Abstract
Studies of neurologic disease induced by simian immunodeficiency virus (SIV) in Asian macaques have contributed greatly to the current understanding of human immunodeficiency virus (HIV) pathogenesis in the brain and the peripheral nervous system (PNS). Detailed investigations into SIV-induced alterations in the spinal cord, a critical sensorimotor relay point between the brain and the PNS, have yet to be reported. In this study, lumbar spinal cords from SIV-infected pigtailed macaques were examined to quantify SIV replication and associated neuroinflammation. In untreated SIV-infected animals there was a strong correlation between amount of SIV RNA in the spinal cord and expression of the macrophage marker CD68, as well as key pro-inflammatory mediators tumor necrosis factor and CCL2. We also found a significant correlation between SIV-induced alterations in the spinal cord and the degree of distal epidermal nerve fiber loss among untreated animals. Spinal cord changes also were present in SIV-infected antiretroviral-treated animals, including elevated glial fibrillary acidic protein immunostaining and enhanced CCL2 expression despite SIV suppression. A fuller understanding of the complex virus and host factor dynamics in the spinal cord during HIV infection will be critical in the development of new treatments for HIV-associated sensory neuropathies and studies aimed at virus eradication from the central nervous system.
Keywords: Glial activation, HIV-AIDS, Macaque, Neuroinflammation, Neuropathy, Simian immunodeficiency virus (SIV), Spinal cord
INTRODUCTION
Although a multitude of studies thoroughly describe the morphologic and molecular alterations that develop in the brain and peripheral nerves of human immunodeficiency virus (HIV)-infected patients and simian immunodeficiency virus (SIV)-infected macaques (1–8), there have been few detailed analyses of the effects of HIV/SIV infection on the spinal cord. The majority of studies that address HIV-associated spinal cord disease were published during the pre-combination antiretroviral therapy (cART) era, when opportunistic central nervous system (CNS) infections, fulminant HIV encephalomyelitis, and an enigmatic degenerative condition known as vacuolar myelopathy were common findings at autopsy (9–14). While the frequency of serious complications directly attributable to spinal cord disease has waned since the deployment of modern cART, investigators have recently begun to consider the role of spinal cord alterations in the pathogenesis of what is currently the most common neurologic complication of HIV infection, HIV-associated sensory neuropathy (HIV-SN). HIV-SN is reported to affect up to 60% of individuals living with HIV infection and causes a range of uncomfortable, difficult to manage symptoms including “painful numbness” or burning sensations, hyperalgesia, and allodynia (15, 16). Although the pathogenesis of this condition is incompletely understood, the development of chronic neuropathic pain likely involves sensitization peripherally at the level of the nerves and ganglia, as well as centrally, within the spinal cord (17). Notably, 3 recent studies by Tang et al demonstrated increased glial activation, enhanced pro-inflammatory signaling, and elevated concentrations of HIV envelope glycoprotein gp120 at autopsy in the spinal cords of patients with histories of HIV-associated pain vs. “pain-negative” HIV patients (18–20).
In-depth analysis of the HIV/SIV infection in the spinal cord is also highly relevant to ongoing investigations of the CNS as a reservoir for residual viral replication and latent, integrated provirus in patients receiving cART. It is widely recognized that macrophages and microglia, (and potentially astrocytes), in the brain serve as reservoirs for viral replication and reactivation, and that neuroimmune activation often persists in the brain despite systemic viral suppression (21–23). There are, however, currently no reports addressing whether a similar situation exists in the spinal cord. With its positioning behind the blood-CNS-barrier and a full complement of potentially infectable and immunoactive glial cells, residual HIV infection of the spinal cord could significantly contribute to viral persistence in the CNS and impact therapeutic strategies aimed at viral eradication.
In the present study, we performed morphologic and molecular analyses of the spinal cord in SIV-infected pigtailed macaques (Macaca nemestrina). This well-established animal model has been shown to recapitulate HIV-induced lesions in the brain and PNS within a consistent time course of 12 weeks (24, 25). In contrast to human studies, use of macaques allows for comprehensive sampling of key CNS and PNS components at progressive time points throughout the course of infection, with and without concurrent cART, and in the absence of potentially confounding comorbid conditions. Because symptomatic HIV-SN occurs predominantly in patients with advanced disease, as well as in patients on cART (26, 27), we focused on groups of animals killed during the terminal stage of SIV infection (84 days post-infection [p.i.]), and a group of infected animals that received virally suppressive cART for several months. Individual animal’s spinal cord alterations were then compared to PNS changes including loss of epidermal nerve fiber (ENF) density, a commonly used measure of peripheral nerve injury. We hypothesized that spinal cords of infected animals would show evidence of neuroinflammation such as glial cell activation (i.e. of microglia and astrocytes), as well as induction of soluble pro-inflammatory mediators that have been associated with pain-facilitating signaling pathways, specifically tumor necrosis factor (TNF) and the chemokine CCL2. Furthermore, we postulated that certain neuroinflammatory parameters would remain elevated in the spinal cords of macaques receiving long-term cART as compared to uninfected control animals, similar to persistent neuroinflammation found in the brains of cART-treated, SIV-infected macaques.
MATERIALS AND METHODS
Animal Studies
Twenty male pigtailed macaques were intravenously inoculated with both the neurovirulent clone SIV/17E-Fr and the immunosuppressive swarm SIV/DeltaB670, as previously described (28). Fifteen animals did not receive antiretroviral treatment (untreated, SIV-infected group) and were killed at 12 weeks p.i. Five animals were treated with antiretroviral medications beginning at day 12 p.i. and were killed at approximately 170 days p.i. (long-term cART group). The 4-drug combination therapy consisted of the nucleotide reverse transcriptase inhibitor tenofivir (Gilead, Foster City, CA), 2 protease inhibitors, saquinavir (Roche, Basel, Switzerland) and atazanivir (Bristol-Myers Squibb, NY, NY), and the integrase inhibitor L-870812 (Merck, White House Station, NJ [29]). Doses and routes of administration were previously detailed (23). Five additional uninfected pigtailed macaques served as untreated, virus-negative controls.
