Abstract
Colorectal cancer (CRC) is one of the most common cancers in the world. CD147, a transmembrane protein, has been reported to be correlated with various cancers. In this study, we aimed to investigate the mechanism of CD147 in regulating drug resistance, cell invasion and epithelial-to-mesenchymal transition (EMT) in CRC cells. qRT-PCR and western blotting were used to evaluated the expression of CD147 in 40 CRC cases and 4 cell lines. Increased expression of CD147 at both mRNA and protein levels was found in CRC samples, and the level of CD147 was correlated with lymph node metastasis. CD147 overexpression increased the 5-Fluorouracil (5-FU) resistance, enhanced the invasion and EMT of CRC cells by regulating EMT markers and MMPs. Adverse results were obtained in CD147 knockdown CRC cell line. Further investigation revealed that CD147 activated MAPK/ERK pathway, ERK inhibitor U0126 suppressed the CD147-induced cell invasion, migration and MMP-2, MMP-9 expression. Taken together, our study indicates that CD147 promotes the 5-FU resistance, and MAPK/ERK signaling pathway is involved in CD147-promoted invasion and EMT of CRC cells.
Keywords: CD147, 5-FU resistance, invasion, EMT, MAPK/ERK pathway, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer in men (746,000 cases, 10.0% of the total) and the second in women (614,000 cases, 9.2% of the total) worldwide [1]. Every year nearly 0.7 million people died of CRC, the high mortality is mainly due to the chemoresistance and tumor metastasis. It is reported that approximate 50% of the CRC patients would develop distant metastasis eventually [2]. Chemotherapy is an essential treatment to metastatic cancer patients, however, drug resistance leads to treatments failure and cancer progression [2]. So better understanding of the molecular mechanism underlying CRC chemoresistance and metastasis is critical to the treatments.
CD147 (also named EMMPRIN, basigin, M6 and tumor cell-derived collagenase stimulatory factor), is a glycosylated cell surface transmembrane protein of the immunoglobulin superfamily (IgSF) [3]. The CD147 protein has 269 amino acids, exists in two forms: glycosylated form (HG, ~40-60 kDa) and core-glycosylated form (LG, ~32 kDa), the ratio is variable in different cell types, while HG-CD147 is considered to be the functional form [4]. CD147 has a broad tissue distribution, implicating in many physiological processes [5]. Aberrant CD147 expression has been observed to correlate with several diseases, especially cancers [6]. During tumorigenesis, CD147 contributes to cell proliferation, metastasis, drug resistance and angiogenesis [7-9], moreover, its level may also predict tumor relapse and patient outcome [10,11].
CD147 has been shown to interact with hyaluronan, multidrug transporters of the ABC family and monocarboxylate transporters to mediate drug resistance [12]. In addition, CD147 is capable of inducing the expression of several matrix metalloproteinases (MMPs), including MT1-MMP, MMP-1, MMP-2 and MMP-9 [13]. MMPs are major proteases in degrading the extracellular matrix (ECM) and basement membrane, which are vital to tumor initiation, progression and invasion [14]. It was reported that CD147 was upregulated in CRC [15], nevertheless, whether the aberrant expression of CD147 correlates with drug resistance and metastasis of CRC are still unclear.
In the present study, we aimed to evaluate the effects of CD147 on drug resistance and metastasis of CRC cells. We assessed the expression pattern of CD147 and analyzed its correlation with clinicopathological factors of CRC patients. Next the effects of CD147 on drug resistance, cell invasion and EMT were elucidated. Further we investigated the involvement of MAPK/ERK signaling pathway in CD147-induced cell invasion and migration.
Materials and methods
Tissue specimens and cell culture
All tissue samples were obtained from Shandong provincial hospital, consent forms were obtained from all patients. The study was approved by the Ethical Committee of Shandong Provincial Hospital. Cell lines were purchased from the Cell Bank of Chinese Academy of Sciences. Cells were cultured in RPMI 1640 and DMEM medium, respectively, with 10% fetal bovine serum, at 37°C in a humidified incubator with 5% CO2.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted with the TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. Complementary DNA was reverse-transcribed using a reverse transcription kit (Takara, Japan). qRT-PCR was performed using SYBR Premix Ex Taq Kit (Takara, Japan) on ABI 7300. β-actin was used as endogenous control for the normalization of gene expression. The forward and reverse primers for CD147 were 5’-CAGCGGTTGGAGGTTGT-3’ and 5’-TTTGAGGGTGGAGGTGG-3’, for β-actin were 5’-AAAGACCTGTACGCCAACAC-3’ and 5’-GTCATACTCCTGCTTGCTGAT-3’. The PCR cycle conditions consisted of an initial denaturation step at 95°C for 30 sec, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. Relative mRNA expression levels were determined by the 2-ΔΔCt method in comparison with control cells.
