Abstract
Triple-negative breast cancer (TNBC) is a heterogeneous disease; gene expression (GE) analyses recently identified six distinct TNBC subtypes, each displaying a unique biology. Exploring novel approaches for the treatment of these subtypes is critical, especially as the median survival for women with metastatic TNBC is less than 12 months, and virtually all women with metastatic TNBC will ultimately die of their disease despite systemic therapy. To date, not a single targeted therapy has been approved for the treatment of TNBC, and cytotoxic chemotherapy remains the standard treatment. In this review, we will discuss recent developments in subtyping TNBC and the current and upcoming therapeutic strategies being explored in an attempt to target TNBC.
Keywords: Triple-negative breast cancer, targeted therapies, subtyping TNBC
Background
The term “triple-negative breast cancer” (TNBC) is used to identify the approximately 15% of breast cancers that lack expression of estrogen receptor (ER) and progesterone receptor (PR) and do not show amplification of the human epidermal growth factor receptor 2 (HER2) gene. TNBCs are a heterogeneous group of tumors with one common feature: a distinctly aggressive nature with higher rates of relapse and shorter overall survival in the metastatic setting compared to other subtypes of breast cancer. TNBCs more frequently affect younger patients and are more prevalent in African-American women, generally of a higher grade, and associated with BRCA gene mutations1,2. The median survival for women with metastatic TNBC is less than one year, and almost all die of their disease despite aggressive and toxic systemic chemotherapy1.
The benefits of targeted therapies, as evidenced by improving survival rates for patients with ER/PR positive and HER2 amplified breast cancers, have eluded patients with TNBC due to the absence of well-defined molecular targets. Cytotoxic chemotherapy is currently the only treatment option for TNBC. Although some patients respond, the treatment is toxic and a large percentage treated in the early stage eventually relapse. In the metastatic setting, after one or two lines of chemotherapy, meaningful responses are rare and all patients eventually succumb to their disease.
The identification of molecular targets will be critical to improving survival in patients with TNBC. A major obstacle to identifying actionable targets in TNBC is the vast disease heterogeneity, both inter- and intra-tumor heterogeneity; years of study have failed to show a single unifying alteration that is targetable in TNBC. There is clearly a major need to better understand the molecular basis of TNBC and to develop effective therapeutic strategies against it. In this review, we discuss the recent discoveries that have furthered our understanding of TNBC, with a focus on the subtyping of TNBC. We also explore the implications of these discoveries for future treatments and highlight the need for clinical trials focusing on subtypes of TNBC.
Characterization of TNBC
It has been well over a decade since Perou et al. published their seminal paper that categorized breast cancer by gene expression profiling into “intrinsic subtypes” and later developed into the PAM50 assay3. The basal-like subtype was found to be a particularly aggressive and is characterized by lack of ER/PR/HER2 expression. Various studies have shown an association of basal-like breast cancers with expression of cytokeratins 5/6, 14, and 17, P-cadherin, p53, and EGFR4–8. Mutations and genomic deletions in TP53 and RB1 are common in this subtype, along with high proliferation indices 9–11. Although most TNBCs classify in the “basal-like” subtype based on the intrinsic subtype classification, the terms “TNBC” and “basal-like breast cancer” are not synonymous; approximately 20–30% of clinical TNBCs are not basal-like by microarray analysis, and a significant number of basal-like breast cancers express ER/PR or HER212–14.
Several newer studies have refined our understanding of TNBCs. The Cancer Genome Atlas (TCGA) Research Network analyzed primary breast cancers using six platforms including genomic DNA copy number arrays, DNA methylation, exome sequencing, messenger RNA arrays, microRNA sequencing and reverse-phase protein arrays15. By integrating information across platforms, TCGA was able to examine the genomic heterogeneity of tumors. TCGA analysis showed that the most frequent loss-of-function and gain-of-function alterations in TNBC involve genes associated with DNA damage repair and phosphatidylinositol 3-kinase (PI3K) signaling pathways, respectively15. Alterations in DNA damage repair genes include loss of TP53, RB1, and BRCA1 function. Aberrant activation of the PI3K pathway occurs due to loss of negative regulators such as the lipid phosphatases PTEN or INPP4B16,17 or activating mutations in PIK3CA18, along with other genes in the PI3K/TOR signaling network4,18. In another study, Aparicio and colleagues sequenced and analyzed over 100 TNBC tumors at the time of diagnosis and confirmed the high rate of TP53 mutations; however, they showed that 12% of cases did not have somatic mutations in any established “driver” genes, suggesting that primary TNBCs are mutationally heterogeneous from the outset19.
