Abstract
Background
Growing evidence indicates that the presence of TLR4 on platelets is a key regulator of platelet number and function. Platelets exposed to TLR4 agonists may serve to activate other cells such as neutrophils and endothelial cells in sepsis and other inflammatory conditions. The functional significance of platelet TLR4 in hemorrhagic shock, however, remains unexplored.
Methods and Results
Using thromboelastography and platelet aggregometry, we demonstrate that platelet function is impaired in a mouse model of hemorrhagic shock with resuscitation (HS-R). Further analysis using cellular specific TLR4 deletion in mice revealed that platelet TLR4 is essential for platelet activation and function in HS-R and that platelet TLR4 regulates the development of coagulopathy after hemorrhage and resuscitation. Transfusion of TLR4 negative platelets into mice resulted in protection from coagulopathy and restored platelet function. Additionally, platelet-specific TLR4 knock-out mice were protected from lung and liver injury and exhibited a marked reduction in systemic inflammation as measured by circulating IL-6 following HS-R.
Conclusions
We demonstrate for the first time that platelet TLR4 is an essential mediator of the inflammatory response as well as platelet activation and function in hemorrhagic shock and resuscitation.
Keywords: platelet, coagulopathy, inflammation, hemorrhagic shock, TLR4
Introduction
Platelets have been extensively studied as hemostatic regulators, and in the context of hemorrhagic shock are best recognized for their role in clot formation following vascular endothelial injury. A concept that has taken longer to establish, but is rapidly evolving, is that platelets are also key effecter cells in systemic inflammatory processes both as instigators of local and systemic inflammatory reactions and also participants in the inflammation that contributes to tissue injury.1,2 Platelets express a number of receptors common to other immune cells including members of the toll-like receptor family (TLRs).3–6 These receptors not only sense molecules of microbial origin but also molecules of host origin released by damage associated molecular pattern molecules (DAMPs).3–6 Several TLRs, including TLR2, TLR3, TLR4, and TLR9 contribute to the inflammatory response to hemorrhagic shock and other forms of sterile injury.6–8
TLR4, the receptor for the bacterial endotoxin lipopolysaccharide (LPS), is expressed on platelets and the effect of LPS signaling on platelet function has been extensively studied.9–14 Through TLR4, platelets act as inflammatory sentinels, surrounding and isolating an infection, while modulating proinflammatory cytokine release. These events endow platelet TLR4 with a pivotal role in sepsis.15,16 However, to date, no work has focused on the function of platelet TLR4 in hemorrhagic shock, where activation of TLR4 has also been shown to play a vital role.17–21
In the present work, we report on investigations into the role of TLR4 on the activation of platelets, as well as the role of platelet TLR4 on organ injury and inflammatory cytokine production following HS-R. Using a platelet-specific TLR4 knockout mouse, we demonstrate that TLR4 is necessary for platelet activation and functional changes induced by HS-R as well as for coagulation abnormalities. Furthermore, selective deletion of TLR4 from platelets was sufficient to reduce platelet sequestration and organ injury as well as markers of systemic inflammation. Taken together, these data demonstrate a novel and dominant role for TLR4 on platelets and identify a key mechanistic component in the pathogenesis of inflammation and end organ dysfunction following hemorrhagic shock.
Materials and Methods
Animal care
Animal handling and care complied with published regulations by the National Institutes of Health and were approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh. All mice were 8–12 weeks old, weighed 25–30g, and were maintained with a 12:12-h light-dark cycle and free access to standard laboratory chow and water. Male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Generation of TLR4loxP/loxP and cellular specific TLR4−/− mice
TLR4loxP/loxP mice were generated as we have previously described.22 Transgenic mice expressing cre recombinase linked to the platelet factor 4 (Pf4), albumin (alb), lysozyme (lyz) and CD11c (cd11c) were obtained from Jackson Laboratory. TLR4loxP/loxP mice were interbred with stud transgenic males to generate cellular-specific deletion of TLR4 creating the following five cellular-specific groups: 1) Wild-type control (TLR4loxP/loxP); 2) platelet specific (TLR4loxP/Pf4-cre); 3) hepatocyte specific (TLR4loxP/Alb-cre); 4) macrophage specific (TLR4loxP/Lyz-cre); and 5) dendritic cell specific (TLR4loxP/CD11c-cre).
Genotyping was performed using standard genomic PCR genotyping techniques. Global TLR4−/− mice were generated as described.22
Hemorrhagic shock model
To study the influence of HS models on platelet function, WT mice were divided into the following three groups (n=8–10): 1) sham (control); 2) hemorrhagic shock (HS); and 3) hemorrhagic shock and resuscitation (HS-R).
HS was performed as we have previously described.23 Briefly, bilateral inguinal dissections were performed, a small femoral arteriotomy was made, and the femoral arteries were cannulated with sterile PE-10 catheter. Animals were hemorrhaged to a mean arterial pressure (MAP) of 25 mmHg for 120 min. The contralateral cannula was used for continuous hemodynamic monitoring. For the resuscitation group, animals were transfused with three times the volume of shed blood with Ringer lactate after HS and body temperature was maintained at 37°C. Animals were sacrificed 6 hours after HS-R and blood and tissues were collected for analysis. Control animals received cannulation only without hemorrhage.
Sample collection
At the conclusion of the experiment, animals were anesthetized with isoflurane and blood was harvested by cardiac puncture. Serum was collected by centrifugation at 13,000×g (10min, 4°C). The same liver and lung lobe was harvested, snap-frozen in liquid nitrogen or treated with 2-methylbutane, and then stored at −80°C. The liver and lung were also removed and fixed in 10% neutral formalin for hematoxylin and eosin (H&E) staining. For immunofluorescence staining, liver and lung were flushed with PBS and then perfused with 2% paraformaldehyde followed by standard sucrose treatment.
Thromboelastography (TEG) analysis
Platelet function and coagulopathy were measured by TEG analysis using Haemoscope 5000 analyzers (Haemonetics, Braintree, MA) . An aliquot of 340 μl sodium-citrated whole blood [(3.2% sodium citrate added in a ratio of 1:9 (v/v)] recalcified with 20μl of 0.2 mol/l CaCl2 was added to room temperature disposable TEG cups containing 2.0 IU of Heparinase I, and the TEG were done simultaneously on two channels. Coagulation profiles (which plot clot amplitude vs. time) and clotting velocity curves (first derivative of the amplitude curve) were generated using TEG software (version 4.2.3, Haemonetics). Maximal amplitude (MA, in mm) was reported as a measurement of clot strength and platelet aggregation/function. Blood samples were obtained at the time of shock initiation and then following resuscitation, thus each mouse served as its own internal control.
Platelet depletion
Recipient mice were treated with a platelet depleting antibody (anti-CD41, BD biosciences, San Diego, CA) at a final concentration of 1μg/g body weight, diluted in 200μl sterile normal saline (0.9% [wt/vol] sodium chloride) via penile vein. Injection of this mixture reduced the number of circulating platelets to less than 0.05 ×106μL(normal value,1.0 to 1.2×106μL) (data not shown) consistent with previously published values.24
Platelet isolation and transfusion
Seventy-two hours after the platelet depletion, the entire circulating blood volume, approximately 1.0ml/mouse, was collected from donor mice into sterile tubes containing 0.1ml acid citrate dextrose(ACD, Sigma, St. Louis, MO) and 1μM Prostaglandin E1(PGE1,Sigma). Platelet-rich plasma (PRP) was obtained from the mixture by centrifugation at 260×g for 8 minutes and 260×g for an additional 3 minutes, collecting supernatant after each centrifugation. The PRP was then spun at 740×g for 10 minutes and the pellet was then re-suspended in 120μl of sterile PBS. Platelet concentration was determined using the Unopette collection system (Becton Dickinson, Franklin Lakes, NJ, 1:00 dilution) and counting the platelets with a hemocytometer. As reported previously, this method of platelet isolation results in <0.01% of leukocytes in the platelet suspensions.25 Platelets (value=1.0 to 1.2×106μL) from 2 same strain donor mice were then diluted with PBS to a volume of 200μl, and transfused via penile vein into recipient mice just prior to the model.26 HS-R was performed on the transfused mice within 3h following transfusion (n=5 recipient mice per group).
