Abstract
Merkel cell carcinoma (MCC) is an aggressive, virus-associated, neuroendocrine tumor of the skin mainly affecting immunocompromised patients. Higher intratumoral infiltration with CD3 and CD8 positive T-cells is associated with a better prognosis, highlighting the relevance of the immune system for MCC development and progression. In this study 21 primary MCCs were stained with immune cell markers including CD3, CD4, CD8, CD68, CD20, and S100. Furthermore, tumor-infiltrating neutrophils, tertiary lymphoid structures and PD-L1 expression were analyzed and correlated with overall and recurrence free survival. All MCCs were Merkel Cell Polyomavirus positive. Overall and recurrence-free survival did not correlate with intra- and peritumoral CD3 and CD8 T-cell infiltration. In addition, no significant association regarding prognosis was found for tumor-associated neutrophils, tumor-associated macrophages or PD-L1 positivity in MCCs. Interestingly, the presence of tertiary lymphoid structures (TLS) in the tumor microenvironment significantly correlated with recurrence-free survival (P=0.025). In addition, TLS were significantly associated with a higher CD8/CD4 ratio in the tumor periphery (P=0.032), but not in the center of the tumor (P > 0.999). These results demonstrate for the first time that TLS, easily assessed in paraffin-embedded tissue in the tumor periphery of MCCs, may be a valuable prognostic factor indicating prolonged recurrence free survival.
Keywords: Merkel cell carcinoma, immune cell infiltration, tertiary lymphoid structures, PD-L1
Introduction
Merkel cell carcinomas (MCCs) are rare and highly aggressive neuroendocrine neoplasms of the skin that mainly develop in elderly or immunocompromised patients [1]. The association between MCC development and immunosuppression led to the groundbreaking idea that MCC might belong to oncovirus-induced cancers. In 2008, Feng et al. described for the first time Merkel Cell Polyomavirus (MCPyV) in 8 out of 10 MCCs [2]. Most MCPyV-positive MCCs express several MCPyV proteins, among them large T antigen and small T antigen which potentially stimulate tumor cell proliferation. This and other observations support a pathogenic role of MCPyV for MCC development [3-7]. Dependent on the detection method used, MCPyV is found in about 80-100% of MCCs [8]. It is still unclear whether in negative MCCs the detection method was not sensitive enough or whether such MCCs have another etiology or loose MCPyV oncogene expression during tumor progression [9].
Several lines of evidence support the notion that a strong immune response correlates with better outcome in patients with MCC. A gene expression analysis comparing mRNA of MCCs from patients with good and poor prognosis showed a prominent immune response gene signature in those with a good prognosis [10]. In particular, up-regulation of CD8a and granzymes were associated with better outcome, which points to a central role of CD8 lymphocytes for a successful anti-tumor immune response. No association with M2 macrophage infiltration characterized by CD68/CD163 positivity was found [11]. It has not been studied so far whether other innate immune cells such as myeloid-derived suppressor cells or tumor-associated neutrophils (TANs) are implicated in MCC progression, but their tumor promoting properties have been shown for many other tumor entities [12,13].
Additional histopathological features previously associated with a better prognosis of patients with different kinds of tumors are tertiary lymphoid structures (TLS) present in the tumor environment [14-16]. Tertiary lymphoid structures (TLS) are known to play an important role in autoimmunity, organ transplantation and infection. They constitute important ectopic antigen-presenting formations with structural similarities to lymph nodes. Infiltrating CD20-positive B cells cluster with T cells and dendritic cells and form germinal center-like patterns that lead to the development of specific humoral and cell-mediated immune responses, thereby sustaining long-term immunity [17]. Recent studies have identified TLS in the tumor microenvironment as a site of dendritic cell (DC), B cell and T cell priming and maturation with a resultant stronger anti-tumor immune response and longer overall survival [18-20]. However, the presence of TLS and their significance for overall and recurrence-free survival has so far not been studied in MCC.
Evidence that the immune system significantly contributes to MCC development and progression implies that novel therapeutic options for MCC should support the development of an anti-tumor directed immune response. It has already been revealed in patients with advanced melanoma that such therapies can be effective [21]. Immune checkpoint blockade with therapeutic antibodies targeting CTLA-4, PD-1 and its ligand PD-L1 leads to durable objective tumor response in up to one third of melanoma patients [22]. Recently, MCPyV-specific CD8+ T-cells have been identified in the blood of patients with MCC [23]. These T-cells expressed higher levels of the coinhibitory receptor PD-1. In addition, PD-L1 expression has been described in MCCs, tumor-infiltrating and peritumoral leucocytes [23,24]. These findings imply the possibility that blocking antibodies against PD-1 or PD-L1 could enhance an endogenous anti-tumor response leading to tumor regression.
In the present study we investigated the correlation of intratumoral and peritumoral CD3+ lymphocytes, CD8+ cytotoxic cells, CD4+ T-helper cells, CD68+ macrophages, and tumor-associated neutrophils with overall and recurrence-free survival in 21 patients with primary MCC. In addition, we examined whether PD-L1 expression in MCC and the presence of tertiary lymphoid structures in the tumor microenvironment was associated with a better prognosis.
