Abstract
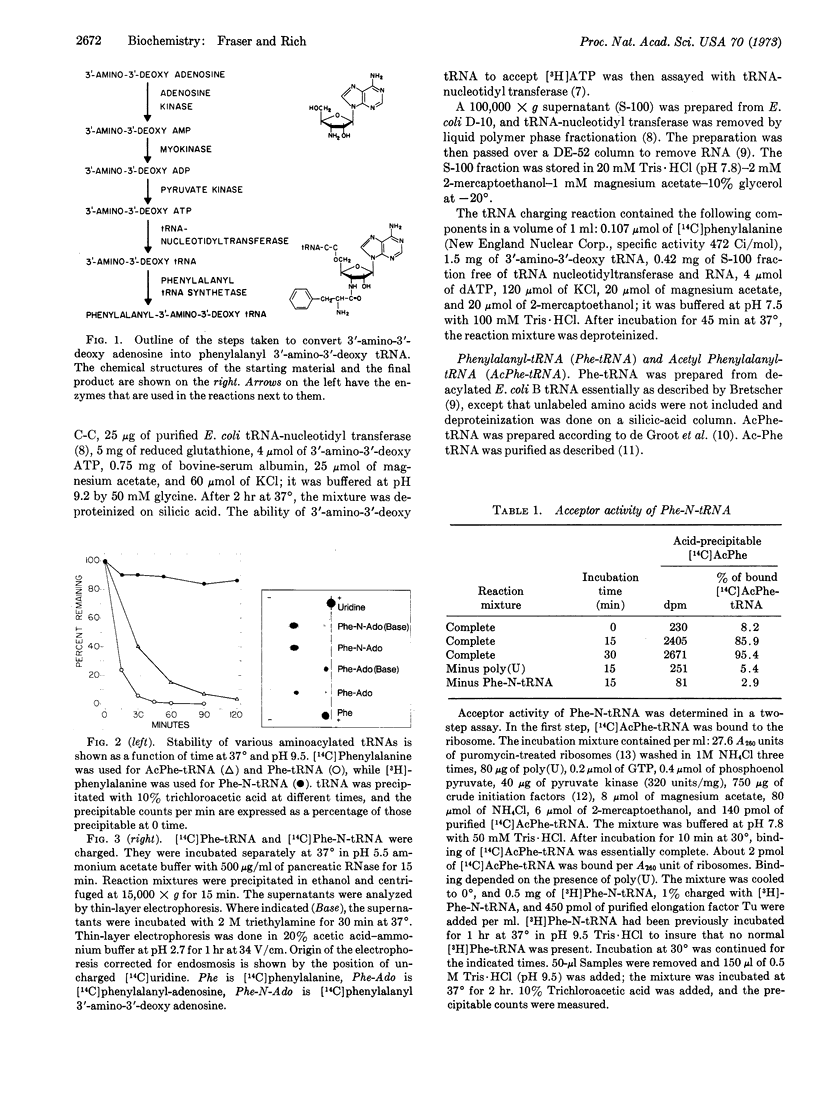
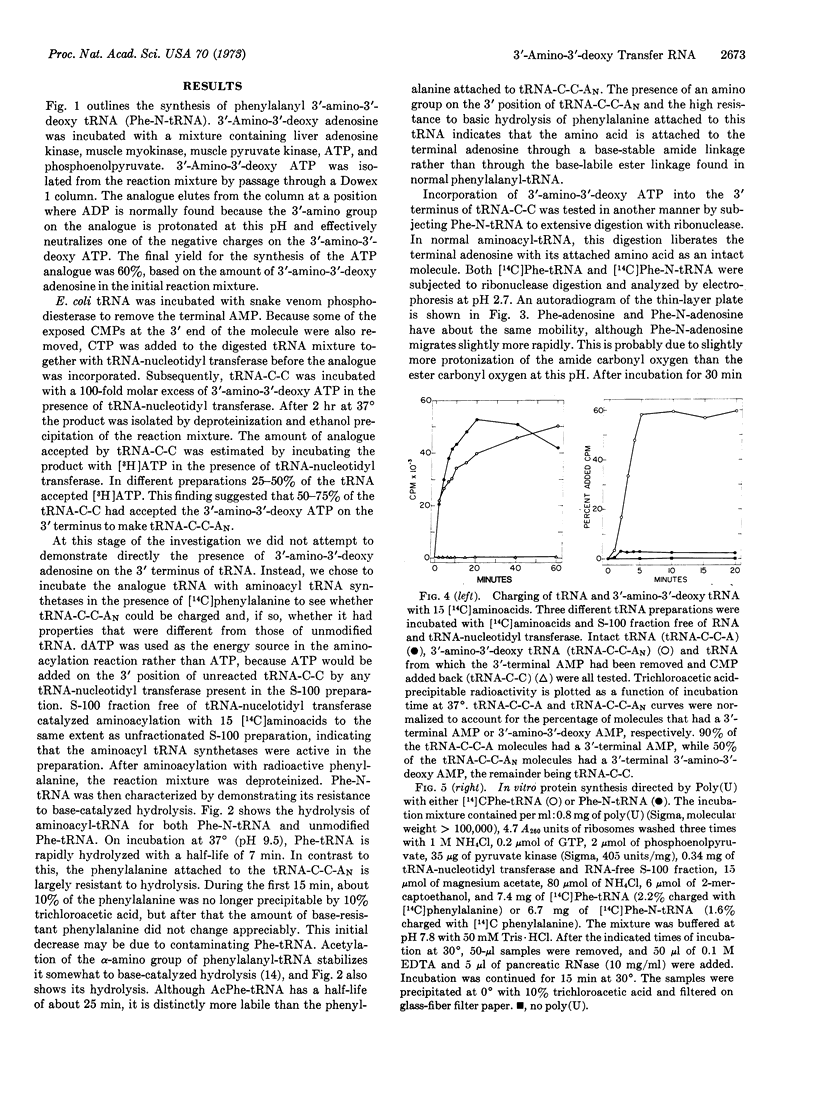
3′-amino-3′-deoxy adenosine was enzymatically converted into 3′-amino-3′-deoxy ATP. This ATP analogue was incorporated into the 3′-terminal adenosine position of Escherichia coli tRNA. The modified tRNA was aminoacylated with phenylalanine by use of E. coli phenylalanyl tRNA synthetase. The phenylalanine was attached to the 3′-amino group of the tRNA, as shown by its high resistance to base-catalyzed hydrolysis in contrast with the normal lability of phenylalanyl-tRNA. Aminoacyl tRNA synthetases charge the 3′-amino-3′-deoxy tRNA with kinetics that are similar to those of the charging reaction in which normal tRNA is the substrate. When phenylalanyl-3′-amino-3′-deoxy tRNA is used in a protein-synthesizing system directed by poly(U) in vitro, this molecule is capable of receiving an acetyl-phenylalanine from the donor site of the ribosome. However, the ribosome is unable to cleave the amide bond connecting phenylalanine to the tRNA molecule; hence, the phenylalanyl-3′-amino-3′-deoxy tRNA has acceptor but not donor activity in protein synthesis. Failure of the ribosome to cleave the amide bond may be due to its greater stability relative to the normal ester bond. However, it may also be due to the fact that the isomerization of the peptidyl chain between the 2′ and 3′ positions of adenosine is prevented due to the amide bond at the 3′ position, and cleavage may normally occur with the peptidyl chain on the 2′ position of adenosine.
Keywords: puromycin-tRNA, 3′-amino-3′-deoxy ATP, aminoacyl synthetase, ribosomal acceptor site
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Bretscher M. S., Clark B. F., Marcker K. A. A GTP requirement for binding initiator tRNA to ribosomes. Nature. 1967 Jul 29;215(5100):490–492. doi: 10.1038/215490a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Polypeptide chain termination: an active process. J Mol Biol. 1968 May 28;34(1):131–136. doi: 10.1016/0022-2836(68)90239-8. [DOI] [PubMed] [Google Scholar]
- Fahnestock S., Rich A. Ribosome-catalyzed polyester formation. Science. 1971 Jul 23;173(3994):340–343. doi: 10.1126/science.173.3994.340. [DOI] [PubMed] [Google Scholar]