At necropsy, the animals were saline-perfused and collected tissues were either immersion fixed in Streck tissue fixative (Streck Laboratories, Omaha, NE) followed by paraffin embedding or snap frozen in liquid N2 followed by storage at −80°C. The animal procedures in this study were in accordance with the principles set forth by the Institutional Animal Care and Use Committee at Johns Hopkins University and the National Research Council’s Guide for the Care and Use of Laboratory Animals (8th edition).
Immunohistochemistry
Immunohistochemistry was performed on Streck-fixed paraffin-embedded sections of lumbar spinal cord. Tissues were deparaffnized in Histo-Clear (National Diagnostics, Atlanta, GA) followed by rehydration in a gradient series of alcohols. After antigen retrieval in sodium citrate buffer with 8-minute microwave treatment, sections were washed and then blocked prior to incubation in the appropriate primary antibody dilution (anti-CD68, 1:2000; clone KP1 and anti-glial fibrillary acidic protein [GFAP], 1:4,000, from DAKO, Carpinteria, CA, and anti-SIVmac251 transmembrane glycoprotein 41 [gp41], KK41,1:4000, NIH AIDS Research and Reagent Reference Program, Bethesda, MD), for 1 hour at room temperature. Sections were then incubated sequentially in biotinylated secondary multilink antibody and horseradish peroxidase-labeled streptavidin (Biogenex, San Ramon, CA). Chromogen detection was performed by incubating the sections in the substrate 3,3’-diaminobenzidine. The sections were then washed, cleared, and coverslipped with Permount mounting medium (Fisher Scientific, Pittsburgh, PA). Image acquisition and quantification of positive CD68 and GFAP immunostaining were performed using a Nikon DS-Ri1 color camera mounted on a Nikon Eclipse 90i microscope and NIS Elements software (version AR710). A composite image of an entire transverse section of lumbar spinal cord was created by aligning serial images of contiguous 100× fields. Regions of interest were traced manually and the percent of total area occupied by positively stained cells was determined for the entire transverse section of spinal cord, as well as the white and gray matter compartments separately.
Immunofluorescent Staining and Confocal Microscopy
To demonstrate active SIV infection of microglia/macrophages in the lumbar spinal cord, we performed fluorescent double labeling for SIV gp41 and the polyclonal macrophage/microglia marker Iba-1 followed by confocal laser microscopy. The staining protocol was similar to that described for immunohistochemistry except that the primary antibodies and concentrations were KK41 (1:100) and Iba-1 (1:100, WAKO Lab Chemicals, Richmond, VA), and secondary antibodies were fluorescent-tagged AF488 Goat anti-mouse AF546 Goat anti-rabbit (both 1:100, Invitrogen, Carlsbad, CA). Sections were mounted using Prolong Gold Antifade reagent (Invitrogen) and sealed with clear nail polish. Colocalization of the resulting green (KK41) and red (Iba-1) fluorescent labeling was visualized using a Nikon C1 confocal laser microscope system mounted on a Nikon Eclipse TE2000-E microscope.
Quantification of SIV RNA and Cytokine mRNA in Spinal Cord Tissue
Total RNA was isolated from 25 mg of snap frozen tissue from the lumbar spinal cord by first extracting with RNA-Stat 60 (Tel-Test Inc., Friendswood, TX) and chloroform, followed by purification using the MirVana kit (Invitrogen). Genomic DNA was removed from the samples using either RQ1 DNase (Promega, Fitchburg, WI) or Turbo DNAse (Invitrogen) according to the manufacturer’s protocols. All tissue samples for RNA isolation were consistently taken from the dorsal half of the spinal cord (dorsal to the central canal) and included both white and gray matter. Purified RNA was analyzed by real-time PCR using specific primers and probes for SIV gag (28), TNF and CCL2 (23). SIV RNA copy number was determined by comparison to a standard curve. TNF and CCL2 gene expression was determined using the ddCt (cycle threshold) method (30) with normalization of cellular mRNAs to 18s ribosomal RNA levels. Gene expression data are reported as fold change relative to that of control animals.
Measurement of ENF Density
Full-thickness skin samples from the plantar footpad were collected from control and infected animals at necropsy using a 3-mm punch biopsy tool. Sections were obtained from an identical location in all animals. Skin sections were then fixed and cryoprotected, as previously described (5). Cryoprotected samples were sectioned using a freezing-sliding microtome to a thickness of 50 µm and immunostained for the panaxonal marker PGP9.5 (1:2000; Chemicon, Temecula, CA), as previously described (31). ENF densities were measured using a modification of the method described by Kennedy et al (32) and McCarthy et al (31). Briefly, 15 adjacent, non-overlapping collapsed Z-stack images were obtained for each immunostained skin section. Serial Z-stack images for each microscopic field were collected at 0.5-µm intervals using a Zeiss microscope equipped with a z-motor at 400× magnification (Carl Zeiss, Oberkochen, Germany). PGP9.5 immunoreactivity was measured by digital image analysis using iVision software (Version 4.0.14, BioVision Technologies, Exton, PA). To control for variations in thickness among sections, results were normalized to the thickness of each skin sample.
Statistics
All statistical inferences were calculated using nonparametric methods and GraphPad Prism Software (Version 5.0d). Group comparisons were performed using the Mann-Whitney test. Relationships between variables were determined using the Spearman rank correlation. For all analyses, statistical significance was assumed when the p value was less than 0.05.