Materials and methods
Patients and tumor tissues
Our sample comprised 43 formalin-fixed paraffin-embedded Merkel cell carcinoma (MCC) specimens from 34 patients, which had been surgically removed or biopsied between 1997 and 2013 at the University Hospital Mannheim, University of Heidelberg, Germany (n=37 tumors from 31 patients) or at the Department of Dermatology of the University Hospital Giessen, Germany (n=6 tumors from 3 patients). Out of the 34 subjects 7 were excluded due to incomplete clinical data, insufficient amounts of paraffin-embedded MCC tissue or inadequate tissue sample quality. The final study cohort contained material of 21 primary carcinomas from 21 subjects as well as 3 local recurrences and 3 metastases from 6 subjects (Figure 1). All tumors were positive for the Merkel cell marker cytokeratin 20.
Figure 1.
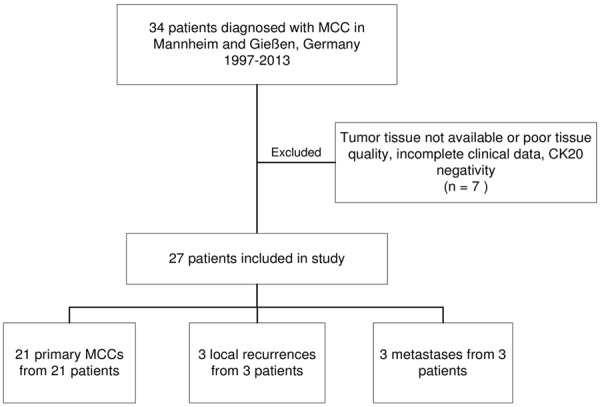
Flowchart of subjects included in the study.
Clinical data including age, sex, immune status, localization and kind of MCC, stage at initial diagnosis and at the end of follow-up period, recurrence, recurrence-free and overall survival were extracted from the patients’ clinical records. Immunosuppression was assumed in patients with inherited or acquired immunodeficiency syndromes, organ transplant, other disseminated malignancies, immunosuppressive medications or chemotherapy. Staging was performed according to the classification suggested by the American Joint Cancer Committee (AJCC) in 2010 [25]. Due to the fact that the size of the primary MCC was only inconsistently reported, patients were categorized into localized disease (stages I and II), regional disease (stage III) and disseminated disease (stage IV). Local recurrences were classified as stage II, lymph node metastases and in-transit metastases as stage III. For patients presenting a primary MCC at initial contact, recurrence-free survival was defined as the disease-free interval between curative treatment of the primary tumor and if applicable concomitant metastases, and relapse of the MCC in months. If patients initially presented local recurrences or MCC metastases, recurrence-free survival was defined as the disease-free interval after curative treatment of the recurrence or the metastases. If patients experienced several relapses in the course of their disease, time until the first relapse was taken into account. Patients who never reached a disease-free state were defined as having a recurrence-free survival of 0 months. All procedures were performed according to the principles of the Declaration of Helsinki and approved by the local medical ethic committee.
Immunohistochemistry and light microscopy
One μm-sections of paraffin-embedded MCC tissues were dried at 37°C overnight and deparaffinized using graded alcohol series according to standard protocols. Subsequent heat-induced antigen retrieval was carried out in either Heat-Induced Epitope Retrieval (HIER) citrate buffer (pH6) for 40 min at 95°C or Tris/EDTA buffer pH9 (Leica Biosystems, UK, Novocastra Epitope Retrieval Solutions) for 20 min at 95°C using a water bath. All MCCs were stained with antibodies against the marker protein cytokeratin 20 (Clone Ks 20.8, Dako M7019, Hamburg, Germany) at a working concentration of 0.25 μg/mL. The percentage of proliferating cells was determined by staining with Ki67 antibody (Ab15580, Abcam, Cambridge, UK) at a concentration of 3 μg/mL. For evaluation of tumor stroma cells the following antibodies were used for staining: Stabilin-1 (custom-made rabbit anti-mouse/human stabilin-1 antibody (RS1), PSL GmbH, Heidelberg, Germany; working dilution 1:1000), CD68 (Clone PG-M1, Dako M0876; 0.1 μg/mL), CD163 (PHA-69786, Dianova, Hamburg, Germany; working dilution 1:100), CD3 (Clone SP7, RM-9107 Thermo Scientific, Ulm, Germany 1:150); CD4 (NCL-CD4-1F6, Novocastra, Wetzlar, Germany; 80 μg/mL); CD8 (Clone 8/144B, Dako M7103, 3 μg/mL), CD31 (Clone JC70A, Dako M0823, 1:100), PD-1 (Ab52587, Abcam, Cambridge, UK; 20 μg/mL) and PD-L1 (anti human B7-H1 clone 5H1, 2 μg/mL, kindly provided by Dr Lieping Chen, Yale Cancer Center, New Haven, USA [26]), CD20 (Clone L26, Dako M0755, working dilution 1:1500), and S100 (Dako Z0311, 1:100). Antigen-bound primary antibodies were visualized by appropriate horseradish-peroxidase (HRP)-coupled secondary antibodies, HRP-coupled polymers or Avidin-Biotin Complex (ABC) in combination with Streptavidin-HRP (PD-L1). AEC Substrate-Chromogen (Dako) was precipitated by HRP-activation, followed by hematoxylin counterstain.