Western blotting analysis
Cells were harvested, washed by cold phosphate-buffered saline (PBS), and lysed in RIPA lysis buffer (Beyotime, China). Total proteins extracted were quantified with BCA Protein Assay Kit (Beyotime, China). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), then transferred to polyvinylidene difluoride (PVDF) membranes and blocked in 5% non-fat milk in TBST buffer for 1 h. Then the membranes were incubated overnight at 4°C with primary antibodies: CD147 (1:500), E-cadherin (1:1000), vimentin (1:500), MMP-2 (1:500), MMP-9 (1:500), ERK (1:500), p-ERK (1:500), β-actin (1:1000), followed by incubation of secondary antibodies (1:5000). Protein bands were detected using ECL detection system (Beyotime, China).
Cell transfection and construction of CD147 downexpression and overexpression CRC cell lines
Plasmids containing CD147 siRNA, CD147 negative control and CD147 cDNA, CD147 negative control were purchased from Genepharma (Genepharma, China). 2 × 105 cells were seeded into 6-well plate. When the cells were 70-90% confluent 24 h later, 2 μg plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen, USA). 48 h after transfection, cells were selected by 400 μg/ml G418 (Sigma, USA) for 1 month to form stable clones. Caco-2 cells transfected with CD147 expression vector and negative control were named Caco-OvCD147 and Caco-cCD147. SW480 cells transfected with CD147 siRNA vector and negative control were labeled as SW480-SiCD147 and SW480-cCD147.
CCK-8 assay
5-FU resistance was measured by Cell Counting Kit-8 (CCK-8). Cells were seeded in 96-well plates at a density of 1 × 105 cells/100 μl/well. After 24 h incubation, cells were treated with different concentration of 5-FU (0, 2, 4, 6, 8, 10 μM). After 48 h, 10 μl CCK-8 (Beyotime, China) was added into the medium, then the supernatants were removed and measured at 450 nm on a microplate reader (Bio-Rad, USA) after 2 h incubation.
Transwell assay
Cell invasion was measured by using transwell chambers (Costar, USA). 1 × 105 cells were suspended in serum-free medium and planted into the upper chamber, then 500 μl complete medium with serum was added to the lower well. Cells were incubated at 37°C for 24 h and the invading cells to the lower surface were stained with 0.1% crystal violet (Beyotime, China) for 30 min. The cells were photographed under a microscope.
Wound healing detection
5 × 105 cells were planted into 6-well plate, incubated overnight until the cells grown to confluent monolayer, a 200 μl eppendorf tip was used to scratch cell wounds, the wounded monolayers were washed with PBS three times to remove non-adherent cells, and incubated in serum-free medium for 48 h. Cell migration were observed and photographed under a microscope.
Statistical analysis
Data were presented as mean ± SD from three independent experiments. Two-sided Student’s t test was used to analyze the different expression of CD147. All statistical analyses were conducted using the SPSS version 17.0 (SPSS Inc. Chicago). P < 0.05 was considered statistically significant.
Results
Increased CD147 expression in CRC tissues and cell lines
CD147 expression level was evaluated in 40 CRC tissues and 4 CRC cell lines by qRT-PCR and western blotting. Results showed that CD147 mRNA expression was upregulated in 55% (22/40) tissues, and protein levels in 62.5% (25/40) tissues (Figure 1A and 1B). In CRC cell lines, we found CD147 was expressed in all the 4 cell lines, SW480 cells exhibited highest expression level of CD147, while Caco-2 cells showed lowest CD147 level (Figure 1C and 1D). These results suggested upregulation of CD147 may play an important role in CRC progression.
Figure 1.
