Abstract
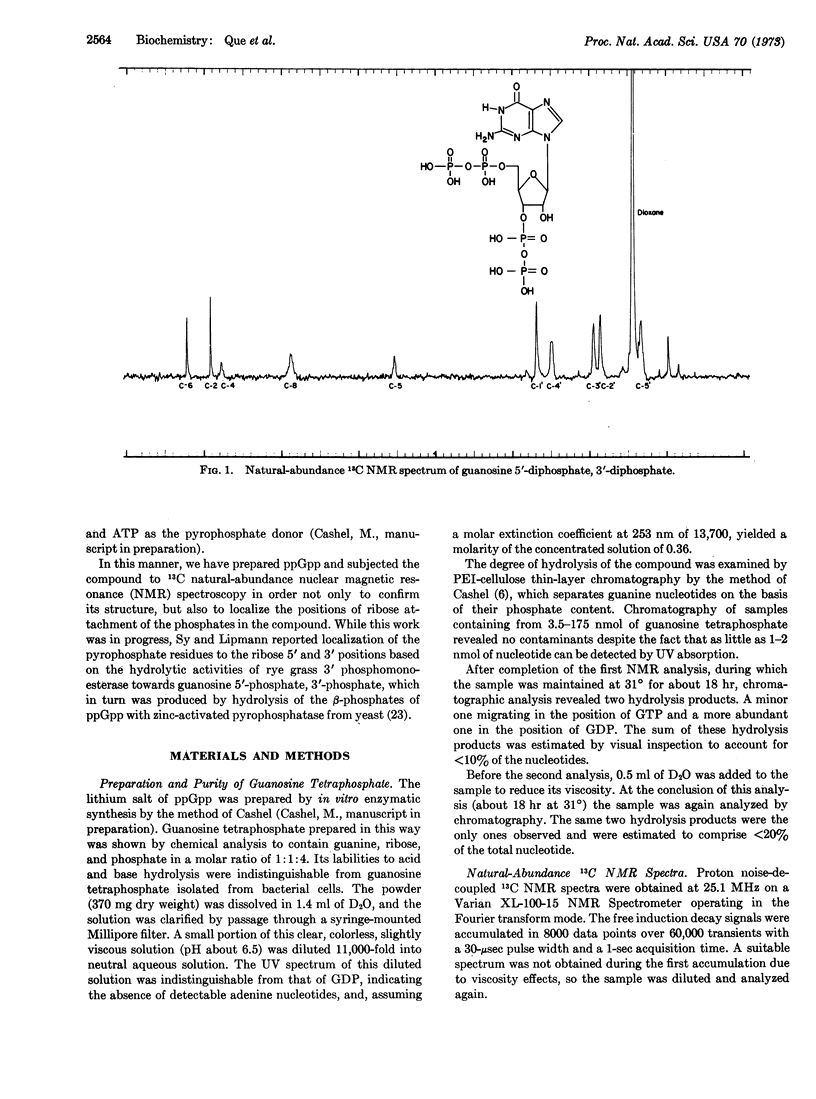
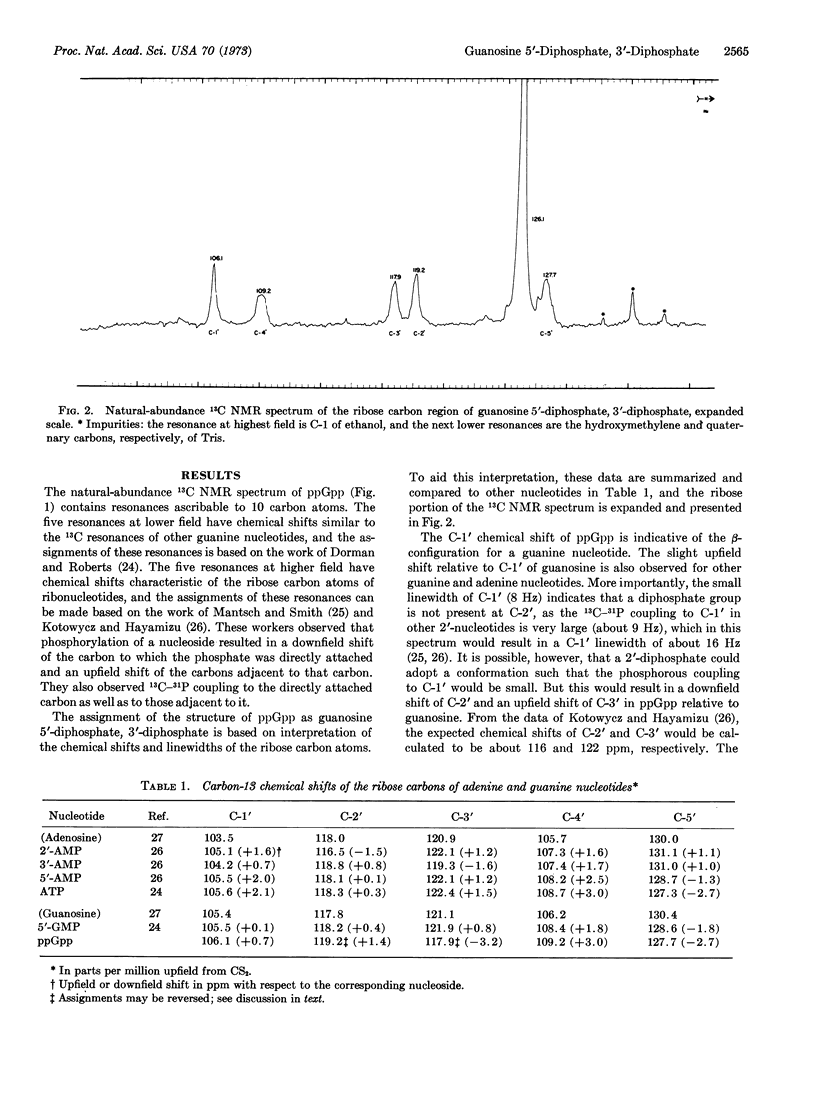
Guanosine tetraphosphate, recently discovered to mediate the regulatory relationship between protein synthesis and RNA accumulation in various bacteria, has been synthesized in vitro in large quantities and analyzed by natural-abundance 13C nuclear magnetic resonance spectroscopy in order to confirm its structure and establish the positions of phosphate attachment. These studies have established its structure as guanosine 5′-diphosphate, 3′-diphosphate.
Keywords: E. coli, stringent control, Magic Spots
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOREK E., RYAN A., ROCKENBACH J. Nucleic acid metabolism in relation to the lysogenic phenomenon. J Bacteriol. 1955 Apr;69(4):460–467. doi: 10.1128/jb.69.4.460-467.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Dorman D. E., Roberts J. D. Nuclear magnetic resonance spectroscopy: 13C spectra of some common nucleotides. Proc Natl Acad Sci U S A. 1970 Jan;65(1):19–26. doi: 10.1073/pnas.65.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Irr J., Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971 Sep 25;246(18):5812–5816. [PubMed] [Google Scholar]
- Gallant J., Margason G. Amino acid control of messenger ribonucleic acid synthesis in Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2289–2294. [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. Formation of ppGpp in a relaxed and stringent strain of Escherichia coli during diauxie lag. Biochemistry. 1971 Oct 12;10(21):3980–3982. doi: 10.1021/bi00797a027. [DOI] [PubMed] [Google Scholar]
