Abstract
We conducted a prospective, observational study of aspirin and COX-2 inhibitor use and survival in stage III colon cancer patients enrolled in an adjuvant chemotherapy trial. Among 799 eligible patients, aspirin use was associated with improved recurrence-free survival (RFS) (multivariable hazard ratio [HR] = 0.51, 95% confidence interval [CI] = 0.28 to 0.95), disease-free survival (DFS) (HR = 0.68, 95% CI = 0.42 to 1.11), and overall survival (OS) (HR = 0.63, 95% CI = 0.35 to 1.12). Adjusted HRs for DFS and OS censored at five years (in an attempt to minimize misclassification from noncancer death) were 0.61 (95% CI = 0.36 to 1.04) and 0.48 (95% CI = 0.23 to 0.99). Among 843 eligible patients, those who used COX-2 inhibitors had multivariable HRs for RFS, DFS, and OS of 0.53 (95% CI = 0.27 to 1.04), 0.60 (95% CI = 0.33 to 1.08), and 0.50 (95% CI = 0.23 to 1.07), and HRs of 0.47 (95% CI = 0.24 to 0.91) and 0.26 (95% CI = 0.08 to 0.81) for DFS and OS censored at five years. Aspirin and COX-2 inhibitor use may be associated with improved outcomes in stage III colon cancer patients.
Randomized trials support the efficacy of aspirin and COX-2 (cyclooxygenase; prostaglandin-endoperoxide synthase-2 [PTGS2]) inhibitors in reducing adenoma and cancer risk in patients with familial colorectal cancer (CRC) syndromes (1–9). Meta-analyses of randomized cardiovascular disease (CVD) prevention trials confirm the protective effect of aspirin against CRC (10–12), and observational studies report improved survival with postdiagnosis aspirin use (13–16). To test the hypothesis that aspirin and COX-2 inhibitors may be effective in the adjuvant setting, we conducted a prospective analysis of aspirin and COX-2 inhibitor use in stage III colon cancer patients enrolled in CALGB 89803 (1999–2001) (17). In an abstract, we reported improved recurrence-free (RFS), disease-free (DFS), and overall survival (OS) associated with these medications with a median follow-up of 2.7 years (18). These findings led to two ongoing phase III trials, Alliance for Clinical Trials in Oncology study CALGB 80702 and the Aspirin for Dukes C and High Risk Dukes B Colorectal Cancers study (ASCOLT) (19), but results are not expected for many years. Herein we report updated findings from CALGB 89803 with mature follow-up.
CALGB 89803 compared fluorouracil (FU) and leucovorin (LV) with irinotecan, FU, and LV for adjuvant treatment of American Joint Committee on Cancer stage III colon cancer and found no statistically significant difference in outcome (17). A self-administered questionnaire assessing diet, lifestyle, and medication use was conducted midway through chemotherapy (Q1) and six months after chemotherapy (Q2). Consistent aspirin use was defined as any aspirin use reported on both Q1 and Q2, and COX-2 inhibitor use as any use reported on Q2 (Supplementary Figure 1, available online). All patients signed informed consent, approved by each institution’s review board.
RFS was calculated as the time from Q2 completion to tumor recurrence, death with recurrence, or development of a new invasive colon cancer (n = 1). DFS was defined as time from Q2 to tumor recurrence, occurrence of a new colon cancer, or death from any cause. OS was defined as time from Q2 to death from any cause. Survival was examined using Kaplan-Meier curves (20) and the log-rank test (21). Cox proportional hazards regression was used to simultaneously adjust for potential confounders (22); proportionality of hazards assumption was satisfied by time-dependent covariables and the Schoenfeld residuals method. Because colon cancer recurrences and deaths are rare after five years (23), we conducted a secondary analysis with DFS and OS events censored at five years to minimize misclassification because of noncancer deaths. Statistical significance was at the .05 level with two-sided P values.
Among 799 patients who responded to the aspirin question, 75 (9.4%) reported use both during and after chemotherapy. Consistent aspirin users were older and more likely to be male. Among 843 patients with data on COX-2 inhibitor use, 59 (7.0%) reported use after chemotherapy. COX-2 inhibitor users were less likely to have a family history of cancer, had higher body mass index, and reported more acetaminophen use (Supplementary Table 1, available online).
