Abstract
Digitonin - fractionated photosystem - I subchloroplasts were titrated potentiometrically between -450 and -610 mV at pH 10. Examination of the titrated subchloroplasts by low-temperature (13°K) electron paramagnetic resonance spectroscopy revealed resonances centered at values of 2.05, 1.94, 1.92, 1.89, and 1.86 on the g-factor scale. The peak heights depended on the potentials at which the chloroplasts were poised. The resonances of at least three iron-sulfur centers can be recognized: one with lines at g = 2.05 and 1.94; one with lines at g = 2.05, 1.92, and 1.89; and one for which only a line at g = 1.86 has been resolved. The midpoint potentials of the iron-sulfur species fall into two distinctly separate regions: the titration profile of the g = 1.94 signal, the first segment of the g = 2.05 plot, and the rise phase of the g = 1.86 signal had a value of -530 ± 5 mV; the upper segment of the g = 2.05 plot, the decrease phase of the g = 1.86 signal, and the g = 1.89 profile had a midpoint potential estimated to be [unk] -580 mV. The oxidation-reduction reaction of each of the bound iron-sulfur species, as represented by the changes of the electron paramagnetic resonance spectra, was reversible and apparently involved a two-electron change.
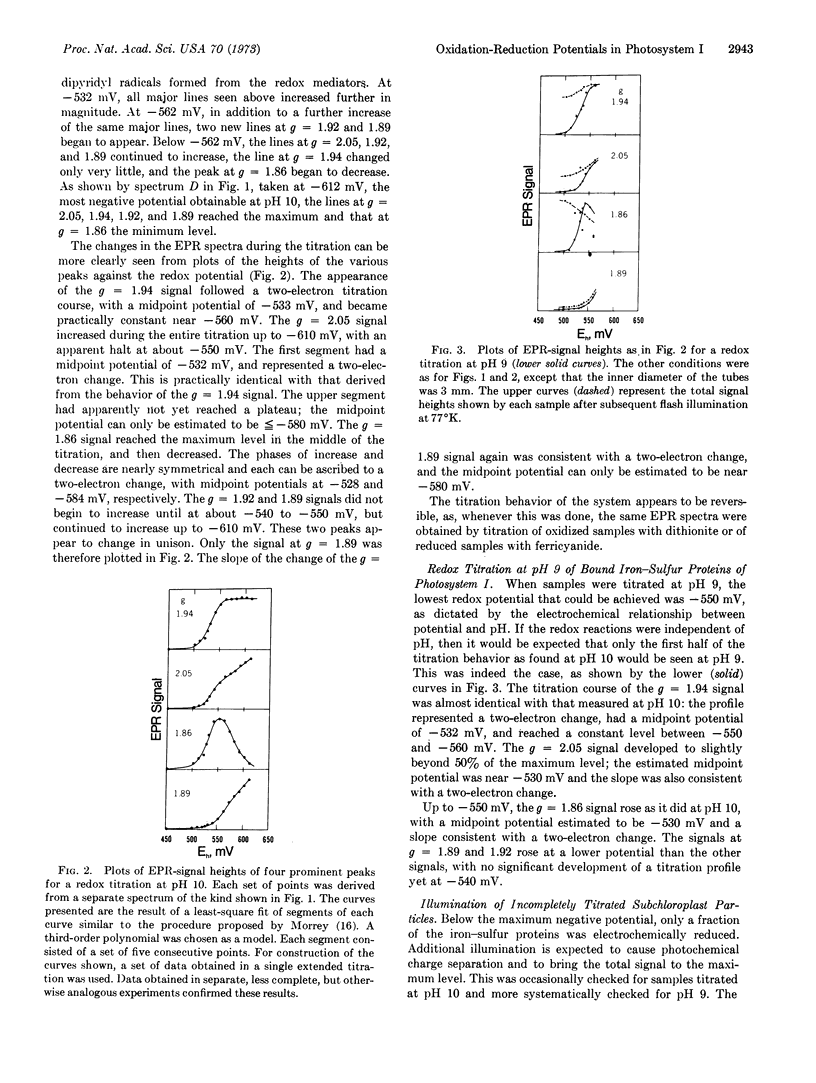
Titration at pH 9 could only be carried to -560 mV, and essentially only the first half of the titration behavior as found at pH 10 was seen. At any given potential more positive than -560 mV, the part of the iron-sulfur protein that was not reduced electrochemically could be reduced photochemically, but only to the maximum extent reduced electrochemically at -560 mV. Whereas, chloroplasts illuminated at room temperature and then frozen while still being illuminated developed a signal similar to that produced by electrochemical reduction at -610 mV, illumination at 77°K did not bring about photoreduction beyond that accomplished electrochemically at about -560 mV.