- Harshman R. B., Yamazaki H. MSI accumulation induced by sodium chloride. Biochemistry. 1972 Feb 15;11(4):615–618. doi: 10.1021/bi00754a023. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Cashel M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J Biol Chem. 1972 Nov 10;247(21):7067–7072. [PubMed] [Google Scholar]
- Irr J. D. Control of nucleotide metabolism and ribosomal ribonucleic acid synthesis during nitrogen starvation of Escherichia coli. J Bacteriol. 1972 May;110(2):554–561. doi: 10.1128/jb.110.2.554-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. J., Grant D. M., Winkley M. W., Robins R. K. Carbon-13 magnetic resonance. XVII. Pyrimidine and purine nucleosides. J Am Chem Soc. 1970 Jul 1;92(13):4079–4087. doi: 10.1021/ja00716a042. [DOI] [PubMed] [Google Scholar]
- Kotowycz G., Hayamizu K. A carbon-13 nuclear magnetic resonance study of nucleotide-metal interactions. Binding of manganese(II) with adenine nucleotides. Biochemistry. 1973 Jan 30;12(3):517–520. doi: 10.1021/bi00727a025. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Smith I. C. Fourier-transformed 13 C NMR spectra of polyuridylic acid, uridine, and related nucleotides--the use of 31 POC 13 C couplings for conformational analysis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):808–815. doi: 10.1016/s0006-291x(72)80213-4. [DOI] [PubMed] [Google Scholar]
- Martin R. G. Polarity in relaxed strains of Salmonella typhimurium. J Mol Biol. 1968 Jan 14;31(1):127–134. doi: 10.1016/0022-2836(68)90060-0. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton M., Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]
- Sy J., Lipmann F. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3'-position in guanosine 5'-diphosphate. Proc Natl Acad Sci U S A. 1973 Feb;70(2):306–309. doi: 10.1073/pnas.70.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]