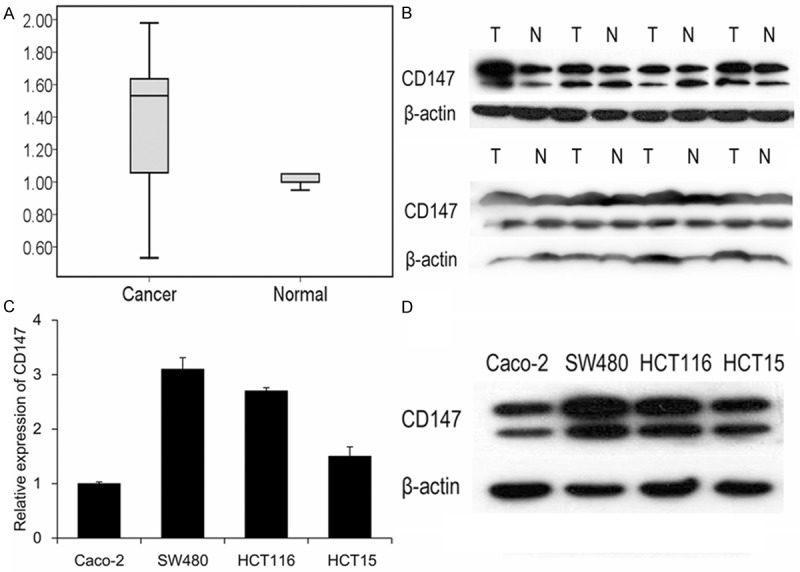
The expression of CD147 was determined in CRC tissues and cell lines by qRT-PCR and western blotting. CD147 was upregulated in mRNA (A) and protein (B) level in 40 CRC tissues. N, non-tumor tissues; T, tumor tissues. (C and D) Detection of CD147 expression in CRC cell lines.
Increased expression of CD147 is associated with lymph node metastasis of CRC
To further explore the relationship between the expression pattern of CD147 and clinicopathological features of patients with CRC, 40 patients were categorized into two groups according to the CD147 expression level difference (≥ 1.5-fold). The correlation was summarized in Table 1. Statistical analyses revealed that overexpression of CD147 is correlated with lymph node metastasis (P = 0.033) of CRC. The results indicated CD147 contributed to CRC metastasis.
Table 1.
Relationship between CD147 expression and clinicopathologic features of CRC patients
| Feature | Relative CD147 expression | P value | |
|---|---|---|---|
|
| |||
| Low | High | ||
| Gender | |||
| Male | 9 | 14 | 0.804 |
| Female | 6 | 11 | |
| Age | |||
| < 60 | 6 | 9 | 0.8 |
| ≥ 60 | 9 | 16 | |
| Differentiation | |||
| Poorly | 5 | 10 | 0.673 |
| Moderately/Well | 10 | 15 | |
| local invasion | |||
| Yes | 4 | 15 | 0.055 |
| No | 11 | 10 | |
| Lymph node metastasis | |||
| Yes | 5 | 17 | 0.033* |
| No | 10 | 8 | |
| Distant metastasis | |||
| Yes | 6 | 14 | 0.327 |
| No | 9 | 11 | |
P < 0.05.
Expression of CD147 in the stable transfected CRC cells
To further study the function of CD147, Caco-2 and SW480 cell lines were chosen to enhance and deplete CD147, respectively. RT-qPCR and western blotting were used to evaluate the relative mRNA and protein expression levels. Results showed that CD147 increased its expression by 2.3 fold in Caco-OvCD147 cells than control (Figure 2A). While CD147 reduced its expression by 0.68 fold in SW480-SiCD147 cells compared to SW480-cCD147 cells (Figure 2B).
Figure 2.