Tumors arising in BRCA1 carriers have many similarities to basal-like sporadic breast tumors, including greater likelihood of being high-grade, ER/PR-negative, HER2-negative, and of having a high frequency of TP53 mutations. Basal keratins are expressed by both sporadic basal-like tumors and tumors with BRCA1 mutations, and both groups cluster together by gene expression profiling20. Other studies support these data, in which familial-BRCA1 breast cancers have shared features with a subset of sporadic tumors, indicating a similar etiology. Hallmarks of this “BRCAness” include basal-like phenotype (associated with the BRCA1 phenotype but not with the BRCA2 phenotype), ER-negativity, EGFR expression, c-MYC amplification, TP53 mutations, loss of RAD51-focus formation, extreme genomic instability and sensitivity to DNA-crosslinking agents21. This intrinsic genomic instability in TNBCs and BRCA associated breast cancers is likely a result of deficient DNA repair and may lead to the success of some chemotherapy regimens22. The translational strategy for this group of tumors with “BRCAness” is the design of rational clinical trials that investigate the role of chemotherapy and biologic agents targeting DNA repair defects.
Subtyping TNBC
In order to better understand the molecular underpinnings of TNBC, our group compiled an extensive number of TNBC gene expression (GE) profiles and initiated molecular subtyping of the disease23. We reported that TNBC is a heterogeneous disease composed of distinct molecular subtypes, each with a unique biology that responds differentially to current therapies. We identified two basal-like TNBC subtypes, one with cell cycle and DNA damage response GE signatures (BL1) and the other enriched in growth factor signaling and myoepithelial markers (BL2), two mesenchymal subtypes with high expression of genes involved in differentiation and growth factor pathways (M and MSL), an immunomodulatory (IM) type, and a luminal subtype driven by androgen signaling (luminal androgen receptor, LAR) (Figure 1). Differential GE was used to designate TNBC cell line models representative of these subtypes and predicted ‘driver’ signaling pathways were pharmacologically targeted in these preclinical models as proof of concept that analysis of distinct GE signatures can inform therapy selection. Cell line models representing each of the TNBC subtypes also displayed different sensitivities to targeted therapeutic agents. We found that representative BL1 subtype cell lines preferentially respond to cisplatin. Mesenchymal and luminal subtype lines with aberrations in PI3K signaling have the greatest sensitivity, in general, to PI3K pathway inhibitors. The mesenchymal subtypes preferentially respond to the nonspecific SRC inhibitor dasatinib. The LAR subtype cell lines express androgen receptor (AR) and are sensitive to the AR antagonist bicalutamide and PI3K inhibitors. Sensitivity to PI3K inhibitors is due to the high frequency of mutations in PIK3CA, the gene encoding the p110α catalytic subunit of PI3K, in LAR cell lines (~40%)23.
Figure 1. Distribution of TNBC subtypes from TCGA with enriched gene ontology and potential therapeutic targets.
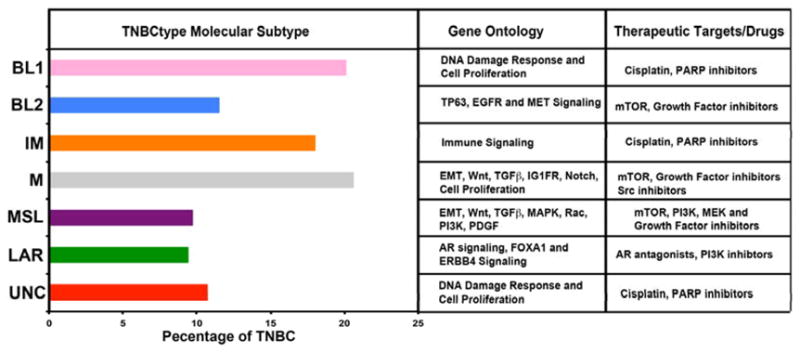
Bargraphs display the subtype percentage relative to TNBC.