RESULTS
SIV Infection Induces Morphologic Changes in the Lumbar Spinal Cord
Histopathologic lesions observed in the lumbar spinal cord of untreated, SIV-infected macaques were predominantly mild and consisted of modest perivascular infiltrates of lymphocytes and macrophages, most notable in the gray matter and meninges (mild lesions in 6 of 15 animals [40%]). A subset of animals exhibited more severe myelitis with lesions similar to those seen in SIV encephalitis including glial nodules (n = 4), pronounced perivascular cuffing (n = 4, Fig. 1A), and multinucleated giant cells (n = 3, Fig. 1B). Interestingly, all animals in which giant cells were observed in the lumbar spinal cord also had severe encephalitis. Immunostaining for the macrophage marker CD68 and SIV gp41 confirmed that foci of inflammation included variable numbers of activated microglia/macrophages often harboring SIV (Fig. 1C, D). Confocal laser scanning microscopy of double-stained spinal cord sections showed clear colocalization of the macrophage/microglia marker Iba-1 and SIV gp41, demonstrating active SIV infection of macrophage-lineage cells. Histologic lesions in the lumbar spinal cords of cART-treated animals were limited to minimal lymphohistiocytic infiltrates in the meninges. However, in 1 cART-treated animal there was mild, bilaterally symmetrical vacuolization of the lateral white matter tracts characterized by frequent, dilated myelin sheaths, similar to changes described in mild cases of HIV-associated vacuolar myelopathy (9).
Figure 1.
Morphologic changes in the lumbar spinal cord of untreated simian immunodeficiency virus (SIV)-infected macaques. (A, B) While histologic lesions observed in hematoxylin and eosin-stained sections of lumbar spinal cord were typically mild, a subset of SIV-infected animals showed evidence of more severe myelitis, including pronounced perivascular cuffing (A) and multi-nucleated giant cells (B). (C, D) Immunostaining for the macrophage marker CD68 and SIV gp41 demonstrated that inflammatory foci included numerous activated macrophages/microglia (C) that were often infected by SIV (D). (E–G) Confocal laser scanning microscopy was performed in sections of lumbar spinal cord that were double-stained for the macrophage marker Iba-1 (D, red) and SIV gp41 (E, green). A composite image (G) showed clear colocalization of Iba-1 and SIV gp41, confirming that macrophages in the lumbar spinal cord are target cells for SIV infection. Scale bars: A–D, 100 µm. Original magnification for E–G, 400×.
Viral Load Is Directly Associated with Increasing CD68, but not GFAP Expression in the Lumbar Spinal Cord of Untreated, SIV-infected Macaques
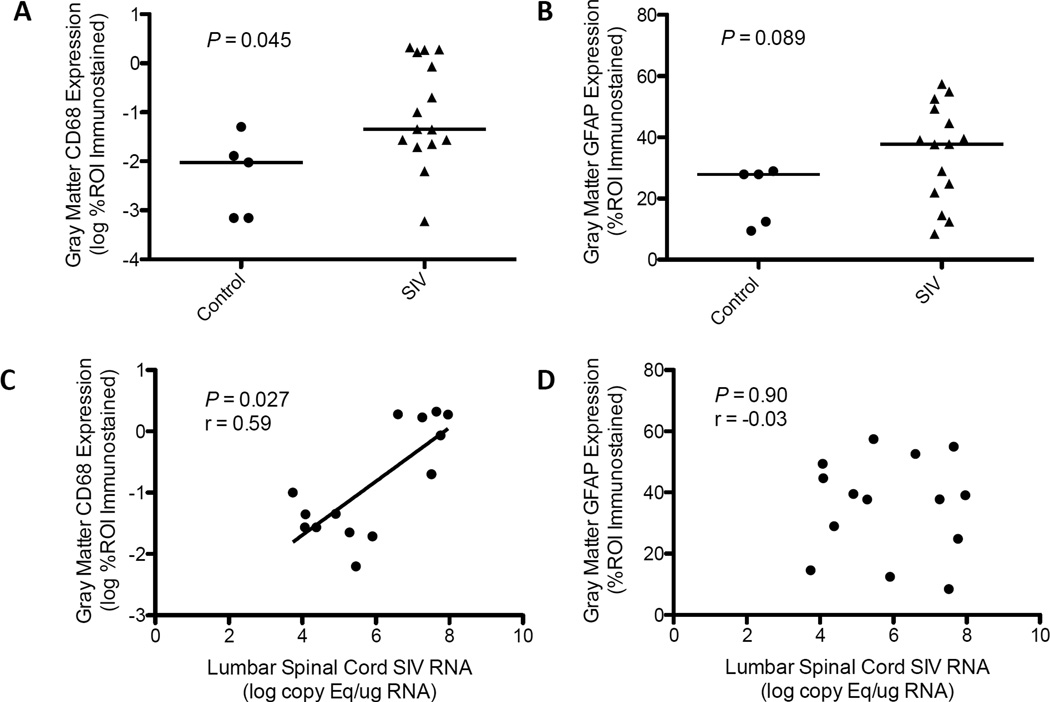
SIV and HIV infection elicit immune activation of resident glial cells in the brain and PNS, as well as recruitment of infiltrating macrophages (13, 25, 26, 33). Because the extent of glial cell activation is often not appreciated on routine histopathologic review, we utilized immunohistochemical staining to assess macrophage/microglial and astrocytic activation in the spinal cord. In untreated, SIV-infected animals there was a significant increase in the amount of CD68 immunostaining, reflecting macrophage/microglial activation, in the gray matter of the lumbar spinal cord compared to control animals (p = 0.045, Fig. 2A) but not white matter (p = 0.29, data not shown). Furthermore, there was a direct correlation between SIV RNA levels and CD68 expression in both the gray matter (p = 0.027, r = 0.59, Fig. 2C) and white matter (p = 0.047, r = 0.54, data not shown). In contrast, there was no significant difference in the level of astrocyte activation, as measured by GFAP expression, between control and untreated, SIV-infected animals (gray matter: p = 0.089, Fig. 2B; white matter: p = 1.0, data not shown), nor was there an apparent relationship between viral load and astrocyte activation (gray matter: p = 0.90, Fig. 2D; white matter: p = 0.20, data not shown).