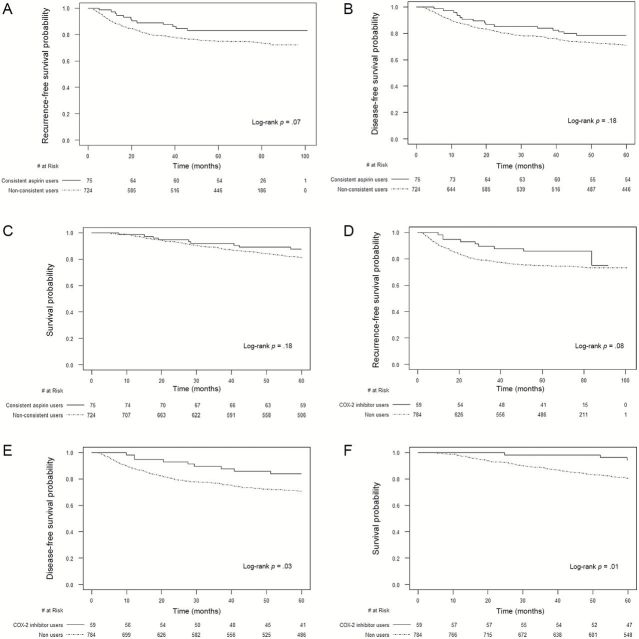
After a median follow-up of 6.5 years, consistent aspirin use was associated with improved RFS (83.1% vs 74.9% at five years, adjusted hazard ratio [HR] = 0.51, 95% confidence interval [CI] = 0.28 to 0.95) (Figure 1A), DFS (78.4% vs 71.1%, HR = 0.68, 95% CI = 0.42 to 1.11), and OS (87.6% vs 80.9%, HR = 0.63, 95% CI = 0.35 to 1.12) (Table 1). HRs for DFS and OS censored at five years were 0.61 (95% CI = 0.36 to 1.04) and 0.48 (95% CI = 0.23 to 0.99) (Table 1; Figure 1, B and C). Patients who used five or more tablets/week had an HR of 0.69 (95% CI = 0.41 to 1.18) compared with nonusers (P trend = .15). COX-2 inhibitor use was also associated with improved RFS (85.6% vs 74.5% at five years, HR = 0.53, 95 % CI = 0.27 to 1.04) (Figure 1D), DFS (83.8% vs 70.6%, HR = 0.60, 95% CI = 0.33 to 1.08), and OS (94.3% vs 80.3%, HR = 0.50, 95% CI = 0.23 to 1.07) (Table 1). Results were stronger when events were censored at five years (HR for DFS = 0.47, 95% CI = 0.24 to 0.91; HR for OS = 0.26, 95% CI = 0.08 to 0.81) (Table 1; Figure 1, E and F). Patients who used one to four tablets/week had an HR of 0.59 (95% CI = 0.22 to 1.59) and those who used five or more tablets/week had an HR of 0.50 (95% CI = 0.21 to 1.23) compared with nonusers (P trend = .14). No statistically significant interactions between aspirin and COX-2 inhibitor use and other covariables were seen for cancer recurrence (Supplementary Tables 2 and 3, available online). There was also no statistically significant increase in cardiovascular events or grade 3 or higher toxicities (17) with medication use, except for leukopenia (P = .008), for unclear reasons (Supplementary Table 4, available online).
Figure 1.
Recurrence-free, disease-free, and overall survival by aspirin or COX-2 inhibitor use. Survival curves were generated by the Kaplan-Meier method and two-sided P values calculated using the log-rank test. A) Recurrence-free survival according to consistent aspirin use. B) Disease-free survival (death events censored at five years) according to consistent aspirin use. C) Overall survival (death events censored at five years) according to consistent aspirin use. D) Recurrence-free survival according to COX-2 inhibitor use. E) Disease-free survival (death events censored at five years) according to COX-2 inhibitor use. F) Overall survival (death events censored at five years) according to COX-2 inhibitor use.
Table 1.