Platelet aggregation
Whole blood samples were utilized for aggregation studies. Blood was stimulated with 20 μM adenosine diphosphate (ADP) or 5ug/ml collagen (Chronolog, Haverton, Pennsylvania) as indicated, and platelet aggregation was assessed using a Chronolog Lumi-aggregometer (Model 490). For HS-R experiments, blood was tested from the time of initial blood draw (pre-shock) or at the termination of resuscitation.
Flow cytometry
PRP was isolated as described above from either sham or HS-R mice and was incubated with FITC-anti-mouse-TLR4, PE-anti-mouse-CD41 and APC-anti-mouse-CD62P (BD bioscience, San Diego, CA) antibodies following the manufacturer's protocol. After incubation, the platelets were washed in washing solution (PBS containing 0.1% sodium azide and 1% FBS) and centrifuged for 5 minutes at 2,000×g. The pellet was resuspended with 500µl platelet washing solution (PBS containing 2 mM EDTA) and read on the Guava easyCyte 8HT flow cytometry system (Millipore, Billerica, MA) using Guava soft 2.2.2. For ex vivo activation studies, PRP was activated with ADP (20–100uM), thrombin (0.1–0.5U/ml), or collagen (5ug/ml).
Platelet counting
Before and after the induction of HS-R, 300μl whole blood was collected and circulating platelets counting was performed using an Advia 120 hematology analyzer (Bayer Diagnostics, Tarrytown, NY).
Western blotting
PRP from TLR4loxP/loxP mice or TLR4loxP/Pf4-cre mice was centrifuged at 800×g for 20 minutes. Pellets were resuspended in platelet lysis buffer [20 mmol/l Tris(pH 7.4), 150 mmol/l NaCl, 1% Triton X-100, and 1 mmol/l EDTA]. Liver and lung samples were homogenized in 1×RIPA cell lysis buffer (Cell Signaling, Danvers, MA). Protein content of cell lysates was determined by BCA protein assay (Pierce, Rockford, IL). Equal protein amounts were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunostaining with optimized dilutions of a rabbit monoclonal anti-mouse CD41 (Abcam, Cambridge, MA) or TLR4 (Abcam). Horseradish peroxidase-conjugated secondary antibodies were used in a standard enhanced chemiluminescence reaction according to manufacturer’s instructions (Pierce, Rockford, IL).
Immunofluorescence
Livers and lung were fixed in 2% paraformaldehyde solution and stained with rat anti-CD41 antibody and rabbit anti-F-actin antibody (Abcam, Cambridge, MA). Samples were washed using PBS + 0.5% BSA followed by incubation in the appropriate Cy3 (1:1000, Invitrogen, Carlsbad, CA) and Cy5 (1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies. Samples were then thoroughly washed before incubation with Hoechst nuclear stain. Positively stained cells in six random fields were imaged with Olympus Fluoview 1000 microscopy (Olympus, Melville, NY).
Histopathology
Ethanol fixed samples were processed in a Shandon Excelsior ES tissue processor (Thermo Scientific, Waltham, MA) with alcohol dehydration and xylene infiltration. Standard hematoxylin-eosin staining was performed to assess for necrosis and inflammatory infiltrate.
Lung myeloperoxidase activity
As a marker for neutrophil infiltration, myeloperoxidase (MPO) activity in lung tissue lysates was determined with a mouse MPO ELISA kit (Hycult Biotech, Plymouth Meeting, PA) according to the manufacturer’s protocol. Briefly, 10mg of frozen lungs were homogenized in lysis buffer with a Tissue Tearor (Biospec Products, Bartlesville, OK) and then centrifuged at 1500×g for 15 min. MPO activity was measured in the supernatant.
Peritoneal macrophage isolation and treatment
Peritoneal macrophages were isolated from mice 4 days after the sterile intraperitoneal injection of 1ml of 3% thioglycollate by washing the peritoneum with sterile PBS (5ml) and culturing cells in DMEM (GIBCO, 11995). Adherent macrophages were cultured overnight prior to exposure to LPS (50ng/ml, 3hrs). Supernatant was isolated for analysis of cytokine production.
Endotoxemia
Endotoxemia was induced by intraperitoneal injection of lipopolysaccarhide (LPS) (Escherichia coli 0111:B4 purified by gel filtration chromatography, >99% pure, Sigma-Aldrich) at a dose of 3 mg/kg for 6 hours into 8 week old male mice. Control animals received saline injections as vehicle alone. Serum was obtained for cytokine analysis following sacrifice.
Assessment of IL-6 protein levels
Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) were used to determine IL6 concentrations in cell supernatant or serum according to the manufacturer’s instructions. All samples were assayed in duplicate.
Measurement of serum ALT and AST levels
To assess hepatic function and cellular injury following HS-R, plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using the Dri-Chem 7000 Chemistry Analyzer (Heska Co, Loveland, CO; slides from Fujifilm Japan).
Statistical analysis
Results are expressed as means±standard error (SEM). Normality of sampled data was assessed using the Shapiro-Wilk test and analysis was performed using SigmaPlot 11.0 (Systat Software, Point Richmond, CA). Comparisons of two groups under the same treatment were performed by Student's t-test, and one-way ANOVA analysis was used for multiple group comparisons. Significance was established at p<0.05.
Results
Platelet function is impaired in HS-R mouse model
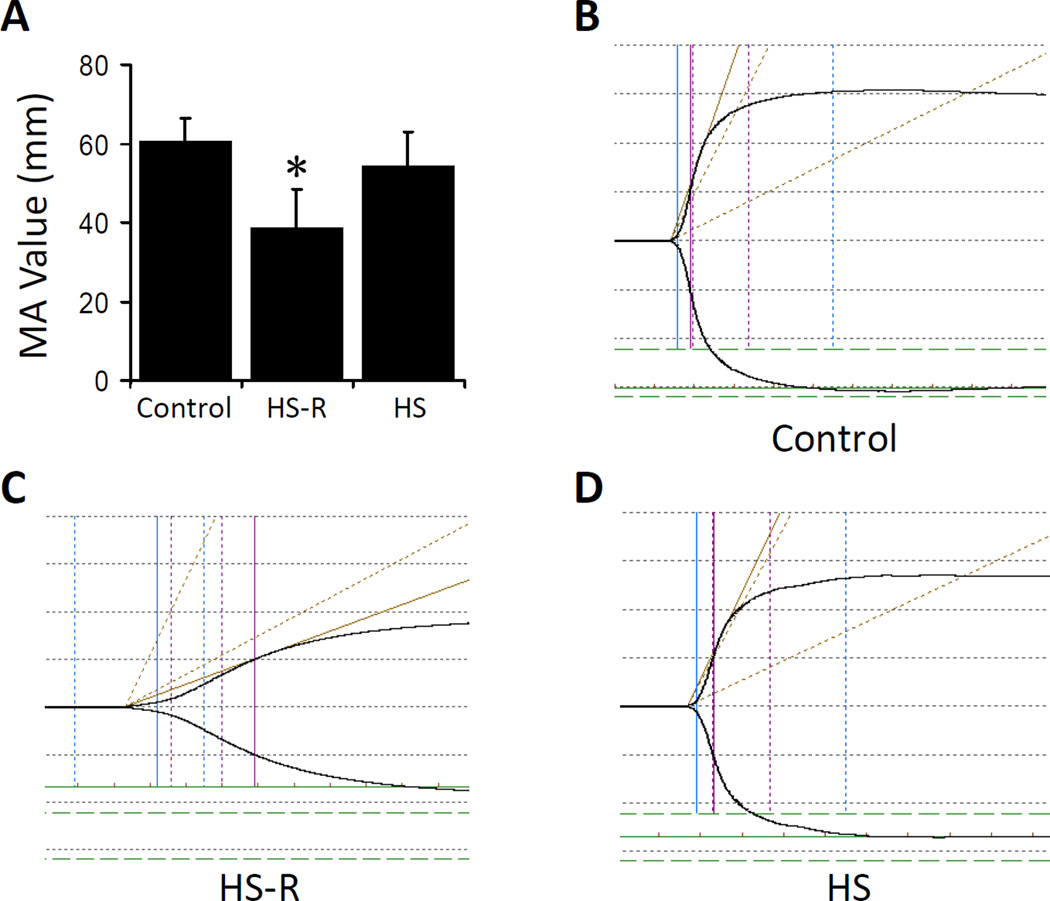
To explore platelet function changes in HS and HS-R, coagulation was measured by whole blood TEG. Maximum amplitude was chosen as the primary parameter for assessing platelet function by TEG.27 HS-R led to a significantly lower maximum amplitude (MA=38.6±9.7mm, p<0.05), indicating smaller and weaker clot formation compared to control mice (60.4±6.2mm). This was not seen with HS without resuscitation where the MA value did not show significant changes compared to normal control (56.4±8.5mm, p>0.05) (Figure 1A). Representative TEG tracings of each group are displayed (Figure 1B–D). Thus, HS-R leads to a marked change in the coagulation profile primarily consistent with altered platelet function.