Figure 2.
Glial activation in the lumbar spinal cord of untreated simian immunodeficiency virus (SIV)-infected macaques. (A) Reflective of enhanced macrophage/microglia activation, untreated SIV-infected macaques had significantly elevated CD68 immunostaining in the gray matter of the lumbar spinal cord vs. control animals (p = 0.045, Mann-Whitney). (B) Glial fibrillary acidic protein (GFAP) immunostaining, an indicator of astrocyte activation, was not significantly different between control and untreated SIV-infected macaques (p = 0.089, Mann-Whitney). (C) Similarly, there was a significant, direct correlation between SIV viral load and CD68 expression in the spinal gray matter of untreated animals (p = 0.027, r = 0.59, Spearman rank correlation), but there was no significant relationship between GFAP expression and viral load (D) (p = 0.90, r = −0.03, Spearman rank correlation). Bars in (A) and (B) represent median values.
SIV Induces Expression Of Soluble Pro-Inflammatory Mediators, TNF and CCL2, in the Lumbar Spinal Cord of Untreated, SIV-Infected Macaques
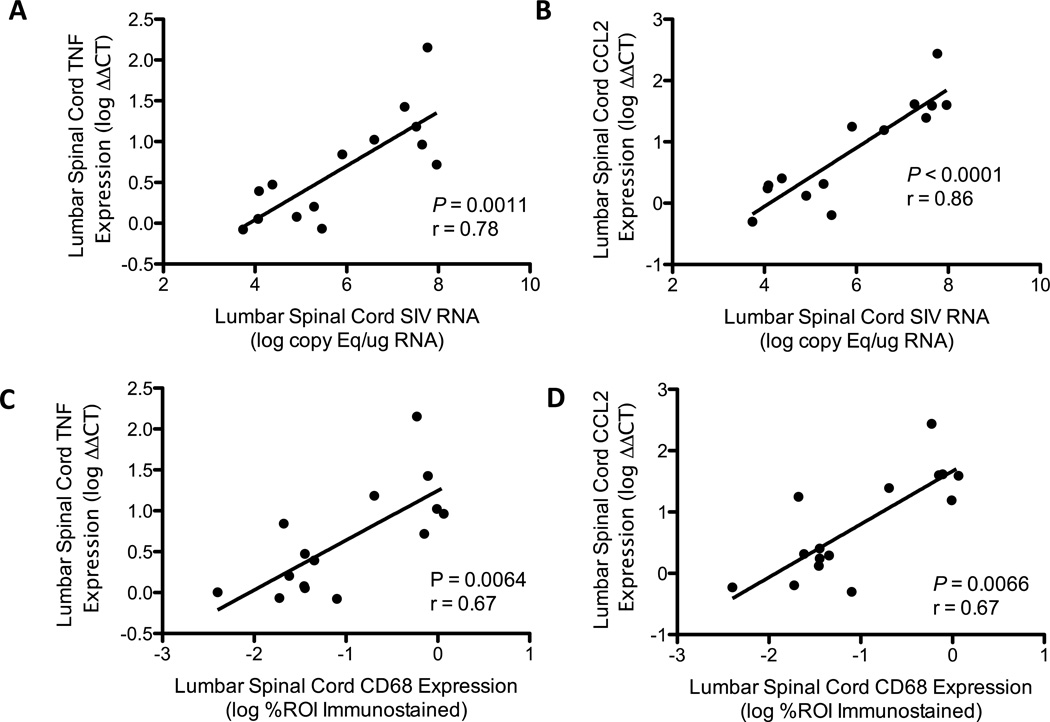
The pro-inflammatory cytokine TNF and the monocyte-attracting chemokine CCL2 (also known as monocyte chemoattractant protein-1, MCP1) have previously been shown to be elevated in the brain tissue of SIV infected macaques (23, 34). Additionally, enhanced expression of these molecules in the spinal cord has been implicated as a key factor in the genesis of neuropathic pain in a number of rodent models (35–37). Gene expression analysis by qRT-PCR revealed marked induction of these soluble mediators in the lumbar spinal cords of untreated SIV-infected macaques. The degrees of both TNF and CCL2 expression showed strong direct correlation with SIV RNA levels (TNF: p = 0.0011, r = 0.78, Fig. 3A; CCL2: p < 0.0001, r = 0.86, Fig. 3B), as well as CD68 expression in the lumbar spinal cord (TNF: p = 0.0064, r = 0.67, Fig. 3C; CCL2: p = 0.0066, r = 0.67, Fig 3D). There was no significant association between TNF or CCL2 expression with GFAP in the cART-naïve animals (p = 0.42 and p = 0.58, respectively, data not shown).
Figure 3.
Simian immunodeficiency virus (SIV) induces expression of tumor necrosis factor (TNF) and CCL2 in the lumbar spinal cord of untreated SIV-infected macaques. (A, B) Quantitative reverse transcription PCR (qRT-PCR) analysis showed strong, direct correlations between SIV viral load and expression of the soluble pro-inflammatory mediators TNF and CCL2 (TNF: p = 0.0011, r = 0.78; CCL2: p < 0.0001, r = 0.86, Spearman rank correlation). (C, D) Expression of TNF and CCL2, as measured by qRT-PCR, also showed a significant, direct correlation with the degree of CD68 expression in the lumbar spinal cord, indicating that expression of these mediators and macrophage/microglia activation are covariant in this group of animals (TNF: p = 0.0064, r = 0.67; CCL2: p = 0.0066, r = 0.67, Spearman rank correlation).