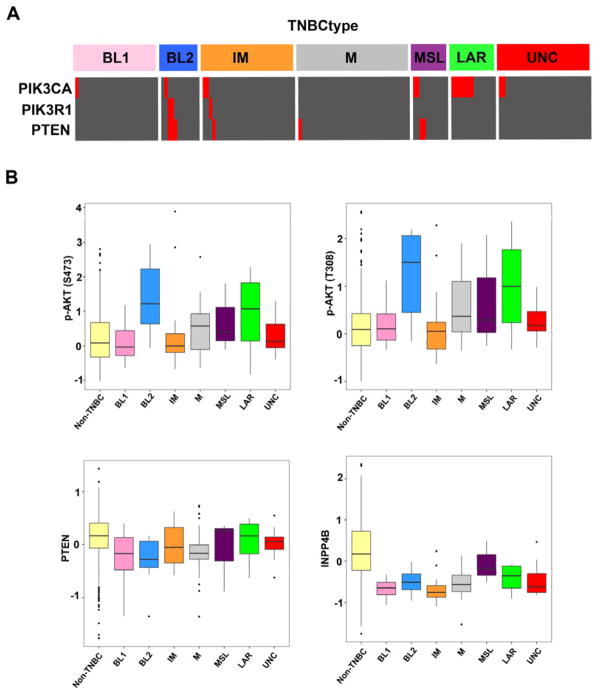
We validated our subtyping using TNBC RNA-sequencing data from TCGA. We determined a statistically similar distribution of subtypes across 163 TNBC cases from TCGA (Figure 2A). When we analyzed DNA-sequencing data for these TCGA cases, we found that 50% of the AR+ tumors had PIK3CA mutations. Also of note were the enrichment of PIK3CA, PIK3R1 and PTEN mutations in the BL2 subtype. Further analysis suggests increased PI3K pathway activity, as reverse-phase protein arrays demonstrate that both the BL2 and LAR subtypes have increased levels of phoshoryalated AKT (S473 and T308). PTEN protein levels are slightly lower in TNBC, however INPP4B are substantially decreased across all TNBC subtypes (Figure 2B). These data demonstrate that despite having a lower PIK3CA mutation rate, that TNBC and especially specific subtypes display active PI3K pathway signaling.
Figure 2. PI3K pathway is mutated and active in distinct TNBC subtypes.
(A) Heatmap displays the PI3K pathway mutations (red) across TNBC molecular subtypes extracted from the TCGA. (B) Boxplots show protein expression levels for phosphorylated AKT (top) and negative regulators PTEN and INPP4B in non-TNBC and TNBC subtypes. Mutation and RPPA data obtained from the cBioPortal (www.cbioportal.org) on 1–12–14.
We also performed a direct comparison of 374 TNBC samples to determine the relationship between the PAM50 intrinsic and TNBC molecular subtypes24. As anticipated, the majority of the TNBC samples are indeed classified as basal-like by PAM50 (80.6%). However, a significant number classified in other categories: normal-like (14.6%), luminal B (3.5%), luminal A (1.1%), and HER2 (0.2%). With exception to MSL and LAR, all other TNBC subtypes are primarily composed of the basal-like intrinsic subtype. Of MSL TNBCs, approximately 50% are basal-like, 27.8% are normal-like, 13.9% are luminal B, and the remaining approximately 8% are HER2 and luminal A by intrinsic subtyping. Unlike other subtypes, the LAR subtype is primarily classified as HER2 (74.3%) and luminal B (14.3%) by PAM50 intrinsic subtyping. Therefore, PAM50 intrinsic subtyping alone has the potential to classify ~75% of TNBCs that are AR+ as HER2+24.
TREATMENT IMPLICATIONS
The heterogeneity of TNBCs has made finding actionable targets and the development of targeted therapies particularly difficult. Clinical trials that include all TNBCs based on immunohistochemical staining to target a particular receptor or pathway have largely shown only limited benefit25,26. One of the first of these studies targeted EGFR, which was shown to be upregulated in approximately 60% of basal-like TNBCs4. A clinical trial in which chemotherapy was given with or without the EGFR inhibitor cetuximab showed only a modest improvement in response rate (RR) with the addition of cetuximab (28% versus 33%)27. More recently, a study of cisplatin with or without cetuximab in patients with metastatic TNBC showed that the addition of cetuximab doubled the RR from 10% to 20%, and increased the progression free survival (PFS) from 1.5 to 3.7 months, but neither endpoint reached statistical significance28. Studies that have evaluated cetuximab as a single agent have been disappointing as well25. Although there are many reasons why such studies failed to show a significant benefit, there is a possibility that the heterogeneity of TNBCs diluted the effect of a treatment that would have otherwise been effective in a smaller, selected group of TNBCs. Currently, a number of promising new targets are being explored, as discussed below.