Images of all stained tumor slides were recorded with a Nikon Eclipse Ni-E microscope (Nikon DS-U3 Digital Camera Control Unit) and software system (Nikon, Düsseldorf, Germany). The absolute number of positively stained intratumoral cells was counted using ImageJ count in an area of 1 mm2.
Scoring
Absolute cell counts were assessed for intratumorally-localized cells positive for CD3, CD4, CD8, CD68 cells, for CD31-positive vessels and for PD-L1-positive macrophages. TAN infiltration in the tumor was HE-morphologically evaluated and grouped into two categories (I: < 5%, II: > 5%). The percentage of positive cells compared to the total peritumoral immune cell infiltration was determined for peritumoral CD4, CD8, CD68 cells and TANs and accordingly grouped into categories (I: < 10%; II: 10-50%; III: > 50%). CD8/CD4 ratio was assessed in the tumor center and periphery and was then dichotomized for statistical analysis (CD8/CD4 ≤ 1 or > 1). TLS were identified by HE-morphological appearance and staining with CD4, CD8, CD20 and S100 antibodies (Figure 2B-E).
Figure 2.
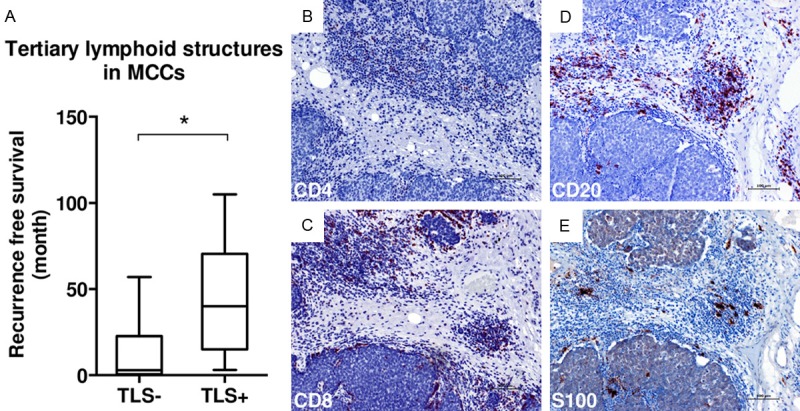
TLS as prognostic markers in MCCs. (A) Box blot showing the association between TLS and recurrence-free survival (p=0.25, Mann-Whitney-U-Test); (B) Immunohistochemical staining of TLS with anti-CD4 antibody; (C-E) Immunohistochemical stainings with antibodies against CD8 (C), CD20 (D), and S100 (E) visualizing TLS. Scale bar: 100 μm.
Membranous PD-L1 expression in MCCs was dichotomized into two categories (absent or low vs. strong). More than 50 PD-L1-positive intratumoral macrophages/mm2 were classified as strong infiltration, ≤ 50 as a limited infiltration. The extent of PD-L1 expression in peritumoral lymphohistiocytic infiltrates was classified into the categories limited (≤ 10%) or strong (> 10%).
Evaluation and counting was performed by two independent investigators (D.S.B. and A.S.). In case of discordance a third investigator (W.K.P.) was consulted and specimens were discussed until unanimous agreement was achieved.
DNA isolation and Merkel cell polyomavirus detection
DNA was isolated from serial paraffin sections using a DNA Isolation Kit (Qiagen, Hilden, Germany). MCC samples were analyzed for the presence of MCPyV by using a TaqMan assay specific for the MCPyV large T antigen and VP1 gene, as described previously [27,28].
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Science (IBM Corp. Released 2012. IBM SPSS Statistics for Mac, Version 21.0. Armonk, NY: IBM Corp.). Mann-Whitney-U tests or Student T-tests were applied for continuous distributions and Fisher’s Exact-Tests for categorical distributions. Spearman’s P was used for correlations with non-normal distribution. All statistical tests were two-sided, and a P-value ≤ 0.05 was considered as significant.
Results
Impact of tumor infiltrating leukocytes on overall and recurrence-free survival
In our patient cohort the mean age at first diagnosis of MCC was 71.9 years and 11.1% of the patients were immunosuppressed (Table 1). The most frequent localization of the primary tumor was the head and neck region (48.1%). At initial diagnosis, 74.1% of the patients had localized disease (stage I or II according to the classification suggested by the AJCC 2010). At the end of the follow-up period 48.1% still had local disease while 14.8% suffered from regional disease and 25.9% from distant metastases. Local recurrence occurred in 25.9%. In the whole patient sample, the mean recurrence-free survival was 19.7 months and the mean overall survival 42.5 months. Patients with primary MCCs had a mean recurrence-free survival of 24.4 months and a mean overall survival of 39.2 months. All MCCs were MCPyV-positive (Table 1).
Table 1.