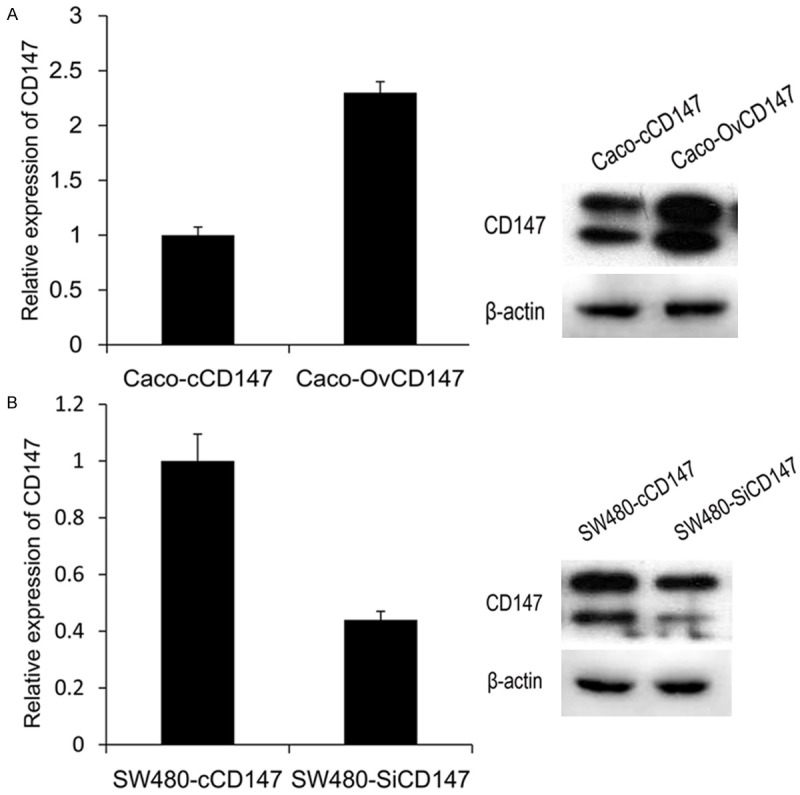
The expression of CD147 in CRC cell lines after stable transfection. A. The expression of CD147 was upregulated in mRNA and protein level in Caco-2 cells. B. The level of CD147 was downregulated in mRNA and protein level in SW480 cells.
CD147 enhanced the 5-FU resistance of CRC cells
CCK-8 kit was employed to detect the effects of CD147 on 5-FU resistance. The data revealed that increased expression of CD147 enhanced the 5-FU resistance of Caco-2 cells (Figure 3A), while reduction of CD147 decreased the SW480 cells sensitivity to 5-FU (Figure 3B). The results indicated CD147 regulates the 5-FU resistance of CRC cells.
Figure 3.
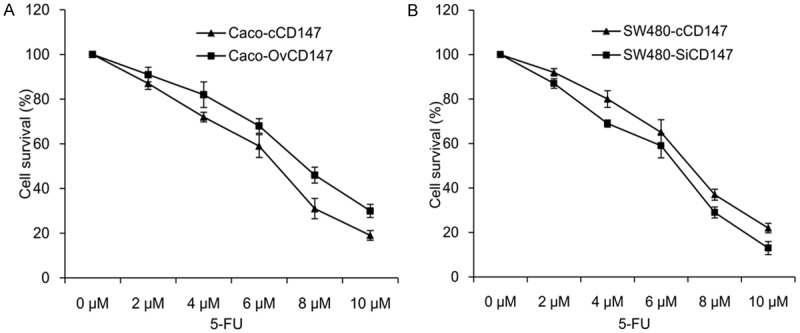
Effects of CD147 on 5-FU resistance in CRC cells. A. CD147 overexpression enhanced the 5-FU resistance of Caco-2 cells. B. CD147 knockdown suppressed the 5-FU resistance of SW480 cells.
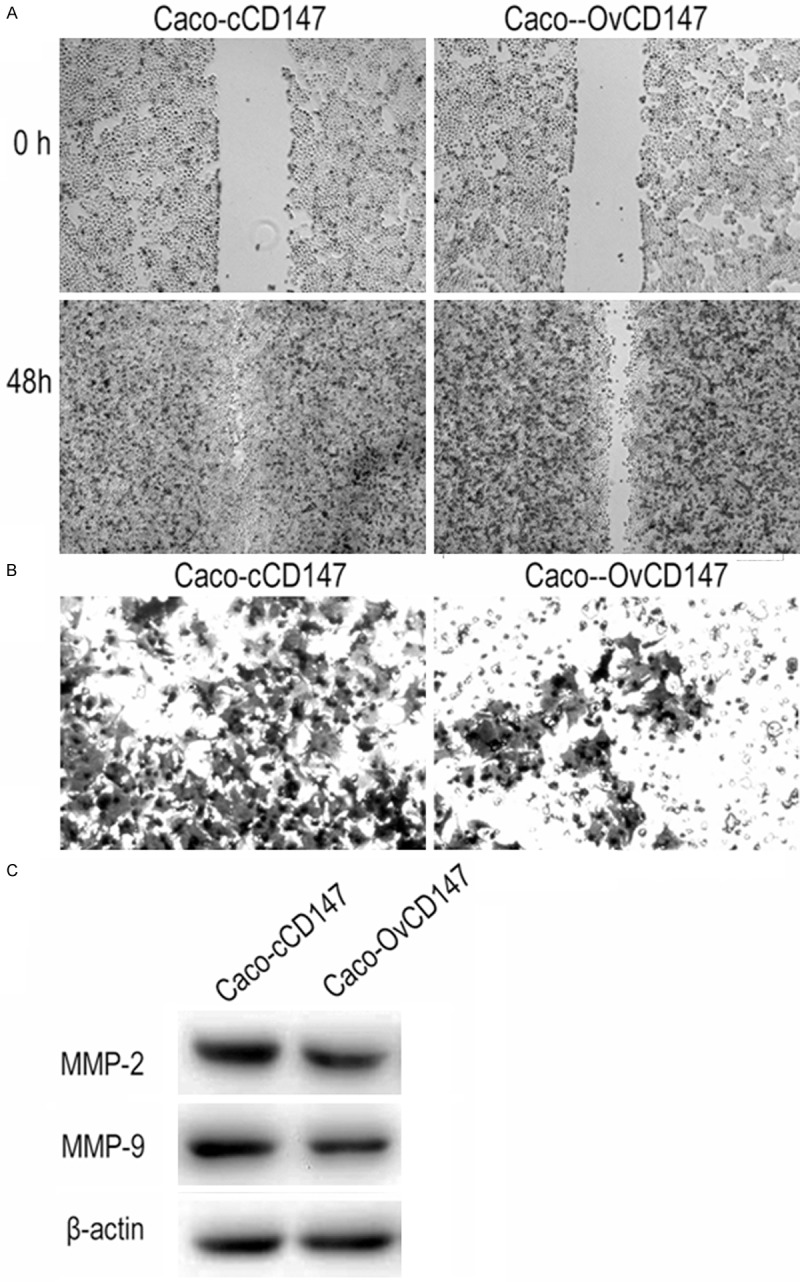
CD147 promoted the invasion and migration of CRC cells