Figure 1.
Changes of platelet function in hemorrhagic shock mouse models. Thromboelastography (TEG) was used to investigate platelet function via maximal amplitude (MA) after HS or HS-R. A: MA value significantly decreased in HS-R mice compared with control and HS alone. Data shown are means ± SEM, n = 8–10 mice/group. *p<0.05 vs. control; B: Representative tracing of control mice; C: Representative tracing of HS-R mice; D: Representative tracing of HS group. x axis, time (min); y axis, maximum amplitude (mm).
TLR4 contributes to platelet function impairment in HS-R
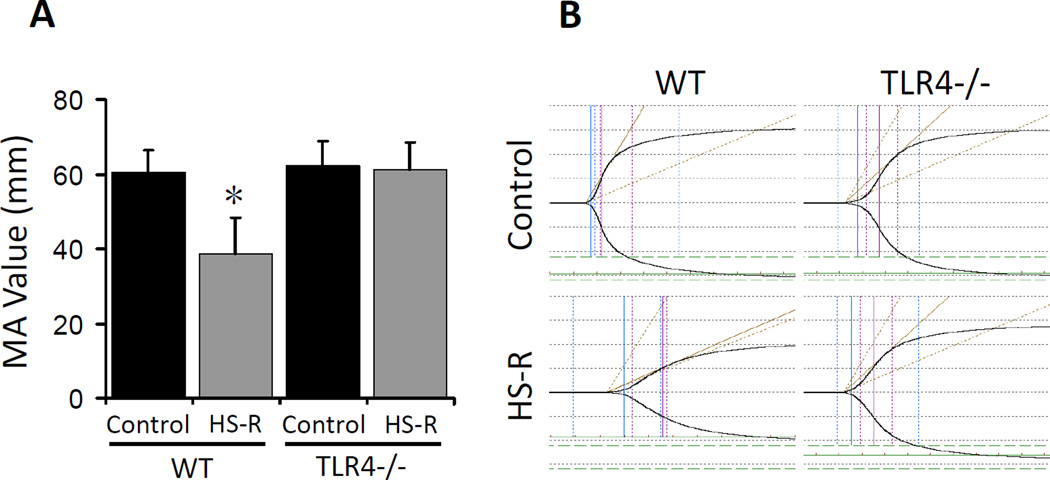
We next investigated whether TLR4 was involved in the impairment of platelet function in the HS-R mouse model. Whereas MA values dropped significantly in WT mice (38.6±9.7mm, p<0.05), no significant changes of MA values were observed in global TLR4−/− mice subjected to HS-R compared to control (Figure 2A, tracings in 2B).
Figure 2.
TLR4 contributes to platelet function impairment in HS-R. A: TEG from wide type (WT) mice and TLR4−/− subjected to HS-R or unmanipulated control. A: MA values showing reduction WT but not TLR4−/− mice after HS-R. Data shown are means ± SEM, n= 8–10 mice/group. *p<0.05 vs. control. B: Representative TEG tracings. x axis, time (min); y axis, maximum amplitude (mm).
Generation of TLR4loxP/Pf4-cre mice
To study the role of TLR4 specifically on platelets, TLR4loxP/Pf4-cre mice were generated as described above3. Deficiency in platelet TLR4 was confirmed by Western blotting and flow cytometry. TLR4 protein was detectable in lysates of TLR4loxP/loxP but not TLR4loxP/Pf4-cre platelets (Supplemental Figure 1A). Staining for TLR4 in TLR4loxP/loxP and TLR4loxP/Pf4-cre platelets demonstrated specific deletion in TLR4loxP/Pf4-cre platelets (Supplemental Figure 1B). Importantly, TLR4loxP/Pf4-cre mice showed intact TLR4 signaling capacity on other cells types confirming similar characterization performed by others3. Intraperitoneal injection of LPS, resulted in a significant increase in serum IL-6 levels in both TLR4loxP/loxP and TLR4loxP/Pf4-cre mice (TLR4loxP/loxP 224±26 pg/ml vs TLR4loxP/PF4-cre 217±19 pg/ml, p=NS) confirming the finding of others3. Furthermore, to show that TLR4 signaling is intact on other cell types, peritoneal macrophages harvested from both TLR4loxP/loxP and TLR4loxP/Pf4-cre mice were stimulated with LPS and demonstrated non-significant differences in IL-6 production (Supplemental Figure 1C).
Platelet TLR4 contributes to platelet function impairment in HS-R
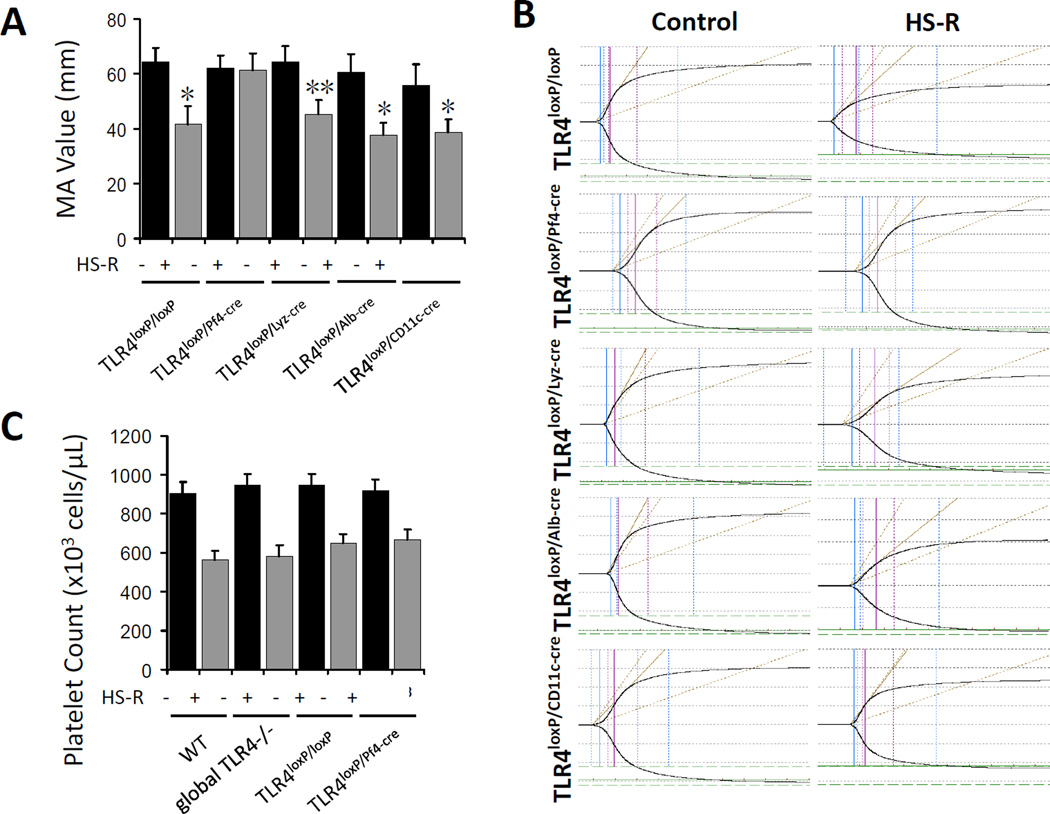
To define cell type specificity of TLR4 involved in platelet dysfunction in HS-R, four kinds of cell specific TLR4−/− mice, including TLR4loxP/Pf4-cre mice (platelet), TLR4loxP/Lyz-cre mice (myeloid), TLR4loxP/Alb-cre mice (hepatocyte), TLR4loxP/CD11c-cre (dendritic cell) mice and their control (TLR4loxP/loxP) mice were subjected to HS-R and the TEG was measured. The additional cell specific deletion mice were included as controls to demonstrate that the effects of removal of TLR4 from platelets were specific to platelet function rather than an epiphenomenon of transgenic manipulation. As expected, significantly lower MA values were observed in the HS-R in TLR4loxP/Lyz-cre mice, TLR4loxP/Alb-cre mice and TLR4loxP/CD11c-cre mice compared with control mice, but HS-R failed to induce significant changes of MA values in TLR4loxP/Pf4-cre mice (Figure 3A) indicating a preservation of platelet function when TLR4 is removed specifically from platelets rather than other cell types (Figure 3B). To exclude that platelet function impairment was simply due to dilutional thrombocytopenia, circulating platelet counts were performed in WT, global TLR4−/−, TLR4loxP/loxP and TLR4loxP/Pf4-cre mice subjected to HS-R or untreated control. Importantly, there were no significant differences of platelet counts (Figure 3C).