Characteristics of the study cohort
| All MCCs(n=27) | Primary MCCs (n=21) | |
|---|---|---|
| Age (y), mean (SD) | 71.9 (12.7) | 71.5 (12.3) |
| Female, n (%) | 15 (55.6) | 10 (47.6) |
| Immunosuppression, n (%)* | 3 (11.1) | 3 (14.3) |
| Diabetes Mellitus, n (%) | 5 (18.5) | 3 (14.3) |
| Localization of primary tumor, n (%) | ||
| Head and Neck | 13 (48.1) | 9 (42.9) |
| Upper extremities | 3 (11.1) | 2 (9.5) |
| Trunk | 5 (18.5) | 4 (19.0) |
| Lower extremities | 6 (22.2) | 6 (28.6) |
| Stage at initial diagnosis, n (%) | ||
| Localized disease (stage I/II) | 20 (74.1) | 15 (71.4) |
| Regional disease (stage III) | 5 (18.5) | 4 (19.0) |
| Disseminated disease (stage IV) | 1 (3.7) | 1 (4.8) |
| Missing | 1 (3.7) | 1 (4.8) |
| Stage at end of follow up, n (%) | ||
| Localized disease (stage I/II) | 13 (48.1) | 12 (57.1) |
| Regional disease (stage III) | 4 (14.8) | 3 (14.3) |
| Disseminated disease (stage IV) | 7 (25.9) | 4 (19.0) |
| Missing | 3 (11.1) | 2 (9.5) |
| Recurrence, n (%) | ||
| Yes | 7 (25.9) | 5 (23.8) |
| Unknown | 7 (25.9) | 4 (19.0) |
| Follow-Up | ||
| Available, n (%) | 25 92.6 | 19 90.5 |
| Duration (months), mean (SD) | 21.2 (28.5) | 22.1 (32.2) |
| Survival | ||
| Recurrence-free (months, n=22), mean (SD) | 19.7 (27.1) | 24.4 (30.5) |
| Overall (months, n=24), mean (SD) | 42.5 (40.1) | 39.2 (39.5) |
| Treatment, n (%) | ||
| Surgery | 25 (92.6) | 19 (90.5) |
| Radiationtherapy | 13 (48.1) | 8 (38.1) |
| Chemotherapy | 3 (11.1) | 1 (4.8) |
| Merkel Cell Polyomavirus, n (%) | 27 (100) | 21 (100) |
SD: standard deviation;
The three immunocompromised patients suffered from mycosis fungoides or from non-Hodgkin lymphoma or received an immunosuppressive therapy for rheumatoid arthritis (leflunomid and prednisolone).
Since it has been suggested that a high intratumoral T-cell (CD3+) and cytotoxic T-cell (CD8+) count is associated with a favorable prognosis of MCC patients [10,29], we assessed the composition of the inflammatory infiltrate in the 21 primary MCCs of our cohort and its correlation with recurrence-free and overall survival. Exemplary immunohistochemical stainings are shown in Supplementary Figure S1, and raw data of CD3, CD4, CD8, CD68, CD31 and ki67 cell counts in Supplementary Table S1. PD-1 positive T-cells were scares and only found in the stroma of 4 out of 21 MCCs. Nearly all macrophages present in the MCCs stained positive for CD163. Interestingly, no Stabilin-1+ macrophages were detected in the MCCs, only the surrounding tissue macrophages expressed Stabilin-1. None of the leukocyte antigens examined (CD3, CD4, CD8, CD8/CD4 ratio, CD68) were significantly associated with overall survival or recurrence-free survival when counted intratumorally (Table 2) or in the tumor periphery (data not shown). Furthermore, the intratumoral density of CD31+ vessel and the proliferation rate assessed by Ki67 staining did not correlate with recurrence-free survival (CD31: Spearman’s ρ=-0.037, P=0.892, ki67: Spearman’s ρ=0.457, P=0.075) or overall survival (CD31: Spearman’s ρ=-0.123, P=0.627, Ki67: Spearman’s ρ=0.08, P=0.752).
Table 2.
Correlation of intratumoral immune markers with overall and recurrence-free survival
| Overall survival | Recurrence-free survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | Spearman’s ρ | P-value | N | Spearman’s ρ | P-value | |
| CD3 | 17 | 0.156 | 0.55 | 15 | -0.271 | 0.329 |
| CD4 | 17 | -0.296 | 0.248 | 15 | -0.355 | 0.194 |
| CD8 | 18 | 0.07 | 0.783 | 16 | -0.194 | 0.472 |
| CD4/CD8 | 17 | -0.25 | 0.333 | 15 | 0.002 | 0.995 |
| CD68 | 18 | 0.152 | 0.548 | 16 | -0.323 | 0.222 |
| CD31 | 18 | -0.123 | 0.627 | 16 | -0.037 | 0.892 |
| Ki67 | 18 | 0.08 | 0.752 | 16 | 0.457 | 0.075 |
Correlation of tumor-associated neutrophils, tertiary lymphoid structures and PD-L1 with survival
Recent studies point to a negative correlation of tumor-associated neutrophil (TAN) infiltration with tumor prognosis [12] and to a favorable prognosis when tertiary lymphoid structures (TLS) are found in the tumor periphery [17-19] or when MCCs are PD-L1 positive [24].