Since CD147 expression was positively correlated with lymph node metastasis of CRC patients, we further verified the biological function of CD147 on CRC cells. The transwell assay indicated CD147 overexpression increased cell invasion of Caco-2 cells. Adverse results were found in CD147 knockdown cells (Figure 4A and 4B). In wound healing assay, the migratory activity of Caco-OvCD147 cells was induced compared to the Caco-cCD147 cells. By contrast, silencing of CD147 inhibited SW480-SiCD147 cells migration as compared with SW480-cCD147 cells (Figure 4C and 4D). Taken together, our data revealed that CD147 could promote the invasion and migration of CRC cells.
Figure 4.
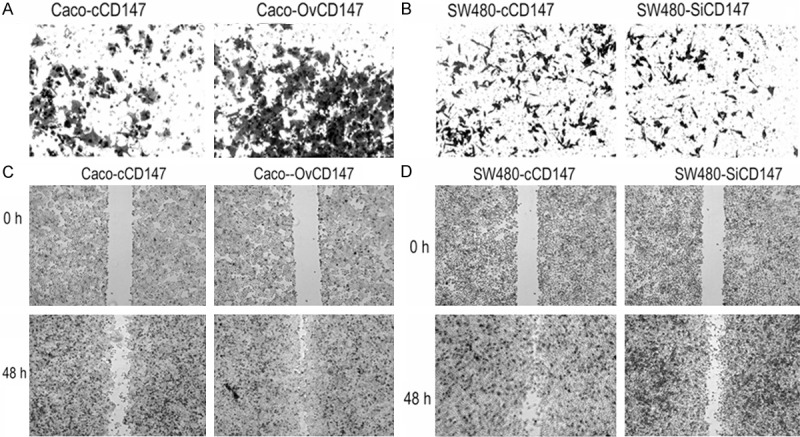
Effects of CD147 on invasion and EMT of CRC cells. A. CD147 overexpression increased the invasive ability of Caco-OvCD147, as compared with Caco-cCD147 cells (200×). B. CD147 knockdown inhibited the invasive ability of SW480-SiCD147, as compared with SW480-cCD147 cells (200×). C. Upregulation of CD147 promoted the migration of Caco-2 cells (40×). D. Downregulation of CD147 inhibited the migration of SW480 cells (40×).