In Untreated, SIV-Infected Macaques, Loss of ENF Is Associated With Increasing Viral Load, CD68, TNF, and CCL2 Expression in the Lumbar Spinal Cord
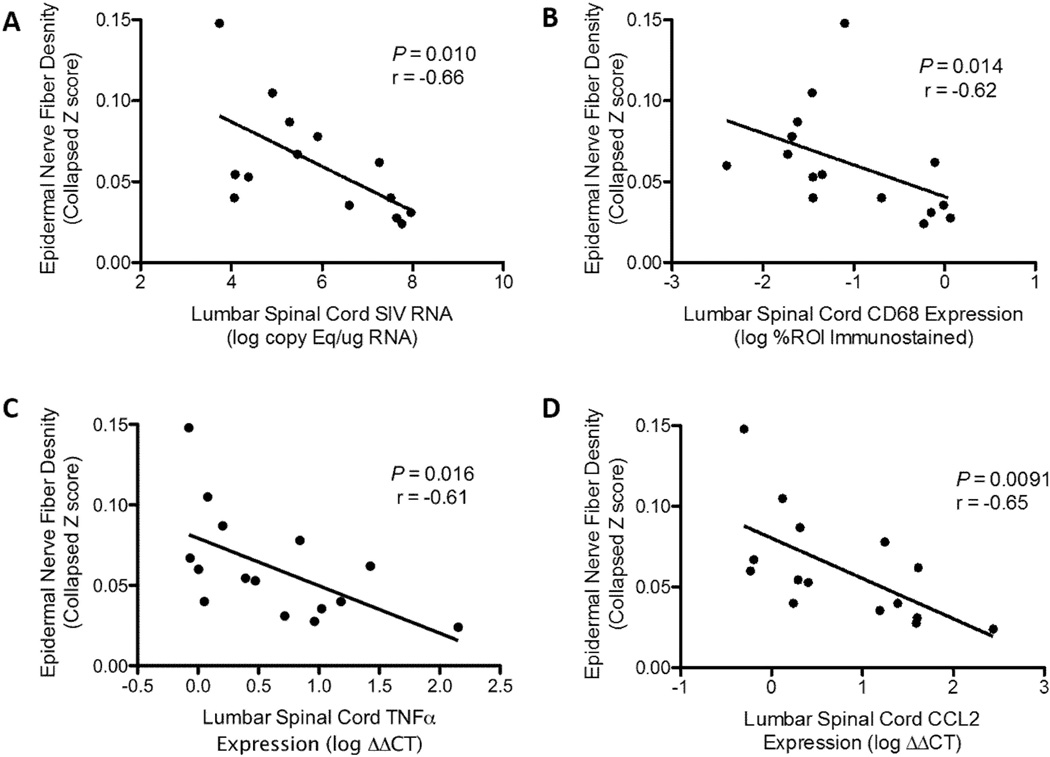
Numerous studies have demonstrated the importance of glial activation and enhanced cytokine and chemokine signaling within the spinal cord during neuropathic pain syndromes (18, 38–40). To investigate the association between pro-inflammatory alterations in the lumbar spinal cord during SIV infection with pathologic changes in the PNS, we compared results of each animal’s spinal cord analyses to its degree of ENF loss, which in HIV patients has been shown to correlate with the development of neuropathic symptoms (41). Striking loss of ENF density has also been previously reported in the accelerated SIV/pigtailed macaque model, with significant decline in epidermal innervation by 8 weeks p.i. (5). In untreated, SIV-infected macaques there was a significant inverse correlation between ENF density and spinal cord viral load (p = 0.010, r = −0.66, Fig. 4A), CD68 immunostaining (p = 0.014, r = −0.62, Fig. 4B), as well as TNF (p = 0.016, r = − 0.61, Fig. 4C) and CCL2 gene expression (p = 0.0091, r = −0.65, Fig. 4D).
Figure 4.
Among untreated simian immunodeficiency virus (SIV)-infected macaques, declining epidermal nerve fiber (ENF) density is associated with increasing viral load and neuroinflammation in the lumbar spinal cord. (A–D) To investigate the relationship between SIV infection and pro-inflammatory alterations in the spinal cord to pathologic changes in the peripheral nerves, we compared results of each animal’s spinal cord analysis to its epidermal nerve fiber density in the footpad. In untreated SIV-infected animals, there were significant inverse correlations between ENF density and (A) SIV viral load (p = 0.010, r = −0.66), (B) CD68 immunostaining (p = 0.014, r = −0.62), (C) Tumor necrosis factor (TNF) expression (p = 0.016, r = −0.61), and (D) CCL2 expression in the lumbar spinal cord (p = 0.0091, r = −0.65). All statistical inferences based on Spearman rank correlation.
Long-term cART-Treated Macaques Show Elevated GFAP and CCL2 Expression in the Spinal Cord Without Detectable Viral Replication
A previous study using the accelerated SIV/pigtailed macaque model (23) demonstrated that long-term cART effectively suppressed viral replication in the brain, along with normalization of some inflammatory parameters measured, including GFAP and major histocompatibility complex class II expression. However, evidence of persistent inflammation was detected in the brains of treated animals in the form of elevated CD68 immunostaining and expression of interleukin-6 (IL-6), TNF, and CCL2, all of which failed to return to normal levels with treatment. Viral DNA was also detected in the brain tissue of these cART-treated animals, suggestive of latent, integrated provirus. Persistent immune activation has also been observed in the brains of virally suppressed HIV patients in the absence of detectable viral replication, and is thought to play a critical role in the progressive neuronal degeneration seen in HIV-associated neurocognitive dysfunction (2, 7, 42, 43). As in the brain, SIV RNA was below the level of detection (<100 copies per µg RNA) in the lumbar spinal cord of cART-treated animals by qRT-PCR, but there was no significant difference in the levels of spinal cord CD68 immunostaining or TNF expression between cART-treated animals and controls (p = 0.11 and p = 0.79, respectively, data not shown). Conversely, there were significantly elevated levels of GFAP immunostaining in both the gray and white matter of cART-treated animals (p = 0.016 and p = 0.021, respectively, Fig. 5A–D), as well as a modest elevation in spinal cord CCL2 expression (p = 0.071, data not shown), indicating that these parameters remain elevated above control values despite long-term suppressive cART.