Relationship between aspirin and COX-2 inhibitor use and colon cancer recurrence and mortality
| Endpoint | Consistent aspirin use* | COX-2 inhibitor use† | ||||||
|---|---|---|---|---|---|---|---|---|
| All subjects | Events censored at 5 y‡ | All subjects | Events censored at 5 y‡ | |||||
| No | Yes | No | Yes | No | Yes | No | Yes | |
| Cancer recurrence (recurrence-free survival) | ||||||||
| No. at risk | 724 | 75 | -- | -- | 784 | 59 | -- | -- |
| No. of events | 182 | 12 | -- | -- | 198 | 9 | -- | -- |
| Unadjusted HR (95% CI) | Referent | 0.59 (0.33 to 1.05) | -- | -- | Referent | 0.55 (0.28 to 1.08) | -- | -- |
| Multivariable-adjusted HR (95% CI)§ | Referent | 0.51 (0.28 to 0.95) | -- | -- | Referent | 0.53 (0.27 to 1.04) | -- | -- |
| Cancer recurrence or death from any cause (disease-free survival) | ||||||||
| No. at risk | 724 | 75 | 724 | 75 | 784 | 59 | 784 | 59 |
| No. of events | 214 | 19 | 203 | 16 | 237 | 12 | 224 | 9 |
| Unadjusted HR (95% CI) | Referent | 0.78 (0.49 to 1.25) | Referent | 0.71 (0.43 to 1.18) | Referent | 0.62 (0.34 to 1.10) | Referent | 0.49 (0.25 to 0.95) |
| Multivariable-adjusted HR (95% CI)§ | Referent | 0.68 (0.42 to 1.11) | Referent | 0.61 (0.36 to 1.04) | Referent | 0.60 (0.33 to 1.08) | Referent | 0.47 (0.24 to 0.91) |
| Overall mortality (overall survival) | ||||||||
| No. at risk | 724 | 75 | 724 | 75 | 784 | 59 | 784 | 59 |
| No. of events | 156 | 14 | 131 | 9 | 179 | 7 | 147 | 3 |
| Unadjusted HR (95% CI) | Referent | 0.80 (0.46 to 1.39) | Referent | 0.63 (0.32 to 1.24) | Referent | 0.48 (0.23 to 1.02) | Referent | 0.25 (0.08 to 0.79) |
| Multivariable-adjusted HR (95% CI)§ | Referent | 0.63 (0.35 to 1.12) | Referent | 0.48 (0.23 to 0.99) | Referent | 0.50 (0.23 to 1.07) | Referent | 0.26 (0.08 to 0.81) |
* Consistent aspirin use defined as any aspirin use reported both during (Questionnaire 1) and after completion of adjuvant chemotherapy (Questionnaire 2). CI = confidence interval; COX = cyclooxygenase; HR = hazard ratio.
† COX-2 inhibitor use defined as any use of celecoxib or rofecoxib reported after completion of chemotherapy (Questionnaire 2).
‡ Secondary analysis performed with death events censored at five years in order to minimize misclassification because of non–cancer-related causes of death.
§ Adjusted for age (in years as a continuous variable), sex (male or female), race (white, African American, or other), adjuvant treatment arm (IFL or FU/LV), family history of colorectal cancer (yes or no), baseline performance status (0 or 1–2), depth of invasion through bowel wall (T1-2 or T3-4), number of positive lymph nodes (1–3 or 4 or more), grade of tumor differentiation (well differentiated, moderately differentiated, or poorly differentiated/undifferentiated), body mass index at time of Questionnaire 2 (in kg/m2 as a continuous variable), and physical activity at time of Questionnaire 2 (in MET-hr/week as a continuous variable).
This analysis of enrolled in a chemotherapy clinical trial is an important addition to the literature, which supports a benefit for aspirin use after CRC diagnosis (13–16,24). The exact dose and duration of aspirin or COX-2 inhibitors required for a potential protective effect remains unclear, however. Although the P trend was not statistically significant, our study suggests a dose-response relationship with increased frequency of aspirin use, while any amount of COX-2 inhibitors was associated with decreased recurrence, consistent with previous reports (3,10,11,15,24–28).
Current knowledge of the biological pathways affected by these medications provides strong support for our findings. Aspirin inhibits PTGS (COX), which converts arachidonic acid to prostaglandins, which modulate tumor growth through alteration of stem cell gene expression (29), hypermethylation of genes involved in proliferation and differentiation (29,30), promotion of angiogenesis and WNT/CTNNB1 signaling (31,32), and inhibition of apoptosis (33–35), among others. Molecular pathological epidemiology studies also report differential benefit depending on BRAF (36) or PIK3CA mutation status (37–39) and tumoral PTGS2 (13) or HLA class I antigen expression (39); results are conflicting and require further study. Unfortunately, we had insufficient power to explore these interactions.
Other limitations of our study include misclassification of the exposure from self-reported data, however, prior studies have demonstrated the reliability of such data (40). Moreover, medication use was recorded before any knowledge of cancer outcomes, thus minimizing reporting biases. Also, we were unable to assess prediagnostic use and total duration of use in this study. Patients who enroll in clinical trials often engage in other healthful behaviors, so we controlled for physical activity, body mass index, and performance status, but residual confounding from unknown variables is possible.
In conclusion, this observational study of stage III colon cancer patients found statistically significant associations between aspirin and COX-2 inhibitor use and reduced cancer recurrence and mortality. Results from the ongoing CALGB 80702 and ASCOLT trials are eagerly awaited. Further exploration of predictive biomarkers of aspirin and COX-2 inhibitor activity is warranted.
Funding
This work was supported by the National Cancer Institute, National Institutes of Health (P50 CA127003 and K07 CA148894 to KN), the Conquer Cancer Foundation of the American Society of Clinical Oncology (to KN), the Damon Runyan Cancer Research Foundation (to ATC), and the Pharmacia and Upjohn Company, now Pfizer Oncology. The research for CALGB 89803 was also supported, in part, by grants from the National Cancer Institute, National Institutes of Health (CA31946) to the Alliance for Clinical Trials in Oncology (Monica Bertagnolli, MD, Chairman) and to the Alliance Statistics and Data Center (Daniel J. Sargent, PhD, CA33601).