Targeting BRCA and “BRCA-ness”
The idea that TNBCs have a “BRCA-ness” has led to clinical trials focusing on platinum salts, including carboplatin and cisplatin, which lead to DNA cross-link strand breaks. BRCA1 plays a key role in DNA double-strand break repair by mediating homologous recombination (HR) and thereby maintaining DNA stability29. Thus, the platinum agents may be especially important in cells that are deficient in homologous recombination repair mechanisms such as BRCA mutated cells and TNBCs. Silver et al. conducted a phase II study of 29 patients that highlighted the activity of single agent neoadjuvant cisplatin in the treatment of patients with locally advanced triple-negative breast cancer. The pathologic complete response (pCR) rate was 22% with cisplatin alone30. In another small study, 9 of 10 patients with stage I-III breast cancer harboring BRCA1 mutations achieved a pathological complete remission after neoadjuvant therapy with cisplatin31.
More recently, the addition of carboplatin to neoadjuvant paclitaxel, anthracycline, and bevacizumab in the GerparSixto trial improved pCR rates from 37.9% to 58.7% with the addition of carboplatin in a subset of TNBC patients32. Biomarker studies in this trial are underway to determine whether certain subsets of TNBC benefit more from the addition of carboplatin and should provide insights to markers of response. CALGB40603 study (NCT00861705) is a randomized phase II study of 454 patients with stage II/III TNBC which examined the addition of carboplatin and/or bevacizumab to neoadjuvant weekly paclitaxel followed by dose-dense adriamcyin and cyclophosphamide33. The primary endpoint was pCR in the breast and a secondary endpoint was pCR in the breast and axillae. The results, presented at the 2013 San Antonio Breast Cancer Symposium (SABC), showed that the addition of carboplatin to standard chemotherapy resulted in a significant improvement in pCR rates for both the breast alone (46% to 60% with carboplatin) and the breast and axillae (41% to 54%, p=.0029). The benefit for carboplatin was independent of bevacizuamb. Bevacizuamb also resulted in a benefit in pCR, but this benefit was significant only in the breast and at the cost of a significant increase in toxicities. A phase II study of neoadjuvant cisplatin and paclitaxel with or without everolimus in patients with early stage TNBC did not show any apparent clinical benefit for the addition of everolimus; however, an overall pCR rate of 38% was noted, again highlighting the effectiveness of the platinum agents in this population34.
In general, patients with TNBC who do not achieve a pCR after neoadjuvant therapy have a significantly worse prognosis, which lends even greater importance to understanding which tumors will have a suboptimal response to platinum agents1. A study which has recently initiated accrual is evaluating how well TNBCs that do not carry a germline BRCA mutation respond to neoadjuvant cisplatin or paclitaxel chemotherapy and whether the use of a Homologous Recombination Deficiency (HRD) assay can predict response to the two chemotherapies (NCT01982448). The HRD assay is a tumor DNA-based assay that can identify underlying defects in the homologous recombination pathway, regardless of etiology, with high sensitivity. The assay evaluates loss of heterozygosity; a higher loss of heterozygosity count reflects more areas of genomic loss in the tumor and more genomic instability due to underlying DNA defects35. The primary objective of the study is to evaluate the correlation between the HRD biomarker and pathologic response to cisplatin or paclitaxel. The HRD assay should, in effect, predict “BRCA-ness” and potentially provide an important test to help predict which tumors are likely to respond to platinum compounds.
BRCA-deficient tumors rely more heavily on poly-AD-ribose polymerase (PARP) to mediate DNA repair because homologous recombination mechanisms of repair are impaired in this group of patients. PARP enzymes are critical for base excision repair of single strand DNA breaks, and since these mechanisms of DNA repair are more important in BRCA-deficient tumors, PARP inhibition results in high antitumor activity36–41. Inhibition of this enzyme by RNA interference or with chemical inhibitors leads to severe, highly selective toxicity in BRCA1 and BRCA2-defective cells, with selectivity being several-fold higher than for conventional chemotherapy drugs42. Reduction in PARP activity leads to chromosomal instability, cell cycle arrest and subsequent apoptosis, most likely due to persistence of DNA lesions normally repaired by homologous recombination. Sensitivity to PARP inhibition depends on homologous recombination deficiency and not necessarily on inherited BRCA1 or BRCA2 deficiency39. Therefore, use of PARP1 inhibitors as a therapeutic strategy in the treatment of sporadic breast cancers with “BRCAness,” including basal-like breast cancers, may be a potential approach.
