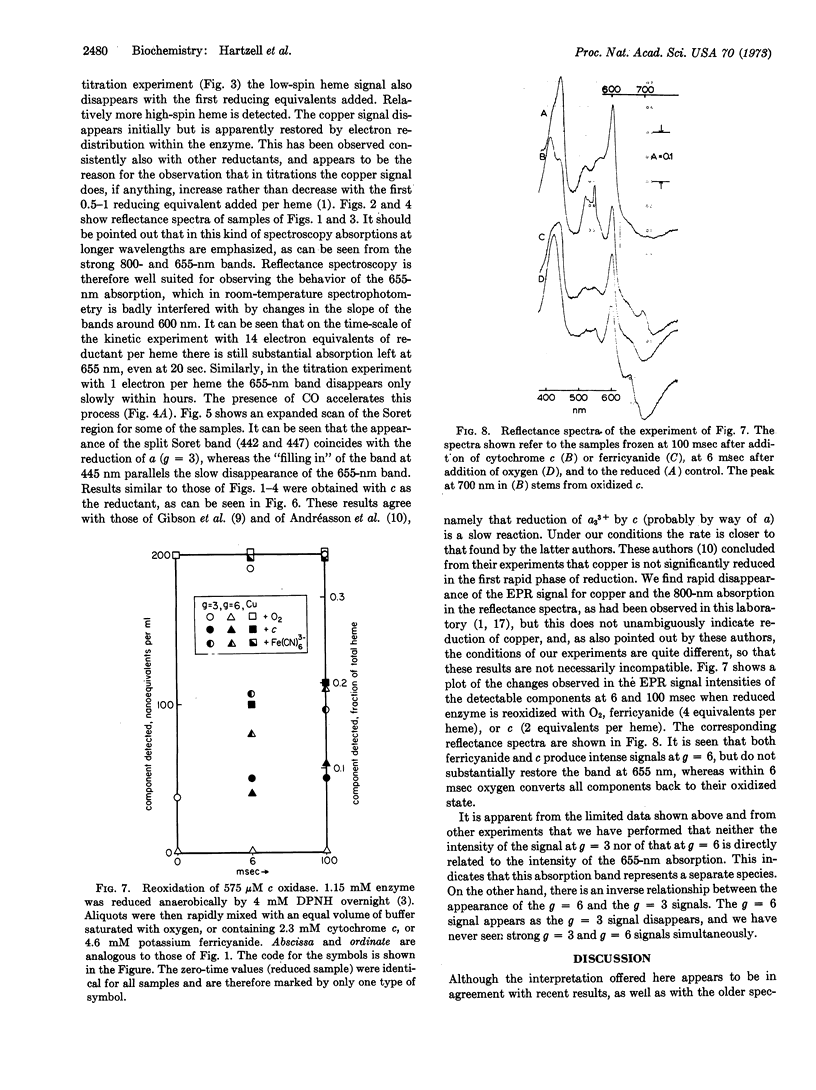
On the basis of oxidoreductive rapid kinetic and titration experiments with purified cytochrome c oxidase (EC 1.9.3.1), monitored by electron paramagnetic resonance (EPR) at 13°K and by spectrophotometry at 100°K, a new assignment of EPR signals is proposed. The bulk of both the low-spin (g = 3.0; 2.2; 1.5) and highspin (g = 6; 2) signals is attributed to the component with the properties of traditional cytochrome a. It is further proposed that the absorption band at 655 nm represents the most unambiguous manifestation of the a3 component.