Supplementary Material
The research presented in this manuscript was previously presented, in part, at the American Society of Clinical Oncology Annual Meeting in 2005.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health, Conquer Cancer Foundation of the American Society of Clinical Oncology, or the Damon Runyan Cancer Research Foundation.
The study funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
References
- 1. Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–1952. [DOI] [PubMed] [Google Scholar]
- 2. Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. [DOI] [PubMed] [Google Scholar]
- 3. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. [DOI] [PubMed] [Google Scholar]
- 4. Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. [DOI] [PubMed] [Google Scholar]
- 5. Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. [DOI] [PubMed] [Google Scholar]
- 6. Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. [DOI] [PubMed] [Google Scholar]
- 7. Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. [DOI] [PubMed] [Google Scholar]
- 8. Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. [DOI] [PubMed] [Google Scholar]
- 9. Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. [DOI] [PubMed] [Google Scholar]
- 11. Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. [DOI] [PubMed] [Google Scholar]
- 12. Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–1612. [DOI] [PubMed] [Google Scholar]
- 13. Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. Jama. 2009;302(6):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reimers MS, Bastiaannet E, van Herk-Sukel MP, et al. Aspirin use after diagnosis improves survival in older adults with colon cancer: a retrospective cohort study. J Am Geriatr Soc. 2012;60(12):2232–2236. [DOI] [PubMed] [Google Scholar]
- 15. Bastiaannet E, Sampieri K, Dekkers OM, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012; 2012/03/29:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walker AJ, Grainge MJ, Card TR. Aspirin and other non-steroidal anti-inflammatory drug use and colorectal cancer survival: a cohort study. Br J Cancer. 2012;107(9):1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25(23):3456–3461. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs CS, Meyerhardt JA, Brady D, et al. Influence of regular aspirin use on survival for patients with stage III colon cancer: Findings from Intergroup trial CALGB 89803. J Clin Oncol. 2005;23(Suppl 16):Abstract 3530. [Google Scholar]
- 19. Ali R, Toh HC, Chia WK. The utility of Aspirin in Dukes C and High Risk Dukes B Colorectal cancer--the ASCOLT study: study protocol for a randomized controlled trial. Trials. 2011;12:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 21. Therneau T, Grambsch P. Modeling survival data. New York, NY: Springer; 2000. [Google Scholar]
- 22. Jones MP, Crowley J. A general class of nonparametric tests for survival analysis. Biometrics. 1989;45(1):157–170. [PubMed] [Google Scholar]
- 23. Sadahiro S, Suzuki T, Ishikawa K, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepato-gastroenterology. 2003;50(53):1362–1366. [PubMed] [Google Scholar]
- 24. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–1601. [DOI] [PubMed] [Google Scholar]
- 25. Zell JA, Ziogas A, Bernstein L, et al. Nonsteroidal anti-inflammatory drugs: effects on mortality after colorectal cancer diagnosis. Cancer. 2009;115(24):5662–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. Jama. 2005;294(1):47–55. [DOI] [PubMed] [Google Scholar]
- 27. Chan AT, Manson JE, Feskanich D, Stampfer MJ, Colditz GA, Fuchs CS. Long-term aspirin use and mortality in women. Arch Intern Med. 2007;167(6):562–572. [DOI] [PubMed] [Google Scholar]
- 28. Coghill AE, Newcomb PA, Campbell PT, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60(4):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herfs M, Hubert P, Delvenne P. Epithelial metaplasia: adult stem cell reprogramming and (pre)neoplastic transformation mediated by inflammation? Trends Mol Med. 2009;15(6):245–253. [DOI] [PubMed] [Google Scholar]
- 30. Hahn MA, Hahn T, Lee DH, et al. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68(24):10280–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310(5753):1504–1510. [DOI] [PubMed] [Google Scholar]
- 34. Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114(11):1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pozzi A, Yan X, Macias-Perez I, et al. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279(28):29797–29804. [DOI] [PubMed] [Google Scholar]
- 36. Nishihara R, Lochhead P, Kuchiba A, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. Jama. 2013;309(24):2563–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Domingo E, Church DN, Sieber O, et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31(34):4297–4305. [DOI] [PubMed] [Google Scholar]
- 39. Reimers MS, Bastiaannet E, Langley RE, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA internal medicine. 2014;174(5):732–739. [DOI] [PubMed] [Google Scholar]
- 40. Satia-Abouta J, Patterson RE, King IB, et al. Reliability and validity of self-report of vitamin and mineral supplement use in the vitamins and lifestyle study. Am J Epidemiol. 2003;157(10):944–954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.