Expression of EMT markers, MMPs and pERK mediated by CD147
To identify the mechanism of CD147 in the metastasis of CRC cells, we further examined the expression of EMT markers and MMPs. Results demonstrated that overexpression of CD147 in Caco-2 cells led to decreased expression of E-cadherin and increased level of vimentin, and the level of MMP-2, MMP-9 were increased (Figure 5A). Knockdown of CD147 in SW480 cells induced the expression of E-cadherin and inhibited the level of vimentin, while the expression of MMP-2 and MMP-9 were suppressed (Figure 5B). Previous study had reported that the MAPK/ERK pathway is involved in modulating the cancer metastasis and drug resistance [16], so we studied the effects of CD147 on ERK expression in CRC cell lines. The data indicated that upregulation of CD147 induced the phosphorylation of ERK (pERK), whereas downregulation of CD147 reduced the expression of pERK, but there were no obvious changes of the total-ERK (tERK) in the 2 cell lines (Figure 5C and 5D). These data suggested CD147 promoted cell EMT, stimulated the expression of MMP-2, MMP-9 and activated the MAPK/ERK pathway.
Figure 5.
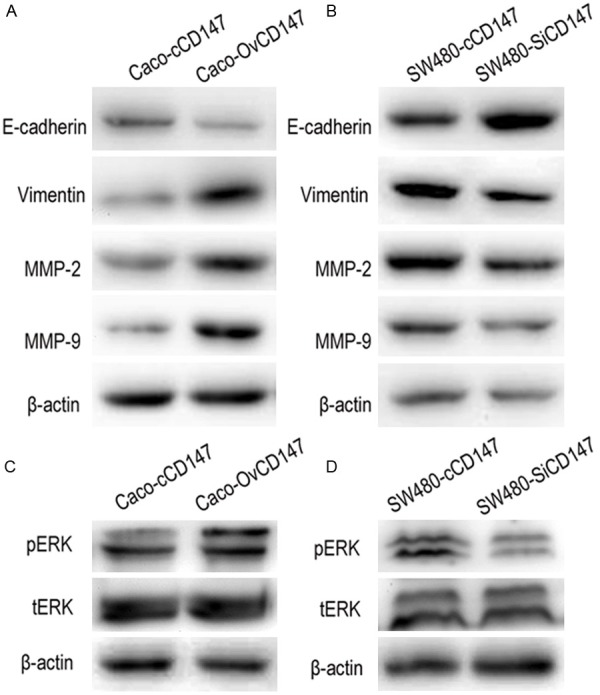
The expression of EMT markers, MMPs and ERK. A. CD147 overexpression decreased the level of E-cadherin and increased vimentin, enhanced the expression MMP-2 and MMP-9 in Caco-2 cells. B. CD147 knockdown upregulated E-cadherin and downregulated vimentin, suppressed the expression MMP-2 and MMP-9 in SW480 cells. C. CD147 overexpression induced the level of p-ERK in Caco-2 cells. D. CD147 knockdown reduced the level of p-ERKin SW480 cells.
MAPK/ERK pathway is involved in regulating the CD147-induced cell invasion, EMT and MMPs expression
To validate whether the CD147-mediated cell invasion, migration and MMPs expression in CRC cells were regulated by MAPK/ERK signaling pathway, CD147 overexpressed Caco-2 cell lines were treated with ERK inhibitor (U0126; 10 μM). We found inhibition of ERK suppressed the invasive and migratory ability of Caco-OvCD147 cells, as compared with the Caco-cCD147 (Figure 6A and 6B). Western blot showed U-0126 treatment attenuated the increased expression of MMP-2, MMP-9 in Caco-2 cells (Figure 6C). The data indicated that U0126 inhibits CD147-induced invasion, migration, and MMP-2, MMP-9 expression of CRC cells. Taken together, MAPK/ERK signaling pathway may involve in CD147-stimulated cell invasion, EMT and MMPs expression of CRC cells.
Figure 6.
Effects of ERK inhibitor on CD147 overexpressed cells. U0126 inhibited the migratory (40×) (A) and invasive (200×) (B) ability of Caco-OvCD147 cells. (C) U0126 suppressed the expression of MMP-2 and MMP-9 in Caco-OvCD147 cells.
Discussion
Drug resistance and tumor metastasis are two important causes of treatment failure and mortality in cancer patients [16]. Tumor metastasis is a complex process includes multiple steps and molecules. Cell invasion and EMT are two important processes, in which cells migrate and invade to nearby tissues, eventually form new metastatic sites [8]. Many molecules, including CD147, MMPs, hyaluronan, are involved in these processes.