Investigational use of PARP inhibitors has shown an impressive clinical response in patients with BRCA1-mutations; however, other TNBC patients that appeared to have a ‘BRCA-deficient phenotype’ have not responded as expected. Significant single agent activity was reported with the PARP inhibitor olaparib in patients with BRCA-deficient metastatic breast cancer. Overall responses ranged from 22% at doses of 100 mg BID, to 41% at doses of 400 mg BID, with minimal toxicity37. Olaparib as a single agent was also evaluated in a phase II study of patients with recurrent, high-grade serous or poorly differentiated ovarian carcinoma or TNBC43. In this latter trial, however, no confirmed objective responses were seen in the 26 patients with TNBC.
A phase II, open-label, two-arm randomized, safety and efficacy trial investigated gemcitabine/carboplatin with or without iniparib in patients with metastatic TNBC44. The final analysis of 123 randomized patients showed that addition of iniparib to gemcitabine/carboplatin improved the clinical benefit rate from 33.9% to 55.7% (p=0.015) and ORR from 32.3% to 52.5% (p=0.023). The addition of iniparib prolonged the median PFS from 3.6 to 5.9 months [hazard ratio (HR), 0.59; p=0.012] and the median overall survival (OS) from 7.7 to 12.3 months (HR, 0.57; p=0.014)44. A subsequent phase III study evaluating gemcitabine/carboplatin ± iniparib with similar inclusion criteria as the phase II study did not meet the pre-specified criteria for significance for the co-primary endpoints of OS and PFS45. Concerns have been raised that the lack of benefit of iniparib in the phase III study are due to the more recent understanding that iniparib is not a PARP inhibitor. Thus the small clinical benefit with iniparib seen in the phase II study, which did not reach statistical significance in the phase III study, may be due to another mechanism of action which does not affect the TNBC group as a whole. It is important to highlight the impressive response rate for a relatively heavily pre-treated population in the control group. Both the phase II and III studies showed a response rate of approximately 30% with carboplatin/gemcitabine alone, again underscoring the efficacy of platinum agents in TNBC. More recently, a single arm phase II study of neoadjuvant gemcitabine, carboplatin, and iniparib in patients with TNBC or BRCA 1/2 mutation associated breast cancer showed impressive responses, especially in BRCA 1/2 carriers46. It is possible that beyond BRCA mutated cancers, the benefit of PARP inhibitors will be limited to a sub-group of TNBCs. Clinical trials and biomarker studies are needed to explore which TNBCs respond to this group of drugs.
Results of the first drug to complete testing in the adaptive design neoadjuvant breast cancer I-SPY 2 study, a PARP inhibitor in combination with carboplatin, were also presented at SABC 201347. This was a study of standard chemotherapy including weekly paclitaxel for 12 weeks followed by an anthracycline-based regimen for four cycles compared to the same regimen with the addition of the carboplatin and the PARP inhibitor veliparib. All patients had a breast tumor measuring at least 2.5 cm and had TNBC or were deemed high risk by MammaPrint results. The pCR for patients with TNBC was 26% with standard chemotherapy, compared to 52% for the standard regimen with the addition of carboplatin and veliparib. The benefit for carboplatin versus veliparib could not be extracted as all patients received either just the standard regimen or both of these drugs. The results correspond to a 99% probability that the addition of carboplatin/veliparib is superior to the control regimen alone and based on these results, a 300 patient phase III neoadjuvant study is planned to initiate in 2014.
Targeting Subtypes of TNBC
Masuda et al. recently performed a retrospective analysis of response rates by TNBC subtypes in 130 TNBC cases treated with neoadjuvant adriamycin/cytoxan/taxol containing chemotherapy48. The overall pCR response was 28%, and interestingly, subtype specific responses differed substantially. The BL1 subtype achieved the highest pCR rate (52%) and the BL2, LAR and MSL subtypes were found to have the lowest response rates (0%, 10%, and 23%, respectively). TNBC subtype was also shown to be an independent predictor of pCR status (p = 0.022) by a likelihood ratio test48. These results speak not only to the heterogeneity of TNBCs, but also for the need to align patients to different therapies based on the subtype of their disease.