Figure 5.
Simian immunodeficiency virus (SIV)-infected macaques on long-term combination antiretroviral treatment (cART) showed evidence of significant astrocyte activation in the lumbar spinal cord despite non-detectable SIV viral load. (A, B) Representative images of lumbar spinal cord from a control (A) and SIV-infected, cART-treated (B) macaques immunostained for the astrocyte marker glial fibrillary acidic protein (GFAP; brown staining indicates positive immunoreactivity). (C) cART-treated animals showed markedly increased GFAP expression in the gray matter compared to controls (p = 0.016, Mann-Whitney). (D) GFAP expression was also significantly higher in the white matter of cART-treated animals, although to a lesser degree (p = 0.021, Mann-Whitney). ROI, region of interest.
Similar to the untreated SIV-infected macaques, animals treated with long-term cART had markedly decreased ENF density at the time of necropsy. We did not find a significant correlation between GFAP and ENF loss among cART-treated animals (p = 0.92, r = 0.20, data not shown); however, this may be related to the small number of cART treated animals from which paraffin-embedded lumbar spinal cord was available (n = 4). Additionally, there was no significant association between spinal cord CCL2 expression and ENF density among cART-treated animals (p = 0.083, r = 0.90, data not shown).
DISCUSSION
SIV-infected macaques have served as the preeminent animal model for studying HIV pathogenesis for over 2 decades; however, spinal cord neuropathology has not been reported in detail in the SIV/macaque model. Together, our findings demonstrate that the spinal cord, like the brain, is an important CNS site of SIV viral replication in untreated animals and that a subset of neuroinflammatory changes persist in the spinal cord despite virally-suppressive cART. In untreated animals killed during terminal disease (84 days p.i.), high levels of SIV RNA strongly correlated with increasing expression of the macrophage/microglia activation marker CD68 and robust induction of the innate immune signaling mediators TNF and CCL2. These findings are similar to those in the brain, and we observed significant correlation between the degree of CD68 expression in the lumbar spinal cord and the basal ganglia of these animals (p = 0.014, data not shown). In animals that received long-term cART, levels of CD68 immunostaining and TNF gene expression in the spinal cord were not statistically different from uninfected controls, but astrocyte activation, as measured by GFAP immunostaining, was markedly elevated along with increased CCL2 gene expression. This is in contrast to findings in the brain of cART-treated animals, as previously reported by Zink et al (23), where GFAP expression was not significant different from control values, but CD68 and TNF expression were elevated.
The lesions present in the PNS of HIV patients with sensory neuropathy and SIV-infected pigtailed macaques have previously been described in detail (1, 5, 24). It is well recognized that these patients and macaques show evidence of morphologic and functional abnormalities of the small diameter nerve fibers in the periphery, which detect and transduce noxious stimuli and conduct nociceptive signals to the CNS, as well as inflammatory changes in the dorsal root ganglia, which house the cell bodies of primary afferent sensory neurons. It has also been proposed that the development of chronic neuropathic pain in HIV patients also involves pathologic alterations in the spinal cord (17), where primary afferent neurons synapse with second order afferents and interneurons in the dorsal horns, and nociceptive signals are processed and modulated prior to transmission to the brain (44). Indeed, many studies using rodent models of nociception have clearly established that spinal glial cell activation is a critical and necessary factor in the generation of acute and chronic pain (45–47). Further, soluble inflammatory mediators released by activated glial cells, such as cytokines TNF, IL-1, IL-6, and chemokines CCL2 and CX3CL1 (fractalkine), have been shown to modulate synaptic transmission of sensory signals in the spinal cord (38−40, 48). While the physiologic functions of glial activation and enhanced immune signaling may be evolutionarily protective in the setting of acute pain and important in the clearance of injurious agents and damaged neurons, prolonged immune stimulation and dysregulation also may contribute to pathologic pain states.
HIV infection induces chronic, systemic immune activation that often involves the nervous system and is not fully normalized by the use of virally-suppressive cART. Thus it is not surprising that a large proportion of HIV-infected patients are currently living with symptoms of chronic, pathologic pain (49). To investigate this challenging phenomenon, Tang et al recently published a series of reports outlining changes present in the spinal cord dorsal horns of HIV patients with painful symptoms of sensory neuropathy, but absent in HIV-infected individuals without pain (18–20). Alterations in the glial activation profile that were specific to “pain-positive” HIV patients included elevated GFAP expression, indicative of astrocyte activation, increased production of cytokines TNF and IL-1β, and upregulation of proteins involved in MAPK signaling. In addition, dorsal horns of HIV patients with pain showed evidence of synaptic loss along with increases in markers of neuronal activity and plasticity, consistent with the process of central sensitization. Interestingly, quantification of HIV viral proteins revealed that patients with pain had significantly higher levels of gp120 in the spinal cord dorsal horns compared to “pain-negative” patients, but relatively lower levels of Tat, suggesting differential viral gene expression and the potential importance of gp120 specifically in the development of painful symptoms. Intrathecal injection of gp120 in the spinal canal of mice resulted in molecular alterations that closely mirrored those seen in the HIV patients with pain (20). Mice also developed marked spinal macrophage/microglial activation, which was not evident in the HIV patients, along with mechanical hypersensitivity and progressive decline in ENF density in the hind paws.