In our cohort of 21 patients with primary MCCs, neither a significant association of overall survival with intratumoral (TAN+ vs. TAN-: median 6 months (IQR 5, 80.5) (n=4) vs. median 34.5 months (IQR 13.75, 58.25), n=14, P=0.524) or with peritumoral (TAN+ vs. TAN-: median 5 months (IQR n.a.) (n=2) vs. median 3.5 months (IQR 9.5, 58.75) (n=16), P=0.16) TAN infiltration, nor an association of recurrence-free survival with intratumoral (TAN+ vs. TAN-: median 3 months (IQR n.a.) (n=3) vs. median 17.0 months (IQR 1, 45.5), n=13, P=0.635) or peritumoral (TAN+ vs. TAN-: median 3 months (IQR n. a.) (n=2) vs. median 18 months (IQR 1, 52.5) (n=14), P=0.522) TAN infiltration was found, although a trend towards a longer recurrence-free and overall survival was surely seen in TAN- MCCs.
Interestingly however, the presence of TLS in primay MCCs significantly correlated with recurrence-free survival (TLS+ vs. TLS-: median 40 vs. 3 months, P=0.025; Table 3; Figure 2A). Patients with MCCs containing TLS also had a somewhat longer overall survival than those with TLS- tumors (median 29 vs. 18 months), but differences were not statistically significant (P=0.651; Table 3). In addition, TLS were significantly associated with a higher CD8/CD4 ratio in the tumor periphery (P=0.032), but not in the center of the tumor (P > 0.999). Clinicopathological characteristics such as age, gender and stage at initial diagnosis did not correlate with the presence of TLS (Table 3).
Table 3.
Associations between tertiary lymphoid structures (TLS) and clinicopathological or prognostic features
| TLS+ | TLS- | P | |
|---|---|---|---|
| n | 8 | 13 | |
| Age (y), mean (SD) | 66.9 (13.4) | 74.3 (11.3) | 0.209* |
| Sex | |||
| Female (n=10), n (%) | 4 (50) | 6 (46.2) | > 0.999† |
| Male (n=11), n (%) | 4 (50) | 7 (53.8) | |
| Stage initial diagnosis, n (%) | |||
| I/II | 5 (62.5) | 10 (76.9) | > 0.999† |
| III/IV | 2 (25.0) | 3 (23.1) | |
| Unknown | 1 (12.5) | 0 (0) | |
| Local, n (%) | |||
| Yes | 1 (12.5) | 4 (30.8) | 0.338† |
| No | 6 (75) | 6 (46.2) | |
| Unknown | 1 (12.5) | 3 (23.1) | |
| Recurrence-free survival (month, n=16) | |||
| Median (IQR 25, 75) | 40 (15, 70.5) | 3 (0.75, 22.75) | 0.025* |
| Min-Max | 3-105 | 0-57 | |
| Overall survival (month, n=18) | |||
| Median (IQR 25, 75) | 29 (5; 59) | 18 (5; 57) | 0.651* |
| Min-Max | 2-105 | 1-135 |
SD: standard deviation;
Mann-Whitney-U-Test;
Fisher’s Exact Test.
TLS have also been described in metastatic tumors including metastatic melanomas [30,31]. We therefore analyzed if TLS were present also in local recurrences and in metastases. Indeed, all local recurrences and all metastases contained TLS.
PD-L1 expression by MCCs has been shown to correlate with improved overall survival of patients with MCC [24]. We therefore analyzed whether PD-L1 expression in tumor cells, intratumoral macrophages and peritumoral immune cells was associated with specific clinicopathological characteristics or survival of our cohort. Eight of 19 primary MCCs (2 not assessed, no more tissue available) were strongly positive for PD-L1 (for exemplary immunohistochemical stainings of PD-L1 positive and PD-L1 negative MCCs see Figure 3A, 3B). In addition, 8 of 19 MCCs showed a strong infiltration with PD-L1 positive macrophages and 7 of 19 MCCs had peritumoral PD-L1 positive immune cells (Table 4, for exemplary immunohistochemichal stainings of PD-L1 positive macrophages and PD-L1 positive immune cells see Figure 3C, 3D). Neither sex nor age nor stage at initial diagnosis significantly correlated with PD-L1 expression in tumor cells and immune cells (Table 4). In addition, PD-L1 positivity in tumor cells or immune cells did not impact the frequency of local recurrence, recurrence-free or overall survival in our cohort (Table 4).
Figure 3.
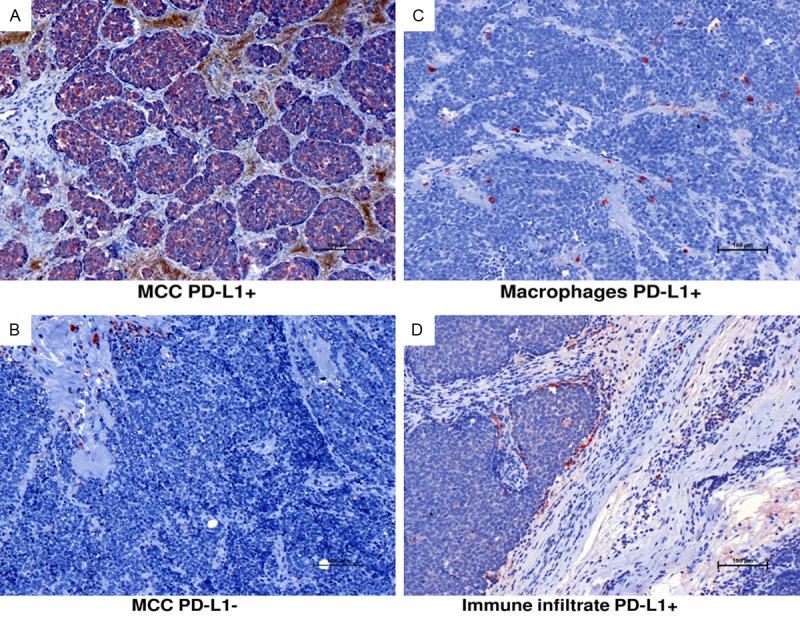
Exemplary immunostainings of primary MCC classified as PD-L1 positive (A) or negative (B), PD-L1 positive intratumoral macrophages (C) and PD-L1 positive peritumoral inflammatory infiltrate (D). Scale bar: 100 μm.