CD147 was reported to implicate in numerous physiological and pathological processes [5], it correlated with the metastasis process of various cancers. In ovarian carcinoma, upregulated expression of CD147 contributed to the pathogenesis by modulating cellular events [17]. Siu A found CD147 modulated the invasive phenotype and may present as therapeutic target of squamous cell carcinoma [18]. In gastric cancer, CD147 mediated cell growth and invasion through ERK1/2 pathway [19]. In this study, we evaluated the expression profile of CD147 in 40 CRC tissues and paired non-cancerous tissues. Western blotting showed an increased expression of CD147 in 62.5% CRC samples. Further statistical analyses indicated CD147 is associated with the lymph node metastasis in CRC patients (P = 0.033). The results indicate CD147 contributes to the progression of CRC and may present as a diagnosis marker of CRC.
Drug resistance in tumor leads to chemotherapy failure, CD147 was reported to implicate in chemoresistance [20]. In this study, we selected 5-FU as an experimental drug. 5-FU is a classic chemotherapy drugs which could restrain the DNA synthesis processing of tumor cell [21]. Data revealed that CD147 decreased the sensitivity of CRC cells to 5-FU. It was reported that CD147 induced drug resistance by stimulating the hyaluronan production [22], further studies are needed to explore the potential mechanism in CD147-mediated 5-FU resistance in CRC cells. Our finding may assist in choosing applicable chemotherapeutics.
Given that CD147 related to the lymph node metastasis, we next examined the impacts of CD147 on CRC cells. Results revealed that CD147 promoted the invasion and migration of CRC cells. Loss of E-cadherin is a common feature of EMT [23]. We found overexpression of CD147 downregulated epithelial marker E-cadherin and upregulated mesenchymal marker vimentin, suggesting CD147 participate in regulating the EMT of CRC cells. One of the most important role of CD147 is the stimulation of MMPs [24]. MMPs are positive regulators of tumor invasion and metastasis [25]. MMP-2 and MMP-9 belong to the gelatinase subclass which can degrade the gelatin, the degradation products then induce multiple cellular signals, thus promote cancer metastasis [26]. Increased expressions of MMP-2 and MMP-9 have been reported to contribute to invasion or metastasis of various cancers [26,27]. In this study, we found CD147 induced the expression of MMP-2 and MMP-9 which suggests that CD147 may activate MMP-2 and MMP-9 to enhance the invasion and EMT in CRC cells. MAPK/ERK signaling pathway is reported to contribute to cell migration, angiogenesis and chemoresistance [28].
In hepatocellular carcinoma, CD147 enhances the secretion of MMP-2 via the activation of ERK, FAK, and PI3K/Akt signaling pathway [24]. In macrophages, CD147 induces the expression of MMP-9 through ERK and NF-κB [29]. Our studies indicate that CD147 promoted the expression of pERK in CRC cells; further research demonstrated that ERK inhibitor abrogated CD147-induced cell invasion, migration and MMPs expression. So we presumed that MAPK/ERK pathway is involved in CD147-induced cell invasion and EMT, but whether MAPK/ERK pathway regulates the cell invasion and EMT by direct mediation MMP-2, MMP-9 still needed to investigate.
In conclusion, we demonstrated that CD147 is upregulated in CRC tissues and associated with the lymph node metastasis. CD147 promotes the 5-FU resistance, and enhance cell invasion and EMT through activating MAPK/ERK pathway.