Figure 3.
Platelet TLR4 contributes to platelet function impairment in HS-R. A: Whole blood of cell specific TLR4−/− mice were subjected to HS-R or unmanipulated control and analyzed by TEG. Data shown are means ± SEM, n= 4–6 mice/group. *p<0.01, **p<0.02 vs. control. B: Representative TEG tracings. x axis, time(min); y axis, maximum amplitude(mm). C: Platelet counts from WT, global TLR4−/−, TLR4loxP/loxP and TLR4loxP/Pf4-cre mice following HS-R or control. Data are means ± SEM, n= 4–6 mice/group. *p<0.05 vs. control.
Platelet TLR4 contributed to platelet activation in HS-R
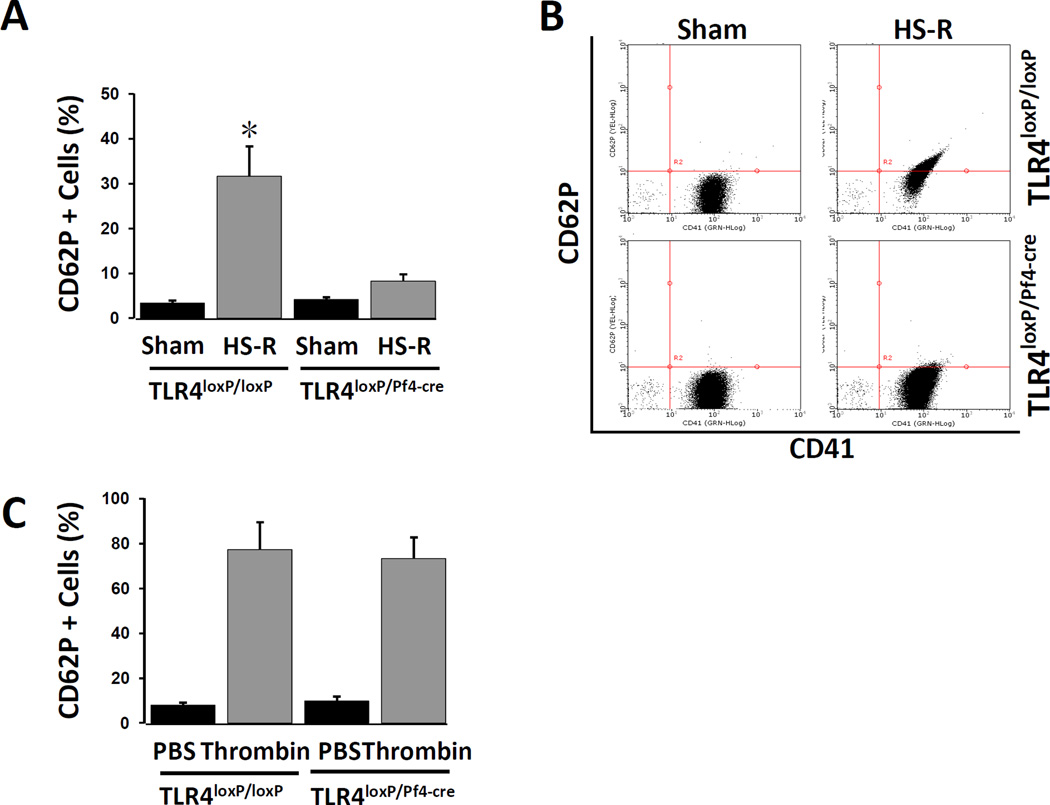
We next assessed the activation of platelets isolated from TLR4loxP/Pf4-cre mice and control TLR4loxP/loxP mice subjected to HS-R. The activation state of the platelets was measured by examining surface CD62P expression using standard flow cytometry analysis. Figure 4A and B demonstrate that platelets from TLR4loxP/Pf4-cre mice did not show significantly increased CD62P expression in response to HS-R, as compared with TLR4loxP/loxP mice, suggesting that HS-R modulates platelet activation through platelet TLR4. Importantly, platelets lacking TLR4 expression were still capable of upregulating CD62P in response to thrombin (Figure 4C). Similar results were obtained using both collagen (%CD62p expression TLR4loxP/loxP: 41±5.8 vs TLR4loxP/Pf4-cre: 48±9.7, p=NS) and 50uM ADP (%CD62p expression TLR4loxP/loxP: 72±4.5 vs TLR4loxP/Pf4-cre: 68±8.2, p=NS).
Figure 4.
Assessment of platelet activation by flow cytometry. Platelet rich plasma from TLR4loxP/Pf4-cre and TLR4loxP/loxP mice was isolated and stained for CD41 and CD62p (100uM ADP). A: Percentage of CD62p positive. Data are means ± SEM, n = 8–10 mice/group. *p<0.05 vs. control. B: Representative dual parameter dot-plot figures obtained by flow cytometry for CD41 and CD62p. C: Treatment of PRP from both TLR4loxP/Pf4-cre and TLR4loxP/loxP mice with 0.1U/ml thrombin confirms equal CD62p upregulation. Data are means ± SEM, n = 5 mice/group. p=NS between thrombin groups.