- Fahnestock S., Rich A. Synthesis by ribosomes of viral coat protein containing ester linkages. Nat New Biol. 1971 Jan 6;229(1):8–10. doi: 10.1038/newbio229008a0. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Harris R., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XXIV. Effects of antibiotics on binding of aminoacyl-oligonucleotides to ribosomes. J Biol Chem. 1973 Feb 25;248(4):1168–1174. [PubMed] [Google Scholar]
- Lindberg B., Klenow H., Hansen K. Some properties of partially purified mammalian adenosine kinase. J Biol Chem. 1967 Feb 10;242(3):350–356. [PubMed] [Google Scholar]
- Lindberg B. Some additional properties of partially purified mammalian adenosine kinase. Biochim Biophys Acta. 1969 Jul 8;185(1):245–247. doi: 10.1016/0005-2744(69)90300-3. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Release of transfer RNA during peptide chain elongation. Proc Natl Acad Sci U S A. 1969 May;63(1):93–97. doi: 10.1073/pnas.63.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Hirst-Bruns M. E., Philipps G. R. Action of venom phosphodiesterase on transfer RNA from Escherichia coli. Biochim Biophys Acta. 1970 Sep 17;217(1):176–188. doi: 10.1016/0005-2787(70)90134-6. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Philipps G. R. Purification of transfer-RNA-nucleotidyltransferase from E. coli B. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1174–1179. doi: 10.1016/0006-291x(70)90363-3. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Philipps G. R. Transfer ribonucleic acid nucleotidyltransferase from Escherichia coli. II. Purification, physical properties, and substrate specificity. J Biol Chem. 1971 Mar 10;246(5):1274–1279. [PubMed] [Google Scholar]
- Sprinzl M., Scheit K. H., Sternbach H., von der Haar F., Cramer F. In vitro in corporation of 2'-deoxyadenosine and 3'-deoxyadenosine into yeast tRNA Phe using t-RNA nucleotidyl transferase, and properties of tRNA Phe -C-C-2'dA and tRNA Phe -C-C-3'dA. Biochem Biophys Res Commun. 1973 Apr 16;51(4):881–887. doi: 10.1016/0006-291x(73)90009-0. [DOI] [PubMed] [Google Scholar]
- de Groot N., Lapidot Y., Panet A., Wolman Y. The synthesis of N-acetylphenylalanyl-sRNA. Biochem Biophys Res Commun. 1966 Oct 5;25(1):17–22. doi: 10.1016/0006-291x(66)90633-4. [DOI] [PubMed] [Google Scholar]