Keywords: cytochrome a, a3; low-temperature spectra; rapid kinetics; redox titrations
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. -E., Malmström B. G., Strömberg C., Vänngård T. The reaction of ferrocytochrome c with cytochrome oxidase: A new look. FEBS Lett. 1972 Dec 15;28(3):297–301. doi: 10.1016/0014-5793(72)80735-x. [DOI] [PubMed] [Google Scholar]
- BEINERT H., PALMER G. OXIDATION-REDUCTION OF THE COPPER COMPONENT OF CYTOCHROME OXIDASE. KINETIC STUDIES WITH A RAPID FREEZING TECHNIQUE. J Biol Chem. 1964 Apr;239:1221–1227. [PubMed] [Google Scholar]
- BRAY R. C., PALMER G., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. II. KINETIC STUDIES EMPLOYING RAPID FREEZING. J Biol Chem. 1964 Aug;239:2667–2676. [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. KINETIC OBSERVATIONS ON THE NEAR INFRARED BAND OF CYTOCHROME C OXIDASE. J Biol Chem. 1965 Jun;240:2694–2698. [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE REACTION OF CYTOCHROME OXIDASE WITH CYTOCHROME C. J Biol Chem. 1965 Feb;240:888–894. [PubMed] [Google Scholar]
- Gilmour M. V., Wilson D. F., Lemberg R. The low-temperature spectral properties of mammalian cytochromes oxidase. II. The enzyme isolated from beef-heart mitochondria. Biochim Biophys Acta. 1967;143(3):487–499. doi: 10.1016/0005-2728(67)90054-0. [DOI] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Wilson D. F. Heme-heme interactions in cytochrome c oxidase: effects of photodissociation of the CO compound. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1266–1272. doi: 10.1016/0006-291x(72)90848-0. [DOI] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Muijsers A. O., Tiesjema R. H., Henderson R. W., Van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VII. The effect of cytochrome c on the oxidation-reduction potential of isolated cytochrome aa 3 . Biochim Biophys Acta. 1972 Apr 20;267(1):216–221. doi: 10.1016/0005-2728(72)90154-5. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Orme-Johnson W. H., Hansen R. E., Beinert H., Hatefi Y. EPR detectable electron acceptors in submitochondrial particles from beef heart with special reference to the iron-sulfur components of DPNH-ubiquinone reductase. Biochem Biophys Res Commun. 1971 Jul 16;44(2):446–452. doi: 10.1016/0006-291x(71)90621-8. [DOI] [PubMed] [Google Scholar]
- Orme-Johson W. H., Beinert H. Anaerobic reductive titrations with solid diluted sodium dithionite in an apparatus suitable for EPR spectroscopy. Anal Biochem. 1969 Dec;32(3):425–435. doi: 10.1016/s0003-2697(69)80010-2. [DOI] [PubMed] [Google Scholar]
- PALMER G., BEINERT H. DIFFUSE REFLECTANCE SPECTROSCOPY OF FROZEN SAMPLES AS AN ADJUNCT TO LOW-TEMPERATURE ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. Anal Biochem. 1964 May;8:95–103. doi: 10.1016/0003-2697(64)90172-1. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Ogawa S., Rachmilewitz E. A., Oltzik R. The effects of protein conformation on the heme symmetry in high spin ferric heme proteins as studied by electron paramagnetic resonance. J Biol Chem. 1971 May 25;246(10):3342–3355. [PubMed] [Google Scholar]
- Tsai R., Yu C. A., Gunsalus I. C., Peisach J., Blumberg W., Orme-Johnson W. H., Beinert H. Spin-state changes in cytochrome P-450cam on binding of specific substrates. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1157–1163. doi: 10.1073/pnas.66.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudzuki T., Wilson D. F. The oxidation-reduction potentials of the hemes and copper of cytochrome oxidase from beef heart. Arch Biochem Biophys. 1971 Jul;145(1):149–154. doi: 10.1016/0003-9861(71)90021-x. [DOI] [PubMed] [Google Scholar]
- Van Gelder B. F., Beinert H. Studies of the heme components of cytochrome c oxidase by EPR spectroscopy. Biochim Biophys Acta. 1969 Sep 16;189(1):1–24. doi: 10.1016/0005-2728(69)90219-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Brocklehurst E. S. The chemical properties of cytochrome c oxidase in intact mitochondria. Arch Biochem Biophys. 1972 Jul;151(1):180–187. doi: 10.1016/0003-9861(72)90486-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Leigh J. S., Jr Heme-heme interaction in cytochrome c oxidase in situ as measured by EPR spectroscopy. Arch Biochem Biophys. 1972 May;150(1):154–163. doi: 10.1016/0003-9861(72)90022-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Lindsay J. G., Brocklehurst E. S. Heme-heme interaction in cytochrome oxidase. Biochim Biophys Acta. 1972 Feb 28;256(2):277–286. doi: 10.1016/0005-2728(72)90058-8. [DOI] [PubMed] [Google Scholar]