Table 4.
Relationship of PD-L1-expression in tumor cells, immune cells with clinicopathological features and survival rates
| Tumor | PD-L1+ intratumoral macrophages | Immune Cells (peritumoral) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| PD-L1+ | PD-L1- | P | PD-L1+ | PD-L1- | P | PD-L1+ | PD-L1- | P | |
| n | 8 | 11 | 8 | 11 | 7 | 12 | |||
| Age (y), mean (SD) | 73.1 (13.2) | 71.3 (11.5) | 0.71* | 67.6 (11.4) | 75.3 (11.8) | 0.14* | 72.6 (11.3) | 73.5 (12.8) | 0.83* |
| Sex, n (%) | |||||||||
| Female | 5 (62.5) | 4 (36.4) | 0.37 | 4 (50) | 5 (45.5) | > 0.99 | 4 (57.1) | 5 (41.7) | 0.65 |
| Male | 3 (37.5) | 7 (63.6) | 4 (50) | 6 (54.5) | 3 (42.9) | 7 (58.3) | |||
| Stage at initial diagnosis, n (%) | |||||||||
| I/II | 7 (87.5) | 6 (54.5) | 0.10† | 6 (75) | 7 (63.9) | > 0.99† | 6 (85.7) | 7 (58.3) | 0.6† |
| III/IV | 0 (0) | 5 (45.5) | 2 (25) | 3 (27.3) | 1 (14.3) | 4 (33.3) | |||
| Unknown | 1 (12.5) | 0 (0) | 0 (0) | 1 (9.1) | 0 (0) | 1 (8.3) | |||
| Local recurrence, n (%) | |||||||||
| Yes | 2 (25) | 3 (27.3) | > 0.99† | 1 (12.5) | 4 (36.4) | 0.12† | 1 (14.3) | 4 (33.3) | 0.58† |
| No | 4 (50 | 6 (54.5) | 7 (87.5) | 3 (27.3) | 5 (71.4) | 5 (41.7) | |||
| Unknown | 2 (25) | 2 (18.2) | 0 (0) | 4 (36.4) | 1 (14.3) | 3 (25) | |||
| Recurrence-free survival (n=14) | |||||||||
| Median (IQR 25; 75) | 17.0 (2; 54) | 3 (1.5; 49.5) | 0.79* | 19 (0; 59) | 3.0 (3; 51) | 0.8* | 19 (1.5; 49.5) | 3 (2; 54) | 0.84* |
| Min-Max | 1-57 | 0-105 | 0-105 | 1-57 | 0-59 | 0-105 | |||
| Overall survival (n=16) | |||||||||
| Median (IQR 25; 75) | 54.5 (13.3; 66.8) | 18.5 (5; 70.5) | 0.75* | 29.5 (7.5; 57.3) | 37 (5.5; 84.3) | 0.75* | 18.5 (4.3; 44.8) | 54.5 (6.5; 96) | 0.30* |
| Min-Max | 2-93 | 4-135 | 2-105 | 5-135 | 2-59 | 4-135 | |||
SD: standard deviation;
Mann-Whitney-U-Test;
Fisher’s Exact Test.
Taken together, the presence of TLS was the only histopathological finding associated with prolonged recurrence-free survival in our patient cohort.
Discussion
Our study shows for the first time that presence of TLS in the tumor periphery of MCCs significantly correlates with prolonged recurrence-free survival and that this is associated with a higher CD8/CD4 ratio in the tumor periphery.
The immune system plays a central role for systemic tumor surveillance; accordingly, immunosuppressed patients bear a considerably higher risk of developing malignancies, including MCCs [1]. In addition, data from diverse studies support an association of a robust T-cell infiltration in the tumor with a better prognosis in malignancies such as colorectal, breast and ovarian carcinoma [32-35]. In MCC presence of a dense CD3+ and CD8+ T-cell infiltrate is a favorable prognostic sign [10,11,29]. The fact that we did not find any significant correlation of recurrence-free or overall survival with intra- and peritumoral CD8+ or CD3+ T-cells may be do to the small sample size of our cohort.