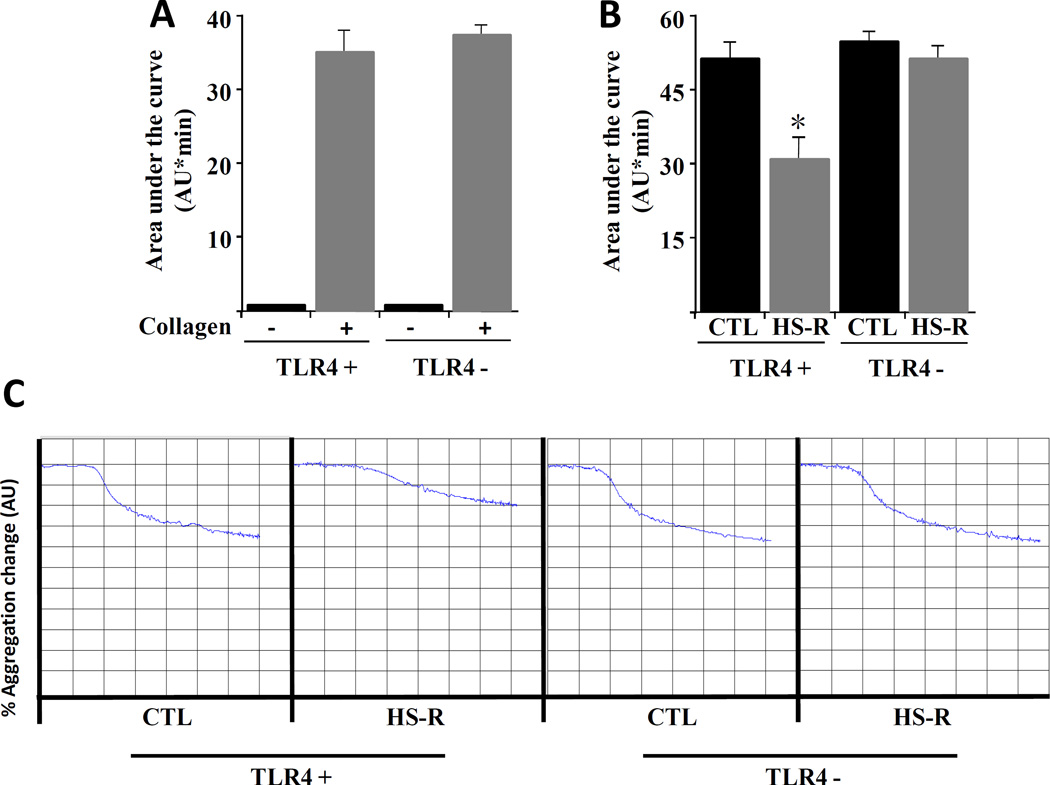
Platelet aggregation is impaired in HS-R and regulated by TLR4
Platelet function following HS-R was assessed using platelet aggregometry. As shown in Figure 5A, ex vivo collagen treatment of blood isolated from unmanipulated mice shows equivalent aggregation response between TLR4 positive and negative mice, indicating that TLR4 deficient platelets response appropriately to traditional aggregation agonists. Similar results were obtained using 20uM ADP and 0.1U/ml thrombin (data not shown). Following HS-R, there was a substantial reduction in the area under the curve for WT mice, indicating impaired platelet aggregation (Figure 5B). Strikingly, however, there was no significant difference in aggregation following HS-R in mice lacking TLR4 on platelets. Taken together, these findings indicate impaired platelet function following HS-R that is not seen in TLR4 deficient mice.
Figure 5.
TLR4 regulates platelet aggregation following HS-R. Aggregometry was performed on wild-type (TLR4 +) or mice lacking TLR4 on their platelets (TLR4 −). Results are expressed as the area under the aggregometry curve, measured as arbitrary units/minute (AU*min). A: Ex vivo collagen (5ug/ml) treatment of platelets demonstrates no significant difference in aggregation between groups. N=8 mice/group. p=NS. B: Aggregometry from either control (pre-shock blood draw) vs HS-R (post-shock). Collagen (5ug/ml) was present as the agonist in all samples. N=5 mice/group. C: Representative aggregometry tracings from control and HS-R. Each y axis block represents 10% change and each x axis block is 1 minute.
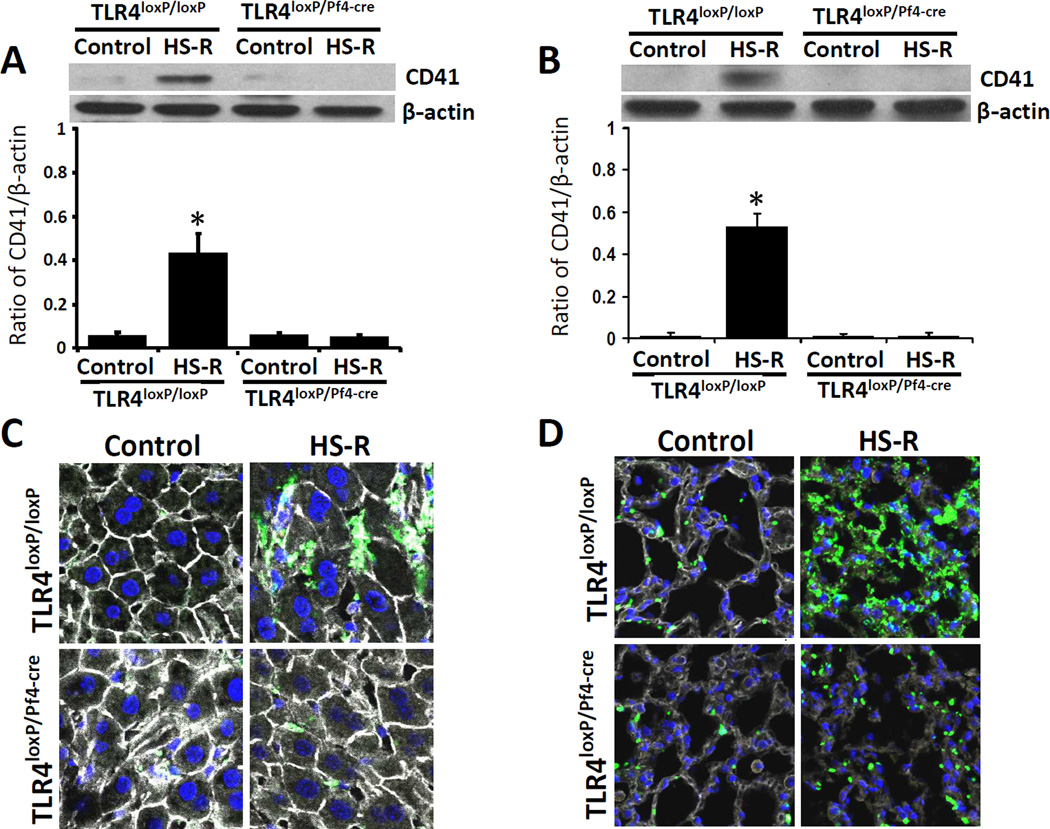
Platelet TLR4 contributed to platelet sequestration into liver and lung in HS-R
To determine whether platelets localized in liver and lung during HS-R, the expression of CD41 was assessed in TLR4loxP/Pf4-cre mice and TLR4loxP/loxP mice by western blotting. In models of sepsis, platelets accumulate in lung and liver where they may contribute to organ damage.28,29 CD41 significantly increased in HS-R group compared to unmanipulated control group in TLR4loxP/loxP mice. However, the expression of CD41 did not show significant changes in TLR4loxP/Pf4-cre mice between HS-R group and control group (Figure 6A,B). Platelets were also labeled with anti-CD41 antibody and examined by immunofluorescence in these tissues. In untreated control TLR4loxP/loxP mice, there was no accumulation of platelets into any tissues. However, a significant accumulation of platelets was noted in liver and lung of TLR4loxP/loxP mice subjected to HS-R. In contrast, there was minimal platelet accumulation into the liver and lung of TLR4loxP/Pf4-cre mice both subjected to HS-R and untreated control (Figure 6C,D).
Figure 6.
Effect of HS-R on platelet sequestration into lung and liver. TLR4loxP/Pf4-cre mice and TLR4loxP/loxP mice were subjected to HS-R or unmanipulated control. Platelet sequestration into lung and liver of the mice were assessed by measuring the expression of CD41 using western blotting and immunofluorescence. A: The expression of CD41 in liver (A) and lung (B), analyzed by Western blotting. Data are means ± SEM, n = 8–10 mice/group. *p<0.05 vs. control. Livers (C) and lungs (D) were stained with CD41 (green), F-actin (white) antibody and counterstained with Hoechst to detect nuclei (blue) and imaged by confocal microscopy (Olympus FV1000 confocal, magnification ×200). Representative images of five individual experiments.
