Abstract
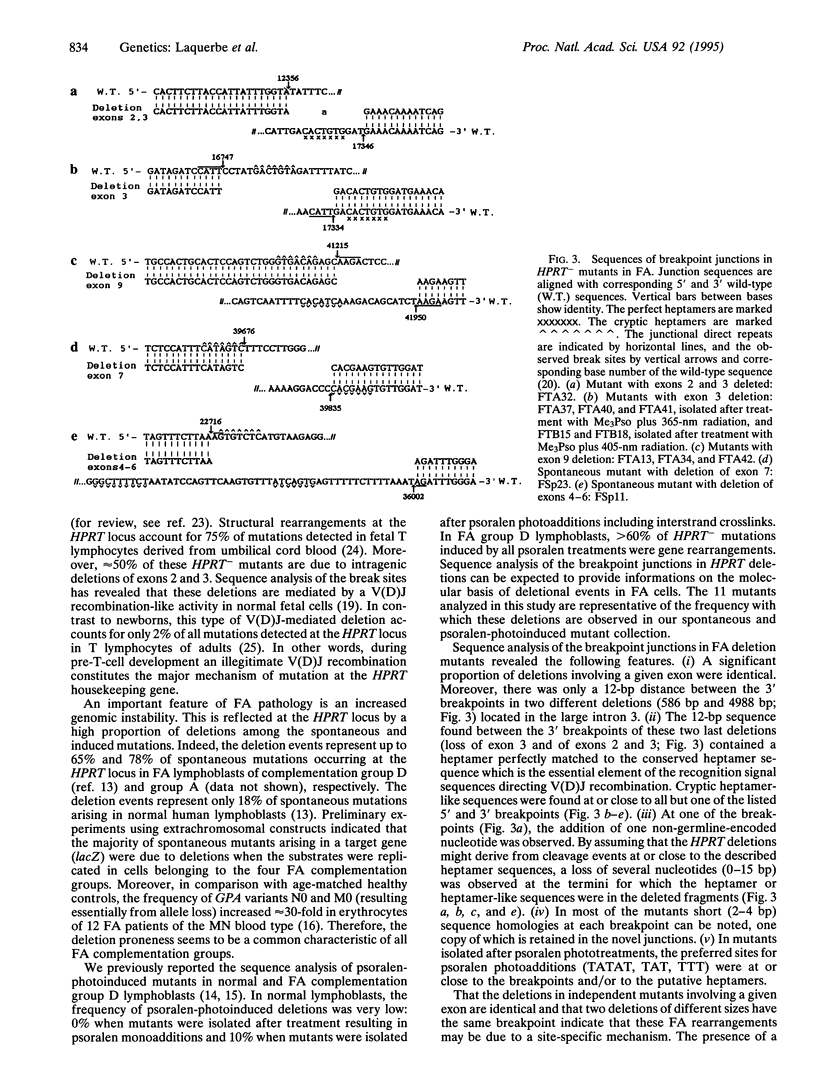
Spontaneous and induced chromosomal breakage is an important cellular feature of Fanconi anemia (FA), an inherited DNA repair disorder characterized by progressive bone marrow failure, developmental abnormalities, and predisposition to leukemia. We have previously reported that in comparison to normal cells, there is a substantial increase in frequency of intragenic deletions at an endogenous locus (HPRT) in FA lymphoblasts. Taken together with the increased chromosomal instability, these observations indicated that the wild-type FA gene(s) plays an important role in the maintenance of the genomic integrity. To obtain information on the mechanism(s) underlying the genomic rearrangements in FA, the breakpoint sites of deletions in 11 FA-derived HPRT- mutants were analyzed. The results indicate that a significant proportion of deletions involving a loss of a given exon are identical and that two deletions of different size have the same 3' breakpoint. Interestingly, it appears that in most of the mutants there is a common deletion signal sequence, which suggests that the mutations in the FA gene(s) may lead to an aberrant site-specific cleavage activity that might be responsible for the deletion proneness and the chromosomal instability characteristic of the FA pathology. From the similarity or even identity of the signal sequence at some of the breakpoints with the consensus heptamer which directs cleavage and joining in the assembly of immunoglobulin and T-cell receptor genes, we speculate that steps in common with the V(D)J recombinational process may be illegitimately involved in FA cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Auerbach A. D., Wolman S. R. Susceptibility of Fanconi's anaemia fibroblasts to chromosome damage by carcinogens. Nature. 1976 Jun 10;261(5560):494–496. doi: 10.1038/261494a0. [DOI] [PubMed] [Google Scholar]
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma D. The genetic defect in DNA repair deficiency syndromes. EACR--Mühlbock Memorial Lecture, 1993. Eur J Cancer. 1993;29A(10):1482–1488. doi: 10.1016/0959-8049(93)90026-c. [DOI] [PubMed] [Google Scholar]
- Edwards A., Voss H., Rice P., Civitello A., Stegemann J., Schwager C., Zimmermann J., Erfle H., Caskey C. T., Ansorge W. Automated DNA sequencing of the human HPRT locus. Genomics. 1990 Apr;6(4):593–608. doi: 10.1016/0888-7543(90)90493-e. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Harrington-Brock K., Burnette L., Moore M. M., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-mediated deletion of the hprt gene in T-lymphocytes from adult humans. Mutat Res. 1992 Sep;283(1):13–20. doi: 10.1016/0165-7992(92)90116-y. [DOI] [PubMed] [Google Scholar]
- Fuscoe J. C., Zimmerman L. J., Lippert M. J., Nicklas J. A., O'Neill J. P., Albertini R. J. V(D)J recombinase-like activity mediates hprt gene deletion in human fetal T-lymphocytes. Cancer Res. 1991 Nov 1;51(21):6001–6005. [PubMed] [Google Scholar]
- Gellert M. Molecular analysis of V(D)J recombination. Annu Rev Genet. 1992;26:425–446. doi: 10.1146/annurev.ge.26.120192.002233. [DOI] [PubMed] [Google Scholar]
- Gerstein R. M., Lieber M. R. Extent to which homology can constrain coding exon junctional diversity in V(D)J recombination. Nature. 1993 Jun 17;363(6430):625–627. doi: 10.1038/363625a0. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., Edwards A., Civitello A. B., Caskey C. T. Multiplex DNA deletion detection and exon sequencing of the hypoxanthine phosphoribosyltransferase gene in Lesch-Nyhan families. Genomics. 1990 Jun;7(2):235–244. doi: 10.1016/0888-7543(90)90545-6. [DOI] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillouf C., Laquerbe A., Moustacchi E., Papadopoulo D. Mutagenic processing of psoralen monoadducts differ in normal and Fanconi anemia cells. Mutagenesis. 