Intratumoral T- and B-cells can specifically recognize tumor antigens [36,37], which has also been shown for CD8+ and CD4+ T-cells in MCCs [38]. However, little is known about the mechanisms controlling such a specific immune activation. Generally it is believed that secondary lymphoid structures such as draining lymph nodes are the major sites of lymphocyte activation. Recently, however, TLS have been identified in direct tumor proximity. The presence of such structures has been associated with a better prognosis in diverse tumor entities such as colon, lung, breast and renal carcinomas as well as in melanomas [31,39-42]. TLS are composed of T- and B-cell areas. In B-cell areas follicular dendritic cells and B-cells are found, while in T-cell areas high endothelial venules, mature dendritic cells as well as T-cells are localized in close proximity [31]. Such a clustering of antigen-presenting cells with cells of the adaptive immune system is thought to facilitate the development of a specific anti-tumor immune response by bringing the whole immune cell repertoire in close proximity to tumor antigens. In our study TLS were analyzed for the first time in MCCs. More than one third of the MCC samples studied contained such structures (8 of 21). Interestingly, the TLS status significantly correlated with the CD8/CD4 ratio in the tumor periphery. It has been reported that the presence of TLS mature dendritic cells is specifically associated with a Th1 and CD8+ cytotoxic T-cell response in non small cell lung carcinoma patients and that this finding correlates with improved long-term survival [43]. Based on our analysis we cannot conclude that the association of a higher CD8/CD4 ratio in the tumor periphery with the presence of TLS is a sign of a specific immune-mediated anti-tumor response. To confirm this hypothesis, it will be necessary to study the T-cell receptor repertoire and T-cell activation status. Interestingly, also all local recurrences and distant metastases studied in this work contained TLS. The presence of TLS in metastases has already been described for melanoma [31]. The authors suggested that TLS might be a sign of an active immune reaction against neoplastic cells. Based on their results they could not say whether the presence of TLS helps or impairs tumor progression as TLS can also be involved in the induction of peripheral immune tolerance [31,44]. Clearly, the association between TLS and recurrence-free survival of MCC patients documented here as well as the impact of TLS on metastasis formation and overall survival need to be verified in larger patient cohorts.
Patients with MCC have a high risk of lymph node and distant metastasis in the course of their disease [45]. Although their tumors are usually chemosensitive with overall response rates of 60-70% [46,47], the median duration of response to currently used chemotherapy regimens is only about 8 months [45]. Therefore new therapeutic options are urgently needed. The promotion of an anti-tumor-directed immune response by blockade of inhibitory co-stimulatory receptors or ligands such as CTLA-4, PD-1 and PD-L1 has proven effective for patients with advanced melanoma and other cancers [21,22,48]. Tumor cells express PD-L1 especially in response to inflammatory stimuli as a sign of immune resistance [49]. It has been suggested that surface expression of PD-L1 on tumor cells might be a useful biomarker to identify patients who benefit from therapeutic PD-1 and PD-L1 blockade [50]. Surface expression of PD-L1 has been reported in about 50% of MCCs [24]. Interestingly, PD-L1 expression in MCCs has been identified as a favorable prognostic marker [22]. It has been postulated that strong PD-L1 expression in tumor cells could point to a strong immune response against MCCs mediated by IFN-gamma secreting CD8+ lymphocytes [24], which leads to up-regulation of PD-L1 in tumor cells in an attempt to reestablish a permissive environment. In our study we were able to confirm the PD-L1 surface expression on 42% of the primary MCCs. However, we did not find any correlation with patients’ survival rates, possibly due to the small sample size. Clearly, the prognostic value of PD-L1 in MCC needs to be further assessed. However, based on our results and the findings by Lipson and colleagues we believe that in patients with advanced MCCs checkpoint blocking agents such as anti-PD-1 or anti-PD-L1 might be promising therapeutic options.
In summary, we were able to confirm surface expression of PD-L1 in a significant proportion of MCCs. In addition, we identified TLS as potentially novel prognostic markers, which are easy to assess in paraffin-embedded tissue. Validation of these findings in larger patient cohorts and tumor samples and exploration of these molecules as tools for routine diagnostic or targets for novel therapies will be of great interest.
Acknowledgements
We would like to thank Lieping Chen, Yale Cancer Center, New Haven, CT, USA, for kindly providing the PD-L1 antibody. This work was supported in part by grants of the Deutsche Forschungsgemeinschaft (DFG), SFB938, project H to SG and project Z2 to FL.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Schrama D, Becker JC. Merkel cell carcinoma--pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol. 2011;25:1121–1129. doi: 10.1111/j.1468-3083.2011.04032.x. [DOI] [PubMed] [Google Scholar]
- 2.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JC, Kauczok CS, Ugurel S, Eib S, Brocker EB, Houben R. Merkel cell carcinoma: molecular pathogenesis, clinical features and therapy. J Dtsch Dermatol Ges. 2008;6:709–719. doi: 10.1111/j.1610-0387.2008.06830.x. [DOI] [PubMed] [Google Scholar]
- 4.Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232–238. doi: 10.1111/dsu.12079. [DOI] [PubMed] [Google Scholar]