As previously discussed, we found that approximately 10% of TNBCs classify to the LAR subtype. This subtype, which is driven by androgen receptor signaling, may be uniquely sensitive to androgen blockers. By immunohistochemistry, LAR subtype cell lines have a significantly higher percentage of tumor cells with nuclear androgen receptor staining and higher intensity of staining compared with other all other TNBC subtypes (>10 fold; p<.004). A study evaluating the anti-androgen bicalutamide in patients with metastatic TNBC which express the androgen receptor by immunohistochemistry showed a six month clinical benefit rate of 19% (95% confidence interval (CI), 7%–39%) for bicalutamide and a median progression free survival of 12 weeks (95% CI, 11–22 weeks)49 An androgen receptor inhibitor, enzalutamide, is also currently being explored in patients with TNBC who express the androgen receptor (NCT01889238). In addition to expression of the androgen receptor, the LAR subtype cell lines have a high rate of PIK3CA activating mutations and exhibit strong sensitivity to PI3K inhibitors23. The co-evolution of PIK3CA mutations with AR-dependency is similar to ER-positive breast cancers, which have a high frequency of PIK3CA mutations50,51. Preclinical data show that the combination of bicalutamide with a PI3K inhibitor produces an additive/synergistic effect, specifically in LAR cell lines.
PI3K inhibition may also be relevant for non-LAR tumors. Studies have provided preclinical rationale for the combined use of a DNA damaging agent with PI3K inhibitors by demonstrating that in addition to regulating cell growth, metabolism, and survival, PI3K also stabilizes double strand breaks by interacting with the homologous recombination complex and, in effect, creates a BRCA deficient state52. PI3K blockade promotes homologous recombination deficiency by down-regulating BRCA1/2 and thus sensitizing BRCA-proficient tumors to PARP inhibition. To capitalize on these findings, a phase I study of the pan-PI3K inhibitor BKM120 (Novartis®) in combination with the PARP inhibitor olaparib in patients with metastatic TNBC is ongoing (NCT01623349). BKM120 would be expected to create a BRCA mutant-like tumor state, thus making it susceptible to PARP inhibition.
Our studies of combinations of PI3K-inhibitors and cisplatin show either additive or synergistic decreases in tumor viability, with significant decreases in pAKT and pS6 levels and a concomitant elevation in cleaved PARP. Altogether, these data suggest that targeting the PI3K pathway could be clinically relevant in TNBC. Our group is now initiating clinical trials in which therapy for patients with metastatic TNBC will be determined by androgen receptor status. In the first study, patients whose tumors express androgen receptor by immunohistochemistry will receive an anti-androgen with a PI3K inhibitor and be spared the toxicity of a chemotherapy regimen with expected limited benefit. In the second study, patients with tumors that do not express the androgen receptor will be randomized to chemotherapy with cisplatin with or without a PI3K inhibitor. The androgen receptor negative study is now active (NCT01918306).
CONCLUSIONS
Despite the promise of targeted therapies, cytotoxic chemotherapy remains the mainstay of treatment for TNBC patients. This is problematic not only due to the numerous toxicities of cytotoxic chemotherapy, but because recurrence rates after early stage disease remain high and survival for metastatic disease remains dismal. Identification of molecular subtypes is essential for understanding the biological characteristics and clinical behaviors of TNBC, as well as for developing personalized treatments.
We recently identified TNBC subtypes with corresponding molecular drivers and preclinical models to develop effective therapeutic approaches. We are translating our preclinical findings to targeted, subtype-specific clinical trials for TNBC patients based on our understanding of the biological drivers of the different TNBC subtypes. In particular, the LAR subtype is predicted to have the least benefit from traditional cytotoxic chemotherapy and based on our preclinical work, will derive great benefit from targeting both the androgen receptor and PI3K, as mutations in PIK3CA may be a major driver for this subtype. It is possible that the mediocre efficacy of drugs targeting TNBCs seen in many previous studies may be due to the nonspecific inclusion of all TNBCs, including the LAR and mesenchymal subtypes. Drugs that would result in stellar responses if only the BL-1 subtype was included in a particular clinical trial might show an overall less robust response due to the inclusion of other subtypes that do not respond to the same drugs. Thus, by including all subtypes of TNBC in clinical trials, we may potentially be eliminating effective therapies for certain subtypes of TNBC by diluting the observed response rates, clinical benefit rates, and ultimately, overall survival.