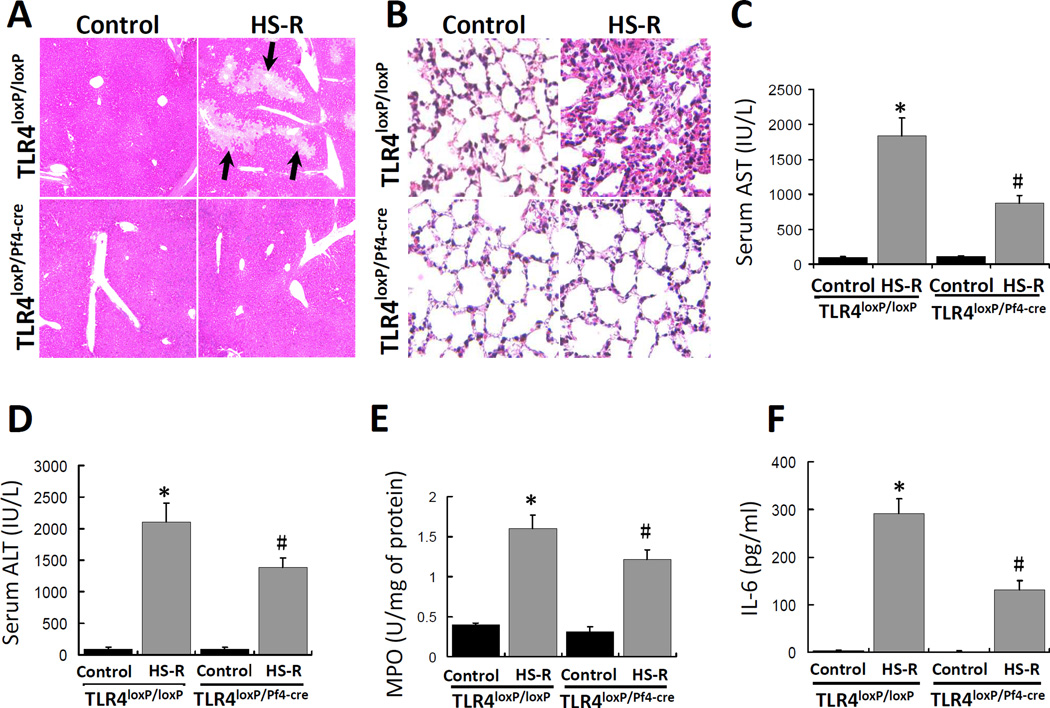
Platelet TLR4 contributes to organ injury and cytokine release in HS-R
To assess the involvement of platelet TLR4 in liver and lung injury, TLR4loxP/Pf4-cre mice and TLR4loxP/loxP mice were subjected to HS-R and liver and lung injury was assessed by histology, circulating ALT and AST concentrations, and lung MPO activity. As shown in Figure 7, HS-R induced significantly hepatic necrosis (arrows) and lung inflammatory injury in TLR4loxP/loxP mice as compared with control. Strikingly, HS-R caused minimal microscopic changes in liver and lung of TLR4loxP/Pf4-cre mice. Serum AST and ALT levels increased significantly after HS/R in TLR4loxP/loxP mice but not in TLR4loxP/Pf4-cre mice (Figure 7C,D). Additionally, HS-R significantly increased the lung MPO activity in TLR4loxP/loxP but notTLR4loxP/Pf4-cre mice (Figure 7E). In accordance with the reduced liver and lung injury, TLR4loxP/Pf4-cre mice had a marked reduction in systemic inflammation as measured by levels of IL-6 in the circulation after HS-R, as compared with TLR4loxP/loxP mice (Figure 7F).
Figure 7.
Effect of HS-R on lung and liver injury and cytokine release. TLR4loxP/Pf4-cre mice and TLR4loxP/loxP mice were subjected to HS-R or unmanipulated control and liver and lung injury was assessed by histology, circulating AST and ALT concentrations, lung MPO activity and circulating IL-6. Liver (A) and lung (B) were sectioned and stained with H&E and tissue damage induced by HS-R was assessed by light microscopy (Nikon FX series). Black arrows show the regions of necrosis. (Original magnification ×100). Serum AST (C) and ALT (D) levels were assayed and are presented as means ± SEM (n = 8–10 mice/group). Aliquots of the mouse lung tissues were homogenized to prepare total protein samples and MPO levels were assayed by ELISA (E). The levels are presented as means ± SEM (n = 8–10 mice/group). Serum samples from mice treated as described above were used to assay IL-6 concentration by ELISA (F) and the levels are presented as means ± SEM (n = 8–10 mice/group). *p<0.05 vs. control. #p<0.05 vs. HS-R group of TLR4loxP/loxP mice.
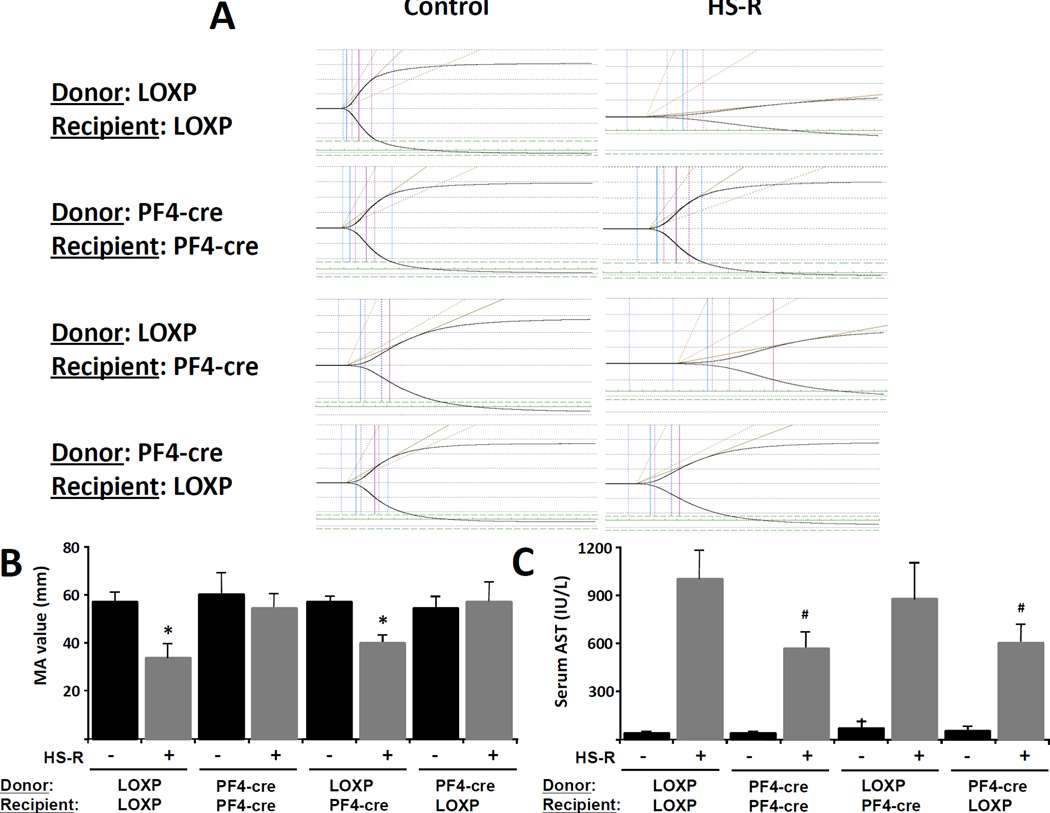
Pre-shock transfusion of TLR4-negative platelets eliminates coagulopathy and organ injury in HS-R
We next sought to establish whether TLR4 specifically on the platelet was necessary and sufficient to the development of coagulopathy and organ injury following HS-R using adoptive transfer. We reduced native platelets from wild type (TLR4loxp/loxp) mice using an anti-platelet antibody to platelet counts of less than 0.05 ×106/μL(normal value,1.0 to 1.2×106/μL). Platelets were then harvested from either wild type or mice lacking TLR4 on platelets (TLR4loxp/PF4cre) and transfused into thrombocytopenic recipients. Post transfusion platelet values were consistently 0.5–0.7×106/μL, indicating that native platelets were less than 10% of circulating in the transfused recipients (data not shown). The tested groups of donor and recipient platelets are noted in Figure 8. For the purpose of clarity in reporting these data, TLR4loxp/loxp will be reported as LOXP and TLR4loxp/PF4cre will be reported as PF4-cre alone. Mice with TLR4(+) platelets after transfusion developed significant coagulopathy (LOXPdonorLOXPrecipient, pre-HS-R MA: 57.3±4.2mm vs post-HS-R MA: 36.1±6.8mm, p<0.01) which was not seen in mice lacking TLR4 on platelets (PF4-credonorPF4-crerecepient, pre-HS-R MA: 61.2±6.9mm vs post-HR-R MA: 59.4±4.9mm, p=NS). Transfusion of TLR4(+) platelets into thrombocytopenic TLR4(−) platelet mice resulted in a significant reduction in clot strength (Figure 8B). Strikingly, however, transfusion of TLR4(−) platelets completely reversed the coagulation abnormality seen in wild type animals after HS-R (PF4-credonorLOXPrecipient, pre-HS-R MA: 58.7±5.9mm vs post-HR-R MA: 60.1±8.1mm, p<0.05) (Figure 8A,B). The effect of platelet transfusion was also reflected in the degree of liver injury, as mice transfused with TLR4(+) platelets had significantly elevated AST levels, while transfusion of TLR4(−) platelets reduced these effects (Figure 8C).
