Abstract
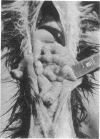
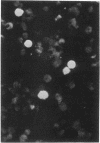
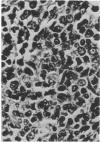
Neoplasia resembling human malignant lymphoma, reticulum cell sarcoma type, occurred in cottontop marmosets inoculated with materials containing Epstein-Barr virus. One of four monkeys that received autologous cells transformed in vitro by Epstein-Barr virus developed lymphoma in mesenteric lymph nodes 7.5 months after inoculation. Three of four marmosets inoculated with cell-free Epstein-Barr virus developed lymphoma. The latent period for detectable tumor formation after addition of virus was 31-46 days. Immunosuppressive drugs given with the virus accelerated the course of disease. Nevertheless, malignant lymphoma occurred in an animal given only cell-free virus. Six of eight marmosets inoculated with the virus demonstrated antibodies to the virus. Four marmosets not exposed to the virus, including two that received immunosuppressive drugs, developed neither tumors nor antibodies to Epstein-Barr virus. Virus antigen detectable by immunofluorescence was found in 5% of cells shed from one tumor maintained in organ culture. These results imply that Epstein-Barr virus is capable of inducing malignant lymphoma in at least one primate species. Additional evidence is required before its oncogenic capacity in this host can be accepted without reservation.
Keywords: immunofluorescence, primate, tumor
Full text
PDF

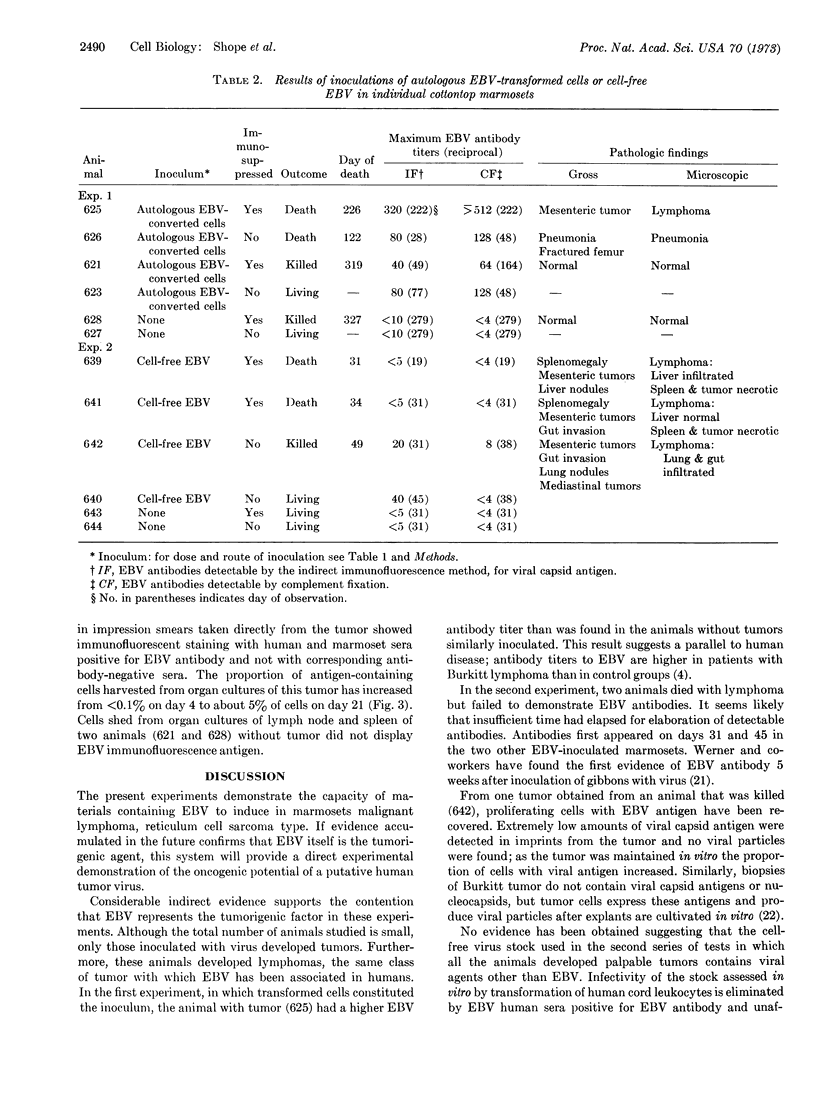

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blacklow N. R., Watson B. K., Miller G., Jacobson B. M. Mononucleosis with heterophil antibodies and EB virus infection. Acquisition by an elderly patient in hospital. Am J Med. 1971 Oct;51(4):549–552. doi: 10.1016/0002-9343(71)90260-9. [DOI] [PubMed] [Google Scholar]
- Diamandopoulos G. T., McLane M. F. The tumor imprint technique for demonstrating SV40 T antigen by immunofluorescence. Proc Soc Exp Biol Med. 1972 Oct;141(1):62–66. doi: 10.3181/00379727-141-36716. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W., Clifford P., Diehl V., Kafuko G. W., Kirya B. G., Klein G., Morrow R. H., Munube G. M., Pike P. Antibodies to Epstein-Barr virus in Burkitt's lymphoma and control groups. J Natl Cancer Inst. 1969 Nov;43(5):1147–1157. [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. Epstein-Barr virus and infectious mononucleosis. N Engl J Med. 1973 Feb 1;288(5):263–264. doi: 10.1056/NEJM197302012880512. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GWATKIN R. B., BIGGERS J. D. A SIMPLE ORGAN CULTURE METHOD WHICH ALLOWS SIMULTANEOUS ISOLATION OF SPECIFIC TYPES OF CELLS. Exp Cell Res. 1964 May;34:440–447. doi: 10.1016/0014-4827(64)90231-9. [DOI] [PubMed] [Google Scholar]
