Abstract
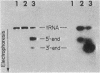
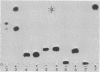
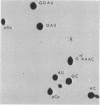
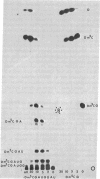
Eukaryotic cytoplasmic initiator tRNAs lack the sequence G-T-Ψ-C-G(A)-, which is in every tRNA of known sequence that is active in protein biosynthesis. In initiator tRNA of yeast cytoplasm, which is the only eukaryotic initiator tRNA of known sequence, this sequence is replaced by G-A-U-C-G-. We now report the sequence of a 30-nucleotide-long, 3′-terminal fragment of cytoplasmic initiator tRNA of rabbit liver obtained by specific cleavage of the tRNA at the site occupied by the modified nucleoside, 7-methyl guanosine. We show (i) that in rabbit-liver initiator tRNA also, the sequence G-T-Ψ-C-G(A)- is replaced by G-A-U-C-G- and (ii) that the sequences of loop IV of both the yeast and rabbit-liver cytoplasmic initiator tRNAs are identical, A-U-C-G-m1-A-A-A-.
Keywords: eukaryotic initiator tRNA, fingerprinting, specific chemical cleavage, nucleotide sequence analysis
Full text
PDF
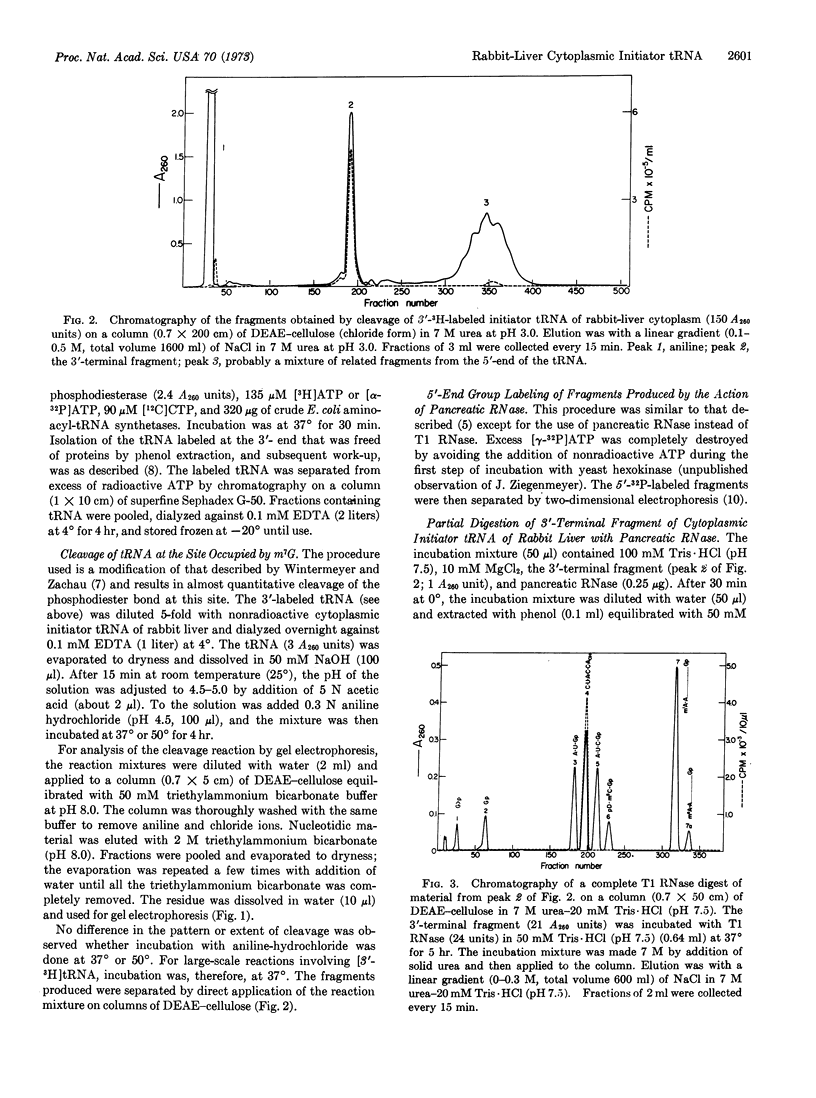

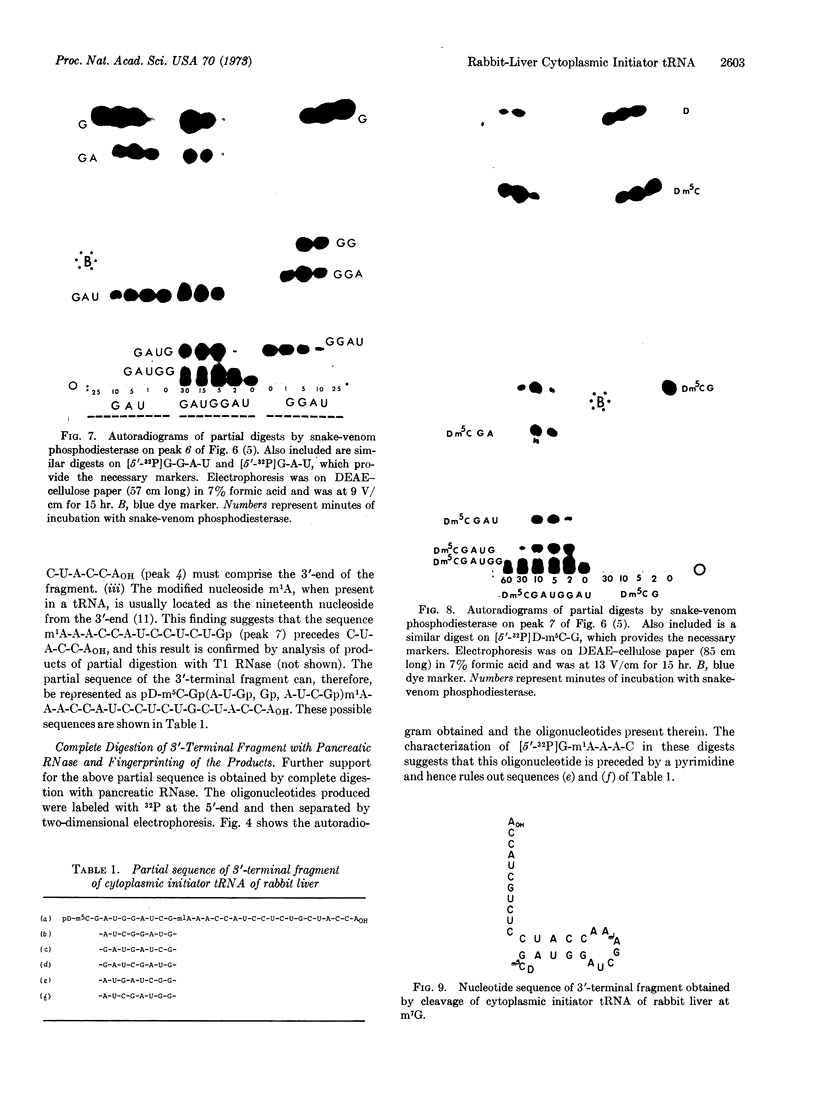

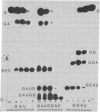
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. C., Woodley C. L., Bose K. K., Gupta K. K. Protein synthesis in rabbit reticulocytes: characteristics of a Met-tRNA Met f binding factor. Biochem Biophys Res Commun. 1972 Jul 11;48(1):1–9. doi: 10.1016/0006-291x(72)90335-x. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Hunt T., Jackson R. J. A complex between met-tRNA F and native 40S subunits in reticulocyte lysates and its disappearance during incubation with double-stranded RNA. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1556–1564. doi: 10.1016/0006-291x(72)90891-1. [DOI] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P., Bonnet J., Gangloff J., Keith G., Krebs B., Kuntzel B., Roy A., Weissenbach J., Werner C. Structure primaire des tRN. Biochimie. 1972;54(2):127–144. doi: 10.1016/s0300-9084(72)80097-x. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Petrissant G. Evidence for the absence of the G-T-psi-C sequence from two mammalian initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1046–1049. doi: 10.1073/pnas.70.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps G. R. Analysis of purified tRNA species by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Dec;44(2):345–357. doi: 10.1016/0003-2697(71)90220-x. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. Lack of Tp-psi-p in loop IV of a mammalian initiator transfer RNA. FEBS Lett. 1973 Mar 15;30(3):265–267. doi: 10.1016/0014-5793(73)80666-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Studies on polynucleotides. CI. Escherichia coli tyrosine and formylmethionine transfer ribonucleic acids: effect of chemical modification of 4-thiouridine to uridine on their biological properties. J Biol Chem. 1972 Aug 10;247(15):4879–4892. [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. A specific chemical chain scission of tRNA at 7-methylguanosine. FEBS Lett. 1970 Dec;11(3):160–164. doi: 10.1016/0014-5793(70)80518-x. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ochoa S. Polypeptide chain initiation in eukaryotes: functional identity of supernatant factor from various sources. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1796–1799. doi: 10.1073/pnas.69.7.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]