Figure 8.
Platelet TLR4 is necessary and sufficient for the induction of platelet dysfunction and organ injury in HS-R. Wild-type (LOXP) and mice lacking TLR4 on platelets (PF4-cre) were depleted of their platelets using anti-CD41 antibody and subsequently transfused donor platelets isolated from either their littermates (LOXPtransLOXP, PF4-cretransPF4-cre) as controls or from donors with opposite TLR4 status with respect to their platelets (LOXPtransPF4-cre, PF4-cretransLOXP) to test the specific role of TLR4 on platelets in HS-R. (A) TEGs pre and post HS-R are shown and the MA are quantified in (B) as means ± SEM (n = 5 mice/group). (C) rSerum AST levels including both sham and HS-R (means ± SEM (n = 5 mice/group)). *p<0.01 vs. control. #p<0.05 vs. HS-R group of LOXPtransLOXP mice.
Discussion
Toll-like receptors transmit danger signals to the innate immune system and mediate inflammatory events that can recruit and activate cells of the adaptive immune system to respond against pathogens.30 Many studies have identified TLR4 on platelets, and the presence of TLR4 on platelets is essential for the LPS- induced thrombocytopenia, cytokine production and platelet accumulation in the liver and lung.11,14,31 Both platelet activation and TLR4 signaling on other cell types have been shown to contribute to the pathogenesis of organ injury following hemorrhagic shock and resuscitation,2,18–20 however, the specific role of TLR4 expressed on platelets had not previously been addressed.
Our results demonstrate that platelet function is impaired in the mouse model of HS-R, as measured by TEG and platelet aggregometry assays. TEG provides comprehensive clotting profile analysis but is also a reliable indicator of clot strength and platelet function using the maximum amplitude.27 The finding of reduced MA in HS-R is directly supported by a substantial impairment in platelet aggregation following HS-R. Further analysis using global TLR4−/− mice revealed that TLR4 is essential for platelet function impairment in HS-R. Interestingly, platelet function was intact in TLR4loxP/Pf4-cre mice while wild-type and transgenic, cell specific deletion controls demonstrated severe functional impairment in HS-R, indicating the specificity of our findings to platelet TLR4 as opposed to other cell types. HS-R also resulted in an up-regulation of CD62P, a marker of platelet activation, in wild type mice while activation was significantly mitigated in TLR4loxP/Pf4-cre mice. This suggests that at least a subset of platelets may be activated through TLR4 signaling following HS-R, although the functional significance of this remains unexplored Collectively, these data indicate a critical role for platelet TLR4 in platelet function following HS-R. In support of this observation, we have shown further that transfusion of platelets lacking TLR4 into thrombocytopenic wild type mice resulted in reversal of coagulopathy following HS-R as measured by TEG. Interestingly, the deposition of platelets in liver and lung, the increase of circulation IL-6 concentration, and liver and lung injury were also attenuated in TLR4loxP/Pf4-cre mice compared with control in response to HS-R. The reduced pro-inflammatory cytokine production likely represents an overall reduction in injury severity following HS-R as opposed to a direct effect of platelet TLR4 on IL-6 production, as the TLR4loxP/Pf4-cre mice demonstrated intact TLR4 signaling and IL-6 production following exposure to LPS3. These data for the first time suggest that platelet TLR4 is necessary and sufficient for the development of platelet dysfunction and coagulopathy following HS-R and may regulate the accompanying systematic inflammation and organ injury.
HS-R causes a global ischemia-reperfusion injury which can result in systemic and end-organ inflammation and eventually organ failure.32,33 Strong evidence for a proinflammatory role for platelets exists,34 and platelet depletion has a beneficial effect on the outcome of ischemia/reperfusion injury.35–38 In the present study, impaired platelet function was only observed in HS-R mice, but not in mice subjected to HS alone. The result suggests that either adequate time had not elapsed in the HS alone group to alter platelet activation, or, more likely, that events associated with reperfusion contribute to platelet activation.
Coagulation abnormalities are common in severe sepsis and it has been suggested that the presence of TLR4 on platelets could be a link between disseminated intravascular coagulation and sepsis.39,40 Early coagulopathy is also a key component of HS-R.27 In the present study, mice deficient in TLR4 showed intact platelet function compared to the platelet impairment seen in WT mice when subjected to HS-R, suggesting that HS-R may also modulate platelet function in a TLR4 dependent manner. Similar observations have been reported in sepsis models where LPS induced platelet aggregation was abolished by an anti-TLR4-blocking antibody or TLR4 knockout in mice.3,10,41
Interestingly, platelets were shown to accumulate in the liver and lungs during HS-R in a TLR4 dependent manner, which was consistent with an inductive effect of HS-R on liver and lung injury and IL-6 production. Previously, the infusion of LPS into animals and humans has been shown to induce severe thrombocytopenia and platelet aggregation formation in the lung and liver microvascular circulation,41 followed by degradation of the platelets and acute inflammation accompanied by tissue destruction.42,43 Furthermore, infused platelets from wild-type but not TLR4 knockout mice accumulate in the lung of LPS-treated wild-type mice.16 Despite the finding of increased CD62p expression and increased platelet sequestration following HS-R, a significant aggregation defect and coagulopathy was present, which may represent similar platelet response to severe sepsis, which presents with both microvascular thrombosis and systemic coagulopathy.16,39 Although we did not establish the ligand or mechanism of TLR4 activation in platelets in HS-R, we suggest in the present study that platelet TLR4 signaling is likely related to the cascade of endogenous mediators that has been extensively characterized.44–47 Candidates include any number of DAMP TLR4 agonists such as HMGB1 which is known to be involved in organ injury and inflammation in HS-R48 or even LPS which can escape from the gut in injury.49
The beneficial effects of TLR4 knockout could be related to reduced recruitment of activated platelets into lungs and liver, where they could cause tissue damage. Indeed, platelets have the capacity to release many different inflammatory mediators to affect local tissue and other inflammatory cells.1,2,7,8–12,14,28 In addition, platelets can adhere to neutrophils or monocytes to induce transcellular biosynthesis between the two cell types to produce mediators that each cell is unable to synthesize alone.50 These mediators could cause ample tissue dysfunction and systematic inflammation. In support of this, TLR4 knock-out animals had reduced neutrophil activity within the lung following HS-R compared to WT animals as measured by MPO levels.
In summary, we have shown for the first time that platelet TLR4 contributes to platelet function impairment, and the deposition of platelets in liver and lung following hemorrhage. Furthermore, deletion of TLR4 specifically from platelets prevents the increase of circulating IL-6, and liver and lung injury following by HS-R. These results suggest that TLR4 expression on platelets may play a previously unrecognized role in inflammatory signaling and that platelet TLR4 signaling may contribute a link between hemostasis and inflammation in HS-R.
Supplementary Material
Acknowledgments
The authors thank Richard L. Simmons, MD for review of the manuscript, Ms. Stacy Stull for technical assistance, and Jason L. Sperry, MD for assistance with thromboelastography.