The reported findings in the spinal cords of HIV patients with pain reflect a combination of the changes present in the spinal cords of both untreated macaques killed during terminal disease and in cART-treated animals. This is not surprising because the HIV patients in these studies died with clinical AIDS, but also had received various antiretroviral drugs throughout the course of their disease. It is widely recognized that sensory neuropathy can arise due to neurotoxic effects of certain antiretroviral drugs, resulting in a syndrome known as antiretroviral toxic neuropathy, which is clinically indistinguishable and likely often concomitant with HIV-SN (50). The predominance of astrocytic reaction in the spinal cords of HIV patients with pain closely resembles that seen in the cART-treated macaques whereas upregulation of pro-inflammatory mediators is more in line with the untreated macaques that had advanced disease. It is possible that astrocyte activation in the spinal cord is associated with toxic neuronal injury rather than a virus-induced inflammatory response. Another explanation would be that GFAP expression was elevated in untreated animals at earlier time points during the course of disease, but declined during the terminal stage. A distinct pattern has previously been reported in the dorsal root ganglia of SIV-infected pigtailed macaques in which there is an early increase in GFAP expression that is sustained throughout asymptomatic infection but wanes by the 84-day p.i. time point (5), possibly reflecting the progression from a state of sustained glial activation to eventual degeneration and loss. Also, although GFAP immunostaining is widely used as a marker of astrocyte activation, GFAP expression does not necessarily correlate with functional changes such as altered expression of surface receptors, ion channels, or production of cytokines and chemokines, which more directly influence pain signaling (38).
Significant correlations between ENF loss and severity of spinal cord inflammation and viral load in the cART-naïve macaques is compelling and, along with the findings of Tang et al in mice, suggest that chronic stimulation and perhaps injury of the central terminus of sensory nerves can contribute to pathologic changes in the peripheral nerve fibers. In cART-treated animals, which also exhibited significant ENF loss, peripheral nerve damage may be attributable to antiretroviral toxicity rather than virus-induced injury to the sensory pathway. Future studies with uninfected, cART-treated macaques will be instrumental in dissecting this complex pathogenesis.
Study of the spinal cord is also germane to another critically important topic in contemporary HIV research, i.e. viral eradication from the CNS. Several studies have demonstrated the presence of low-level viral RNA, integrated viral DNA, and persistent immune activation in the brains of HIV patients and SIV-infected primates on suppressive cART; the authors have hypothesized that microglia and infiltrating macrophages serve as reservoirs for residual viral replication and reactivation in the CNS (2, 21–23, 51–53). However, there are currently no published studies addressing whether the spinal cord, a major component of the CNS, is also a potential viral reservoir in patients on modern cART. Our results show that the spinal cord is an active site of viral infection and replication in some cART-naïve animals. Because microglia and infiltrating macrophages are also abundant in the spinal cord, studies investigating CNS viral latency and persistent neuroinflammation should consider evaluating multiple anatomic locations in the spinal cord as well as the brain. Also, rodent models of acute CNS inflammation have revealed major differences in immune responses, microglial activation, and blood-CNS-barrier breakdown when comparing the brain and spinal cord (54–58). This dichotomy has yet to be investigated in a primate model of chronic CNS inflammation and could have substantial impact on the pathogenesis of HIV infection and persistence in the CNS. Studies based in the SIV/pigtailed macaque model could investigate the presence of residual low-level viral replication, as well as latent, integrated provirus in macrophages and microglia of the spinal cord.
Although widespread use of cART has drastically reduced morbidity and mortality caused by HIV infection, the continued high frequency of chronic neurologic complications and pursuit of systemic viral eradication are enduring challenges. This investigation into the neuropathology of the spinal cord of SIV-infected macaques demonstrates the importance of considering this key CNS compartment in future studies aimed at elucidating the mechanisms underlying painful HIV-SN and the development of novel treatment strategies for this debilitating condition. Furthermore, careful examination of the spinal cord is warranted in the continuing effort to understand CNS viral reservoirs and achieve sterilizing cure of HIV infection.
ACKNOWLEDGMENTS
The authors would like to thank Ming Li and Elizabeth Engle for their excellent technical assistance.
These studies were supported by NIH NS055651, NIH MH070306, NIH NS077869, NIH ORIP P40 OD013117, NIH ORIP T32 OD011089, and NIH R25MH08066108.
REFERENCES
- 1.Pardo AC, McArthur JC, Griffin JW. HIV neuropathy: Insights in the pathology of HIV peripheral nerve disease. J Periph Nerv Sys. 2001;6:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 2.Desplats P, Dumaop W, Smith D, et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burdo T, Orzechowski K, Knight H, et al. Dorsal root ganglia damage in SIV-infected rhesus macaques: an animal model of HIV-induced sensory neuropathy. Am J Pathol. 2012;180:1362–1369. doi: 10.1016/j.ajpath.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laast V, Pardo C, Tarwater P, et al. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J Neurpathol Exp Neurol. 2007;66:26–34. doi: 10.1097/nen.0b013e31802c398d. [DOI] [PubMed] [Google Scholar]
- 5.Laast V, Shim B, Johanek L, et al. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mankowski J, Clements J, Zink M. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J Inf Dis. 2002;186(Suppl 2):208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- 7.McArthur JC, Steiner J, Sacktor N, et al. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 8.Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotoxic Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- 9.Hénin D, Smith T, De Girolami U, et al. Neuropathology of the spinal cord in the acquired immunodeficiency syndrome. Hum Pathol. 1992;23:1106–1114. doi: 10.1016/0046-8177(92)90028-2. [DOI] [PubMed] [Google Scholar]
- 10.Snider W, Simpson D, Nielsen S, et al. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14:403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- 11.Maier H, Budka H, Lassmann H, et al. Vacuolar myelopathy with multinucleated giant cells in the acquired immune deficiency syndrome (AIDS) Acta neuropathologica. 1989;78:497–503. doi: 10.1007/BF00687711. [DOI] [PubMed] [Google Scholar]
- 12.Dal Pan GJ, Glass JD, McArthur JC. Clinicopathologic correlations of HIV-1-associated vacuolar myelopathy: an autopsy-based case-control study. Neurology. 1994;44:2159–2164. doi: 10.1212/wnl.44.11.2159. [DOI] [PubMed] [Google Scholar]