TNBC is clearly a complex disease and its impressive heterogeneity adds to the challenge of finding targets and treatments. To effectively tackle this disease, the focus will need to be clinical trials of increasingly smaller subsets of TNBC patients. The subsets will be defined by molecular and genetic characteristics that classify a patient’s tumor into a subtype (i.e., receptor expression or mutation). Due to cross-talk between pathways, which may be especially relevant in TNBC, combinations of targeted agents will likely ultimately be required to optimally treat the disease. Ongoing and planned studies of various pathway inhibitors in addition to those reviewed above, including c-met inhibitors, PD-1 inhibitors, aurora kinase inhibitors, and others will help determine clinically relevant targets. Clinical trials will need to collect tissue before and after disease progression in order to understand mechanisms of resistance so that we can develop treatment combinations to target all relevant pathways from the onset. Our comprehension of TNBC has grown in the past five years. The goal in the coming years will be to expand this knowledge and to initiate and encourage participation in more clinical trials that align patients to appropriate treatments and capitalize on our ever-increasing understanding of TNBC.
Summary statement.
Triple-negative breast cancer (TNBC) is an aggressive disease with limited treatment options and no approved targeted therapies. This review outlines the current knowledge about TNBC and strategies being explored to specifically target this disease.
Acknowledgments
This research was supported by National Institutes of Health Grant: CA95131 (Specialized Program of Research Excellence in Breast Cancer); Komen for the Cure Foundation Grants SAC110030 (to J.A. Pietenpol) and CCR13262005 (to B.D. Lehmann).
Footnotes
No financial disclosures relevant to this paper from any of the authors.
References
- 1.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Makretsov NA, Huntsman DG, Nielsen TO, et al. Hierarchical clustering analysis of tissue microarray immunostaining data identifies prognostically significant groups of breast carcinoma. Clin Cancer Res. 2004;10:6143–51. doi: 10.1158/1078-0432.CCR-04-0429. [DOI] [PubMed] [Google Scholar]
- 5.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 6.Fulford LG, Easton DF, Reis-Filho JS, et al. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathology. 2006;49:22–34. doi: 10.1111/j.1365-2559.2006.02453.x. [DOI] [PubMed] [Google Scholar]
- 7.Fulford LG, Reis-Filho JS, Ryder K, et al. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007;9:R4. doi: 10.1186/bcr1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnes JB, Brunet JS, Stefansson I, et al. Placental cadherin and the basal epithelial phenotype of BRCA1-related breast cancer. Clin Cancer Res. 2005;11:4003–11. doi: 10.1158/1078-0432.CCR-04-2064. [DOI] [PubMed] [Google Scholar]
- 9.Subhawong AP, Subhawong T, Nassar H, et al. Most basal-like breast carcinomas demonstrate the same Rb-/p16+ immunophenotype as the HPV-related poorly differentiated squamous cell carcinomas which they resemble morphologically. Am J Surg Pathol. 2009;33:163–75. doi: 10.1097/PAS.0b013e31817f9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis-Filho JS, Savage K, Lambros MB, et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol. 2006;19:999–1009. doi: 10.1038/modpathol.3800621. [DOI] [PubMed] [Google Scholar]
- 11.Rakha EA, Ellis IO, Reis-Filho JS. Immunohistochemical heterogeneity of breast carcinomas negative for estrogen receptors, progesterone receptors and Her2/neu (basal-like breast carcinomas) Mod Pathol. 2008;21:1060–1. doi: 10.1038/modpathol.2008.67. author reply 1061–2. [DOI] [PubMed] [Google Scholar]
- 12.Bertucci F, Finetti P, Cervera N, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–40. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 13.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Ronde JJ, Hannemann J, Halfwerk H, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat. 2010;119:119–26. doi: 10.1007/s10549-009-0499-6. [DOI] [PubMed] [Google Scholar]
- 15.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andre F, Job B, Dessen P, et al. Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clin Cancer Res. 2009;15:441–51. doi: 10.1158/1078-0432.CCR-08-1791. [DOI] [PubMed] [Google Scholar]
- 17.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–25. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 19.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matros E, Wang ZC, Lodeiro G, et al. BRCA1 promoter methylation in sporadic breast tumors: relationship to gene expression profiles. Breast Cancer Res Treat. 2005;91:179–86. doi: 10.1007/s10549-004-7603-8. [DOI] [PubMed] [Google Scholar]