Funding Sources: This work was supported by the National Institutes of Health (P50GM053789 to T.R.B, 5R01GM078238 and 5R01DK083752 to D.J.H, Trans-Agency Consortium for Trauma Induced Coagulopathy (TACTIC) UM1 HL120877-01 to the Department of Surgery, University of Pittsburgh); N.D. is supported by the National Natural Science Foundation of China (81272136), the Science and Technology Planning Project of Guangdong Province (2012J4100034) and the Medical Science and Technology Program of Guangzhou (20121A021001).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Smith TL, Weyrich AS. Platelets as central mediators of systemic inflammatory responses. Thromb Res. 2011;127:391–394. doi: 10.1016/j.thromres.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tweardy DJ, Khoshnevis MR, Yu B, Mastrangelo MA, Hardison EG, López JA. Essential role for platelets in organ injury and inflammation in resuscitated hemorrhagic shock. Shock. 2006;26:386–390. doi: 10.1097/01.shk.0000227907.56060.2b. [DOI] [PubMed] [Google Scholar]
- 3.Bauer E, Chanthaphavong RS, Sodhi CP, Hackam DJ, Billiar TR, Bauer PM. Genetic deletion of TLR4 on platelets attenuates experimental pulmonary hypertension. Circ Res. 2014 Mar 17; doi: 10.1161/CIRCRESAHA.114.303662. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 7.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–1905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 8.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Brown GT, McIntyre TM. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. J Immunol. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott T, Owens MD. Thrombocytes respond to lipopolysaccharide through Toll-like receptor-4, and MAP kinase and NF-kappaB pathways leading to expression of interleukin-6 and cyclooxygenase-2 with production of prostaglandin E2. Mol Immunol. 2008;45:1001–1008. doi: 10.1016/j.molimm.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Ståhl AL, Svensson M, Mörgelin M, et al. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108:167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 15.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 16.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 17.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 18.Benhamou Y, Favre J, Musette P, Renet S, Thuillez C, Richard V, et al. Toll-like receptors 4 contribute to endothelial injury and inflammation in hemorrhagic shock in mice. Crit Care Med. 2009;37:1724–1728. doi: 10.1097/CCM.0b013e31819da805. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 20.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, et al. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–417. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 21.McGhan LJ, Jaroszewski DE. The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury. 2012;43:129–136. doi: 10.1016/j.injury.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca M, et al. Intestinal epithelial TLR4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143:708–718. e1–e5. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohut LK, Darwiche SS, Brumfield JM, Frank AM, Billiar TR. Fixed volume or fixed pressure: a murine model of hemorrhagic shock. J Vis Exp. 2011;52:pii 2068. doi: 10.3791/2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieswandt B, Echtenacher B, Wachs FP, Schroder J, Gessner JE, Schmidt RE, et al. Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpIIb/IIIa antibody in mice. Blood. 1999;94:684–693. [PubMed] [Google Scholar]
- 25.Rumbaut RE, Bellera RV, Randhawa JK, Shrimpton CN, Dasgupta SK, Dong JF, et al. Endotoxin enhances microvascular thrombosis in mouse cremaster venules via a TLR4-dependent, neutrophil-independent mechanism. Am J Physiol Heart Circ Physiol. 2006;290:H1671–H1679. doi: 10.1152/ajpheart.00305.2005. [DOI] [PubMed] [Google Scholar]
- 26.Stark RJ, Aghakasiri N, Rumbaut RE. Platelet-derived Toll-like receptor 4 (Tlr-4) is sufficient to promote microvascular thrombosis in endotoxemia. PLoS One. 2012;7:e41254. doi: 10.1371/journal.pone.0041254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65:535–543. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 28.Zarbock A, Ley K. The role of platelets in acute lung injury (ALI) Front Biosci. 2009;14:150–158. doi: 10.2741/3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, Ohtaki Y, Yamaguchi K, Matsushita M, Fujita T, Yokochi T, et al. LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system. Blood. 2002;100:3233–3239. doi: 10.1182/blood-2002-01-0252. [DOI] [PubMed] [Google Scholar]
- 30.Modlin RL, Brightbill HD, Godowski PJ. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999;340:1834–1835. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- 31.Cognasse F, Lafarge S, Chavarin P, Acquart S, Garraud O. Lipopolysaccharide induces sCD40L release through human platelets TLR4, but not TLR2 and TLR9. Intensive Care Med. 2007;33:382–384. doi: 10.1007/s00134-006-0488-8. [DOI] [PubMed] [Google Scholar]
- 32.Subeq YM, Hsu BG, Lin NT, Yang FL, Chao YF, Peng TC, et al. Hypothermia caused by slow and limited-volume fluid resuscitation decreases organ damage by hemorrhagic shock. Cytokine. 2012;60:68–75. doi: 10.1016/j.cyto.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Lv KY, Yu XY, Bai YS, Zhu SH, Tang HT, Ben DF, et al. Role of inhibition of p38 mitogen-activated protein kinase in liver dysfunction after hemorrhagic shock and resuscitation. J Surg Res. 2012;178:827–832. doi: 10.1016/j.jss.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Nachman RL, Weksler B. The platelet as an inflammatory cell. Ann N Y Acad Sci. 1972;201:131–137. doi: 10.1111/j.1749-6632.1972.tb16294.x. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda T, Shiohara E. Leukocyte and platelet depletion protects the liver from damage induced by cholestasis and ischemia-reperfusion in the dog. Scand J Gastroenterol. 1996;31:182–190. doi: 10.3109/00365529609031984. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda T, Shiohara E, Homma T, Furukawa Y, Chiba S. Effects of leukocyte and platelet depletion on ischemia-reperfusion injury to dog pancreas. Gastroenterology. 1994;107:1125–1134. doi: 10.1016/0016-5085(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 37.Shimada Y, Kutsumi Y, Nishio H, Asazuma K, Tada H, Hayashi T, et al. Role of platelets in myocardial ischemia-reperfusion injury in dogs. Jpn Circ J. 1997;61:241–248. doi: 10.1253/jcj.61.241. [DOI] [PubMed] [Google Scholar]
- 38.Lefer AM, Campbell B, Scalia R, Lefer DJ. Synergism between platelets and neutrophils in provoking cardiac dysfunction after ischemia and reperfusion: role of selectins. Circulation. 1998;98:1322–1328. doi: 10.1161/01.cir.98.13.1322. [DOI] [PubMed] [Google Scholar]
- 39.Alves-Filho JC. Toll-like receptors on platelets: the key for disseminated intravascular coagulation in sepsis? Thromb Res. 2005;115:537–538. doi: 10.1016/j.thromres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Funayama H, Huang L, Sato T, Ohtaki Y, Asada Y, Yokochi T, et al. Pharmacological characterization of anaphylaxis-like shock responses induced in mice by mannan and lipopolysaccharide. Int Immunopharmacol. 2009;9:1518–1524. doi: 10.1016/j.intimp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Montrucchio G, Bosco O, Del Sorbo L, Fascio Pecetto P, Lupia E, Goffi A, et al. Mechanisms of the priming effect of low doses of lipopolysaccharides on leukocyte-dependent platelet aggregation in whole blood. Thromb Haemost. 2003;90:872–881. doi: 10.1160/TH03-02-0085. [DOI] [PubMed] [Google Scholar]
- 42.Kawabata Y, Yang TS, Yokochi TT, Matsushita M, Fujita T, Shibazaki M, et al. Complement system is involved in anaphylactoid reaction induced by lipopolysaccharides in muramyldipeptide treated mice. Shock. 2000;14:572–577. doi: 10.1097/00024382-200014050-00013. [DOI] [PubMed] [Google Scholar]
- 43.Ohba M, Shibazaki M, Sasano T, Inoue M, Takada H, Endo Y. Platelet responses and anaphylaxis- like shock induced in mice by intravenous injection of whole cells of oral streptococci. Oral Micro Immunol. 2004;19:26–30. doi: 10.1046/j.0902-0055.2002.00107.x. [DOI] [PubMed] [Google Scholar]
- 44.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 45.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 47.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1–H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 50.Marcus AJ, Safier LB, Ullman HL, Broekman MJ, Islam N, Oglesby TD, et al. 12S,20-dihydroxyicosatetraenoic acid: a new icosanoid synthesized by neutrophils from 12S-hydroxyicosatetraenoic acid produced by thrombin- or collagen- stimulated platelets. Proc Natl Acad Sci U S A. 1984;81:903–907. doi: 10.1073/pnas.81.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.