Acknowledgements
This work was supported by Natural Science Foundation of Shandong Province (2013ZRB14250).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Jeong WJ, Cha PH, Choi KY. Strategies to overcome resistance to epidermal growth factor receptor monoclonal antibody therapy in metastatic colorectal cancer. World J Gastroenterol. 2014;20:9862–9871. doi: 10.3748/wjg.v20.i29.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157–169. [PubMed] [Google Scholar]
- 4.Bai Y, Huang W, Ma LT, Jiang JL, Chen ZN. Importance of N-glycosylation on CD147 for its biological functions. Int J Mol Sci. 2014;15:6356–6377. doi: 10.3390/ijms15046356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadool JM, Linser PJ. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev Dyn. 1993;196:252–262. doi: 10.1002/aja.1001960406. [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005;93:199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 7.Trachtenberg A, Pushkarsky T, Heine S, Constant S, Brichacek B, Bukrinsky M. The level of CD147 expression correlates with cyclophilin-induced signalling and chemotaxis. BMC Res Notes. 2011;4:396. doi: 10.1186/1756-0500-4-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou T, Rosenthal EL. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Exp Cell Res. 2012;318:1788–1798. doi: 10.1016/j.yexcr.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Pan Y, He B, Xu Y, Gao T, Song G, Sun H, Deng Q, Wang S. Downregulation of CD147 expression by RNA interference inhibits HT29 cell proliferation, invasion and tumorigenicity in vitro and in vivo. Int J Oncol. 2013;43:1885–1894. doi: 10.3892/ijo.2013.2108. [DOI] [PubMed] [Google Scholar]
- 10.Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han X, Yao L, Lan M, Li Y, Zhang W. EMMPRIN/CD147 expression is associated with disease-free survival of patients with colorectal cancer. Med Oncol. 2013;30:369. doi: 10.1007/s12032-012-0369-7. [DOI] [PubMed] [Google Scholar]
- 11.Sato M, Nakai Y, Nakata W, Yoshida T, Hatano K, Kawashima A, Fujita K, Uemura M, Takayama H, Nonomura N. EMMPRIN promotes angiogenesis, proliferation, invasion and resistance to sunitinib in renal cell carcinoma, and its level predicts patient outcome. PLoS One. 2013;8:e74313. doi: 10.1371/journal.pone.0074313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–121. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 15.Zheng HC, Wang W, Xu XY, Xia P, Yu M, Sugiyama T, Takano Y. Up-regulated EMMPRIN/CD147 protein expression might play a role in colorectal carcinogenesis and its subsequent progression without an alteration of its glycosylation and mRNA level. J Cancer Res Clin Oncol. 2011;137:585–596. doi: 10.1007/s00432-010-0919-3. [DOI] [PubMed] [Google Scholar]
- 16.Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN. Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res. 2003;1:420–427. [PubMed] [Google Scholar]
- 17.Zhao Y, Chen S, Gou WF, Niu ZF, Zhao S, Xiao LJ, Takano Y, Zheng HC. The role of EMMPRIN expression in ovarian epithelial carcinomas. Cell Cycle. 2013;12:2899–2913. doi: 10.4161/cc.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siu A, Chang J, Lee C, Lee S, Lee C, Ramos DM. Expression of EMMPRIN modulates mediators of tumor invasion in oral squamous cell carcinoma. J Calif Dent Assoc. 2013;41:831–838. [PubMed] [Google Scholar]
- 19.Chen L, Pan Y, Gu L, Nie Z, He B, Song G, Li R, Xu Y, Gao T, Wang S. ERK1/2 signalling pathway is involved in CD147-mediated gastric cancer cell line SGC7901 proliferation and invasion. Exp Biol Med (Maywood) 2013;238:903–912. doi: 10.1177/1535370213493706. [DOI] [PubMed] [Google Scholar]
- 20.Grass GD, Dai L, Qin Z, Parsons C, Toole BP. CD147: Regulator of Hyaluronan Signaling in Invasiveness and Chemoresistance. Adv Cancer Res. 2014;123:351–373. doi: 10.1016/B978-0-12-800092-2.00013-7. [DOI] [PubMed] [Google Scholar]
- 21.Tang Q, Wang Y, Huang R, You Q, Wang G, Chen Y, Jiang Z, Liu Z, Yu L, Muhammad S, Wang X. Preparation of anti-tumor nanoparticle and its inhibition to peritoneal dissemination of colon cancer. PLoS One. 2014;9:e98455. doi: 10.1371/journal.pone.0098455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Hao ZW, Zhao YX, Yang XM, Tang H, Zhang X, Song F, Sun XX, Wang B, Nan G, Chen ZN, Bian H. Full-length soluble CD147 promotes MMP-2 expression and is a potential serological marker in detection of hepatocellular carcinoma. J Transl Med. 2014;12:190. doi: 10.1186/1479-5876-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel) 2014;6:1298–1327. doi: 10.3390/cancers6031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PS, Shih YW, Huang HC, Cheng HW. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS One. 2011;6:e20164. doi: 10.1371/journal.pone.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Kim WJ, Kim H, Suk K, Lee WH. The Stimulation of CD147 Induces MMP-9 Expression through ERK and NF-kappaB in Macrophages: Implication for Atherosclerosis. Immune Netw. 2009;9:90–97. doi: 10.4110/in.2009.9.3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]