- 13.Petito C. Review of central nervous system pathology in human immunodeficiency virus infection. Ann Neurol. 1988;23(suppl):S54–S57. doi: 10.1002/ana.410230715. [DOI] [PubMed] [Google Scholar]
- 14.Santosh CG, Bell JE, Best JJ. Spinal tract pathology in AIDS: postmortem MRI correlation with neuropathology. Neuroradiology. 1995;37:134–138. doi: 10.1007/BF00588630. [DOI] [PubMed] [Google Scholar]
- 15.Ellis R, Rosario D, Clifford D, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S, Chandran A, Jansen J. Epidemiology of HIV-related neuropathy: a systematic literature review. AIDS Res Hum Retrovir. 2012;28:36–48. doi: 10.1089/AID.2011.0116. [DOI] [PubMed] [Google Scholar]
- 17.Kamerman P, Moss P, Weber J, et al. Pathogenesis of HIV-associated sensory neuropathy: evidence from in vivo and in vitro experimental models. J Periph Nerv Sys. 2012;17:19–31. doi: 10.1111/j.1529-8027.2012.00373.x. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Gelman B, Lisinicchia J, et al. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012;32:10833–10840. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Shu J, Gelman B, et al. Wnt signaling in the pathogenesis of human HIVassociated pain syndromes. J Neuroimm Pharm. 2013;8:956–964. doi: 10.1007/s11481-013-9474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan S, Shi Y, Chen J, et al. Gp120 in the pathogenesis of human HIV-associated pain. Ann Neurol. 2014;74:837–850. doi: 10.1002/ana.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rappaport J. Editorial: The monocyte/macrophage in the pathogenesis of AIDS: The next frontier for therapeutic intervention in the CNS and beyond: Part I. Curr HIV Res. 2014;12:75–76. doi: 10.2174/1570162x1202140725100102. [DOI] [PubMed] [Google Scholar]
- 22.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV and AIDS. 2013;8:165–169. doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zink M, Brice A, Kelly K, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Inf Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangus LM, Dorsey JL, Laast VA, et al. Unraveling the pathogenesis of HIV peripheral neuropathy: insights from a simian immunodeficiency virus macaque model. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2013;54:296–303. doi: 10.1093/ilar/ilt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clements JE, Mankowski JL, Gama L, et al. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol. 2008;14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McArthur J, Brew B, Nath A. Neurological complications of HIV infection. Lancet neurology. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 27.Kamerman PR, Wadley AL, Cherry CL. HIV-associated sensory neuropathy: risk factors and genetics. Current pain and headache reports. 2012;16:226–236. doi: 10.1007/s11916-012-0257-z. [DOI] [PubMed] [Google Scholar]
- 28.Zink MC, Suryanarayana K, Mankowski JL, et al. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. Journal of virology. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazuda DJ, Young SD, Guare JP, et al. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–532. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- 30.Schefe JH, Lehmann KE, Buschmann IR, et al. Quantitative real-time RT-PCR data analysis: current concepts and the novel "gene expression's CT difference" formula. J Mol Med. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy JM, Hoke A, Zhu Y, et al. Peripheral neuropathy in lentivirus infection: evidence of inflammation and axonal injury. AIDS. 2004;18:1241–1250. doi: 10.1097/00002030-200406180-00002. [DOI] [PubMed] [Google Scholar]
- 33.Soulas C, Conerly C, Kim W-KK, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zink MC, Coleman GD, Mankowski JL, et al. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Inf Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, Ouyang H, Zheng X, et al. Glial TNFα in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Molecular pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X, Ouyang H, Liu S, et al. TNFα is involved in neuropathic pain induced by nucleoside reverse transcriptase inhibitor in rats. Brain Behav Imm. 2011;25:1668–1676. doi: 10.1016/j.bbi.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y-JJ, Zhang L, Samad OA, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji R-R, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White F, Bhangoo S, Miller R. Chemokines: integrators of pain and inflammation. Nature Rev Drug Disc. 2005;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2012;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polydefkis M. Skin biopsy findings predict development of symptomatic neuropathy in patients with HIV. Nat Clin Pract Neurol. 2006;2:650–651. doi: 10.1038/ncpneuro0353. [DOI] [PubMed] [Google Scholar]
- 42.Edén A, Price R, Spudich S, et al. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Inf Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 43.Yilmaz A, Price RW, Spudich S, et al. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J AIDS. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gosselin R-DD, Suter MR, Ji R-RR, et al. Glial cells and chronic pain. The Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 47.Cao H, Zhang Y-QQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Grace PM, Hutchinson MR, Maier SF, et al. Pathological pain and the neuroimmune interface. Nature Rev Immun. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiebe L, Phillips T, Li J-M, et al. Pain in HIV: an evolving epidemic. J Pain. 2011;12:619–624. doi: 10.1016/j.jpain.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Evans S, Ellis R, Chen H, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clements J, Babas T, Mankowski J, et al. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Inf Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- 52.Nath A, Clements J. Eradication of HIV from the brain: reasons for pause. AIDS. 2011;25:577–580. doi: 10.1097/QAD.0b013e3283437d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Current HIV research. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons SB, Liggitt D, Goverman JM. Cytokine-regulated neutrophil recruitment Is required for brain but not spinal cord inflammation during experimental autoimmune encephalomyelitis. J Immunol. 2014;193:555–563. doi: 10.4049/jimmunol.1400807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson J. Immune response by microglia in the spinal cord. Ann NY Acad Sci. 2010;1198:271–278. doi: 10.1111/j.1749-6632.2010.05536.x. [DOI] [PubMed] [Google Scholar]
- 56.Baskar Jesudasan SJ, Todd KG, et al. Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain. PloS one. 2014;9:e99443. doi: 10.1371/journal.pone.0099443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell SJ, Wilcockson DC, Butchart AG, et al. Altered chemokine expression in the spinal cord and brain contributes to differential interleukin-1beta-induced neutrophil recruitment. J Neurochem. 2002;83:432–441. doi: 10.1046/j.1471-4159.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 58.Schnell L, Fearn S, Klassen H, et al. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]