- Kufe D., Hehlmann R., Spiegelman S. RNA related to that of a murine leukemia virus in Burkitt's tumors and nasopharyngeal carcinomas. Proc Natl Acad Sci U S A. 1973 Jan;70(1):5–9. doi: 10.1073/pnas.70.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D., Magrath I. T., Ziegler J. L., Spiegelman S. Burkitt's tumors contain particles encapsulating RNA-instructed DNA polymerase and high molecular weight virus-related RNA. Proc Natl Acad Sci U S A. 1973 Mar;70(3):737–741. doi: 10.1073/pnas.70.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., King N. W., Barahona H. H., Daniel M. D., Fraser C. E., Garcia F. G. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol. 1972 Feb 9;235(58):182–184. doi: 10.1038/newbio235182b0. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lisco H., Kohn H. I., Stitt D., Enders J. F. Establishment of cell lines from normal adult human blood leukocytes by exposure to Epstein-Barr virus and neutralization by human sera with Epstein-Barr virus antibody. Proc Soc Exp Biol Med. 1971 Sep;137(4):1459–1465. doi: 10.3181/00379727-137-35810. [DOI] [PubMed] [Google Scholar]
- Miller G., Niederman J. C., Andrews L. L. Prolonged oropharyngeal excretion of Epstein-Barr virus after infectious mononucleosis. N Engl J Med. 1973 Feb 1;288(5):229–232. doi: 10.1056/NEJM197302012880503. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni J. S., Nadkarni J. J., Klein G., Henle W., Henle G., Clifford P. EB viral antigens in Burkitt tumor biopsies and early cultures. Int J Cancer. 1970 Jul 15;6(1):10–17. doi: 10.1002/ijc.2910060103. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Identification of the filtrable leukocyte-transforming factor of QIMR-WIL cells as herpes-like virus. Int J Cancer. 1969 May 15;4(3):255–260. doi: 10.1002/ijc.2910040302. [DOI] [PubMed] [Google Scholar]
- Shope T., Miller G. Epstein-Barr virus. Heterophile responses in squirrel monkeys inoculated with virus-transformed autologous leukocytes. J Exp Med. 1973 Jan 1;137(1):140–147. doi: 10.1084/jem.137.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J., Henle G., Pinto C. A., Haff R. F., Henle W. Establishment of continuous lymphoblast cultures from leukocytes of gibbons (Hylobates lar). Int J Cancer. 1972 Nov;10(3):557–567. doi: 10.1002/ijc.2910100315. [DOI] [PubMed] [Google Scholar]
- Werner J., Pinto C. A., Haff R. F., Henle W., Henle G. Responses of gibbons to inoculation of Epstein-Barr virus. J Infect Dis. 1972 Dec;126(6):678–681. doi: 10.1093/infdis/126.6.678. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H., Schulte-Holthausen H. Presence of EB virus nucleic acid homology in a "virus-free" line of Burkitt tumour cells. Nature. 1970 Jul 18;227(5255):245–248. doi: 10.1038/227245a0. [DOI] [PubMed] [Google Scholar]
- de-Thé G., Ambrosioni J. C., Ho H. C., Kwan H. C. Lymphoblastoid transformation and presence of herpes-type viral particles in a Chinese nasopharyngeal tumour cultured in vitro. Nature. 1969 Feb 22;221(5182):770–771. doi: 10.1038/221770a0. [DOI] [PubMed] [Google Scholar]