- 21.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 22.Graeser M, McCarthy A, Lord CJ, et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16:6159–68. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann B, Pietenpol J. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232:142–50. doi: 10.1002/path.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–23. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaklavas C, Abramson VG, Lin NU, et al. TBCRC 019: An open label, randomized, phase II trial of nanoparticle albumin-bound paclitaxel (nab-PAC) with or without the anti-death receptor 5 (DR5) monoclonal antibody tigatuzumab (TIG) in patients with metastatic triple negative breast cancer (TNBC). Poster session presented at - 2013 ASCO Annual Meeting; June 1, 2013. [Google Scholar]
- 27.O’Shaughnessy JWD, Vukelja SJ, et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. Breast Cancer Res Treat; presented data— 2007 San Antonio Breast Cancer Symposium; 2007.p. Abstr 308. [Google Scholar]
- 28.Baselga J, Gómez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–92. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–15. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 30.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrski T, Gronwald J, Huzarski T, et al. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat. 2008;108:289–96. doi: 10.1007/s10549-007-9600-1. [DOI] [PubMed] [Google Scholar]
- 32.Von Minckwitz GSA, Salat C, et al. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). Presented at - 2013 ASCO Annual Meeting Abstract 1004; 2013. [Google Scholar]
- 33.Sikov WMBD, Perou CM, et al. Impact of the addition of carboplatin (Cb) and/or bevacizumab (B) to neoadjuvant weekly paclitaxel (P) followed by dose-dense AC on pathologic complete response (pCR) rates in triple-negative breast cancer (TNBC): CALGB 40603 (Alliance). The 2013 San Antonio Breast Cancer Symposium. Abstract S5-01; December 10–14, 2013. [Google Scholar]
- 34.IAMBJVGA, et al. A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus (an mTOR inhibitor) in patients with stage II/III triple-negative breast cancer (TNBC). Poster presented at - 2013 San Antonio Breast Cancer Symposium; 2013. [Google Scholar]
- 35.Telli M, Jensen K, Abkevich V, et al. Homologous Recombination Deficiency (HRD) score predicts pathologic response following neoadjuvant platinum-based therapy in triple-negative and BRCA1/2 mutation-associated breast cancer (BC). Cancer Res; Presented at - 2012 San Antonio Breast Cancer Symposium; 2012. [Google Scholar]
- 36.Johnson N, Li YC, Walton ZE, et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2011;17:875–82. doi: 10.1038/nm.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 38.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 39.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 40.Tutt A, Ashworth A. Can genetic testing guide treatment in breast cancer? Eur J Cancer. 2008;44:2774–80. doi: 10.1016/j.ejca.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Lord CJ, Garrett MD, Ashworth A. Targeting the double-strand DNA break repair pathway as a therapeutic strategy. Clin Cancer Res. 2006;12:4463–8. doi: 10.1158/1078-0432.CCR-06-1269. [DOI] [PubMed] [Google Scholar]
- 42.Turner N, Tutt A, Ashworth A. Targeting the DNA repair defect of BRCA tumours. Curr Opin Pharmacol. 2005;5:388–93. doi: 10.1016/j.coph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 44.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 45.JOS, LSS, MAD, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol; Presented at - 2011 ASCO Annual meeting; 2011. [Google Scholar]
- 46.MLT, KCJ, AWK, et al. PrECOG 0105: Final efficacy results from a phase II study of gemcitabine and carboplatin plus iniparib (BSI201) as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer. J Clin Oncol; Presented at - 2013 ASCO Annual Meeting; 2013. [Google Scholar]
- 47.Rugo HSOO, DeMichele A, et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the I-SPY 2 TRIAL. The 2013 San Antonio Breast Cancer Symposium. Abstract S5 - 02; December 10 - 14, 2013. [Google Scholar]
- 48.Masuda H, Baggerl KA, Wang Y, et al. Differential pathologic complete response rates after neoadjuvant chemotherapy among molecular subtypes of triple-negative breast cancer. J Clin Oncol; Presented at - 2013 ASCO Annual Meeting; 2013.p. Abstract 1005. [Google Scholar]
- 49.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19:5505–12. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–8. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 51.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibrahim YH, Garcia-Garcia C, Serra V, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–47. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]