- 5.Pipas JM. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, Moore PS, Becker JC. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, Martinez-Fernandez A, Barletta JA, Bellizzi AM, Sadasivam S, Holloway DT, Cooper DJ, Kupper TS, Wang LC, DeCaprio JA. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122:4645–4653. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touze A, Le Bidre E, Laude H, Fleury MJ, Cazal R, Arnold F, Carlotti A, Maubec E, Aubin F, Avril MF, Rozenberg F, Tognon M, Maruani A, Guyetant S, Lorette G, Coursaget P. High levels of antibodies against merkel cell polyomavirus identify a subset of patients with merkel cell carcinoma with better clinical outcome. J. Clin. Oncol. 2011;29:1612–1619. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 10.Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, Schrama D, Simonson WT, Lemos BD, Byrd DR, Koelle DM, Galloway DA, Leonard JH, Madeleine MM, Argenyi ZB, Disis ML, Becker JC, Cleary MA, Nghiem P. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J. Clin. Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sihto H, Bohling T, Kavola H, Koljonen V, Salmi M, Jalkanen S, Joensuu H. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872–2881. doi: 10.1158/1078-0432.CCR-11-3020. [DOI] [PubMed] [Google Scholar]
- 12.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J Cancer. 2013;4:3–11. doi: 10.7150/jca.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pimenta EM, Barnes BJ. Role of Tertiary Lymphoid Structures (TLS) in Anti-Tumor Immunity: Potential Tumor-Induced Cytokines/Chemokines that Regulate TLS Formation in Epithelial-Derived Cancers. Cancers (Basel) 2014;6:969–997. doi: 10.3390/cancers6020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 16.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 17.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 18.Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother. 2014;63:643–62. doi: 10.1007/s00262-014-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud JL, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 20.Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2:e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, Iyer JG, Ibrani D, Koelle DM, Nghiem P. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19:5351–5360. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, Anders RA, Topalian SL, Taube JM. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Joint Committee on Cancer: AJCC cancer staging handbook. New York: Springer; 2010. [Google Scholar]
- 26.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmbold P, Lahtz C, Enk A, Herrmann-Trost P, Marsch W, Kutzner H, Dammann RH. Frequent occurrence of RASSF1A promoter hypermethylation and Merkel cell polyomavirus in Merkel cell carcinoma. Mol Carcinog. 2009;48:903–909. doi: 10.1002/mc.20540. [DOI] [PubMed] [Google Scholar]
- 28.Vlahova L, Doerflinger Y, Houben R, Becker JC, Schrama D, Weiss C, Goebeler M, Helmbold P, Goerdt S, Peitsch WK. P-cadherin expression in Merkel cell carcinomas is associated with prolonged recurrence-free survival. Br J Dermatol. 2012;166:1043–1052. doi: 10.1111/j.1365-2133.2012.10853.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson ME, Zhu F, Li T, Wu H, Galloway TJ, Farma JM, Perlis CS, Turaka A. Absolute lymphocyte count: A potential prognostic factor for Merkel cell carcinoma. J Am Acad Dermatol. 2014;70:1028–1035. doi: 10.1016/j.jaad.2014.01.890. [DOI] [PubMed] [Google Scholar]
- 30.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Flejou JF, Gibault L, Verkarre V, Regnard JF, Pages ON, Oudard S, Mlecnik B, Sautes-Fridman C, Fridman WH, Damotte D. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 31.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 32.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activat ed CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 35.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 36.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52:715–738. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizukami M, Hanagiri T, Baba T, Fukuyama T, Nagata Y, So T, Ichiki Y, Sugaya M, Yasuda M, Takenoyama M, Sugio K, Yasumoto K. Identification of tumor associated antigens recognized by IgG from tumor-infiltrating B cells of lung cancer: correlation between Ab titer of the patient’s sera and the clinical course. Cancer Sci. 2005;96:882–888. doi: 10.1111/j.1349-7006.2005.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, Marshak JO, Dong L, Carter J, Lai I, Farrar E, Byrd D, Galloway D, Yee C, Koelle DM, Nghiem P. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res. 2011;17:6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergomas F, Grizzi F, Doni A, Pesce S, Laghi L, Allavena P, Mantovani A, Marchesi F. Tertiary intratumor lymphoid tissue in colo-rectal cancer. Cancers (Basel) 2011;4:1–10. doi: 10.3390/cancers4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mule JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179:37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottlin EB, Bentley RC, Campa MJ, Pisetsky DS, Herndon JE 2nd, Patz EF Jr. The Association of Intratumoral Germinal Centers with early-stage non-small cell lung cancer. J Thorac Oncol. 2011;6:1687–1690. doi: 10.1097/JTO.0b013e3182217bec. [DOI] [PubMed] [Google Scholar]
- 42.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothe F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, Validire P, Remark R, Hammond SA, Cremer I, Damotte D, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 44.Brown K, Sacks SH, Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor-specific tolerance rather than rejection. Eur J Immunol. 2011;41:89–96. doi: 10.1002/eji.201040759. [DOI] [PubMed] [Google Scholar]
- 45.Prieto Munoz I, Pardo Masferrer J, Olivera Vegas J, Medina Montalvo MS, Jover Diaz R, Perez Casas AM. Merkel cell carcinoma from 2008 to 2012: reaching a new level of understanding. Cancer Treat Rev. 2013;39:421–429. doi: 10.1016/j.ctrv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, Wong SL. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007;110:1–12. doi: 10.1002/cncr.22765. [DOI] [PubMed] [Google Scholar]
- 47.Becker J, Mauch C, Kortmann RD, Keilholz U, Bootz F, Garbe C, Hauschild A, Moll I. Short German guidelines: Merkel cell carcinoma. J Dtsch Dermatol Ges. 2008;6(Suppl 1):S15–16. doi: 10.1111/j.1610-0387.2008.06707.x. [DOI] [PubMed] [Google Scholar]
- 48.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 50.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