1993 Jul;8(4):355–361. doi: 10.1093/mutage/8.4.355. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Cooper D. N. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991 Mar;86(5):425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gellert M. The mechanism of antigen receptor gene assembly. Cell. 1989 Nov 17;59(4):585–588. doi: 10.1016/0092-8674(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Lieber M. R. Site-specific recombination in the immune system. FASEB J. 1991 Nov;5(14):2934–2944. doi: 10.1096/fasebj.5.14.1752360. [DOI] [PubMed] [Google Scholar]
- McGinniss M. J., Nicklas J. A., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes: IV. Studies in newborns. Environ Mol Mutagen. 1989;14(4):229–237. doi: 10.1002/em.2850140404. [DOI] [PubMed] [Google Scholar]
- Motejlek K., Schindler D., Assum G., Krone W. Increased amount and contour length distribution of small polydisperse circular DNA (spcDNA) in Fanconi anemia. Mutat Res. 1993 Mar;293(3):205–214. doi: 10.1016/0921-8777(93)90071-n. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Papadopoulo D., Guillouf C., Mohrenweiser H., Moustacchi E. Hypomutability in Fanconi anemia cells is associated with increased deletion frequency at the HPRT locus. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8383–8387. doi: 10.1073/pnas.87.21.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulo D., Laquerbe A., Guillouf C., Moustacchi E. Molecular spectrum of mutations induced at the HPRT locus by a cross-linking agent in human cell lines with different repair capacities. Mutat Res. 1993 Aug;294(2):167–177. doi: 10.1016/0921-8777(93)90025-c. [DOI] [PubMed] [Google Scholar]
- Papadopoulo D., Porfirio B., Moustacchi E. Mutagenic response of Fanconi's anemia cells from a defined complementation group after treatment with photoactivated bifunctional psoralens. Cancer Res. 1990 Jun 1;50(11):3289–3294. [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E., Moustacchi E. Sequence context effects on 8-methoxypsoralen photobinding to defined DNA fragments. Biochemistry. 1987 Jun 16;26(12):3307–3314. doi: 10.1021/bi00386a010. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat M., Boyse J., Richard P., Papadopoulo D., Moustacchi E. Frequencies of HPRT- lymphocytes and glycophorin A variants erythrocytes in Fanconi anemia patients, their parents and control donors. Mutat Res. 1993 Sep;289(1):115–126. doi: 10.1016/0027-5107(93)90137-5. [DOI] [PubMed] [Google Scholar]
- Schroeder T. M., Anschütz F., Knopp A. Spontane Chromosomenaberrationen bei familiärer Panmyelopathie. Humangenetik. 1964;1(2):194–196. doi: 10.1007/BF00389636. [DOI] [PubMed] [Google Scholar]
- Schuler W., Bosma M. J. Nature of the scid defect: a defective VDJ recombinase system. Curr Top Microbiol Immunol. 1989;152:55–62. doi: 10.1007/978-3-642-74974-2_8. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Duncan A. M., Buchwald M. Evidence for at least four Fanconi anaemia genes including FACC on chromosome 9. Nat Genet. 1992 Jun;1(3):196–198. doi: 10.1038/ng0692-196. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Gavish H., Shannon W. R., Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992 Apr 30;356(6372):763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Toda M., Hirama T., Takeshita S., Yamagishi H. Excision products of immunoglobulin gene rearrangements. Immunol Lett. 1989 Jun 15;21(4):311–316. doi: 10.1016/0165-2478(89)90025-4. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Jones B. K., Capraro M., Chu T. The repair of psoralen monoadducts by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1987 Jun 25;15(12):4957–4971. doi: 10.1093/nar/15.12.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]