Dithionite alone in the dark and under anaerobic conditions brought about a partial reduction to the extent of the first electrochemical reduction step. Dithionite plus illumination at room temperature or dithionite plus methyl viologen in the dark produced the maximum signal. Electron paramagnetic resonance spectra due to either light or electrochemically reduced iron-sulfur proteins showed no detectable decay for at least 3 days when samples were stored in the dark at 77°K.
Keywords: photosynthesis, primary electron acceptor, P700, P430, electron paramagnetic resonance
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden A. J., Malkin R. Quantitative EPR studies of the primary reaction of photosystem I in chloroplasts. Biochim Biophys Acta. 1972 Dec 14;283(3):456–468. doi: 10.1016/0005-2728(72)90262-9. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. The bound ferredoxin of chloroplasts: a role as the primary electron acceptor of photosystem I. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1299–1305. doi: 10.1016/s0006-291x(72)80116-5. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. A further study of P430: a possible primary electron acceptor of photosystem I. Arch Biochem Biophys. 1971 Nov;147(1):99–108. doi: 10.1016/0003-9861(71)90314-6. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. A new photosynthetic pigment, "P430": its possible role as the priary electron acceptor of photosystem I. Proc Natl Acad Sci U S A. 1971 May;68(5):1010–1013. doi: 10.1073/pnas.68.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B., Beinert H. Evidence for the identity of P430 of Photosystem I and chloroplast-bound iron-sulfur protein. Biochim Biophys Acta. 1973 Jun 28;305(3):689–693. doi: 10.1016/0005-2728(73)90094-7. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Ke B. The rise time of photoreduction, difference spectrum, and oxidation-reduction potential of P430. Arch Biochem Biophys. 1972 Sep;152(1):70–77. doi: 10.1016/0003-9861(72)90194-4. [DOI] [PubMed] [Google Scholar]
- Ke B., Vernon L. P., Chaney T. H. Photoreduction of cytochrome b 559 in a photosystem-II subchloroplast particle. Biochim Biophys Acta. 1972 Feb 28;256(2):345–357. doi: 10.1016/0005-2728(72)90065-5. [DOI] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Dutton P. L. The primary electron acceptor in photosynthesis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):414–421. doi: 10.1016/s0006-291x(72)80154-2. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Orme-Johnson W. H., Hansen R. E., Beinert H., Hatefi Y. EPR detectable electron acceptors in submitochondrial particles from beef heart with special reference to the iron-sulfur components of DPNH-ubiquinone reductase. Biochem Biophys Res Commun. 1971 Jul 16;44(2):446–452. doi: 10.1016/0006-291x(71)90621-8. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Heterogeneity of paramagnetic species in two iron-sulfur proteins: Clostridium pasteurianum ferredoxin and milk xanthine oxidase. Biochem Biophys Res Commun. 1969 Aug 7;36(3):337–344. doi: 10.1016/0006-291x(69)90569-5. [DOI] [PubMed] [Google Scholar]
- Orme-Johson W. H., Beinert H. Anaerobic reductive titrations with solid diluted sodium dithionite in an apparatus suitable for EPR spectroscopy. Anal Biochem. 1969 Dec;32(3):425–435. doi: 10.1016/s0003-2697(69)80010-2. [DOI] [PubMed] [Google Scholar]
- Yang C. S., Blumberg W. E. Quantitative studies on the EPR signals of photosynthetic system I and ferredoxin. Biochem Biophys Res Commun. 1972 Jan 31;46(2):422–428. doi: 10.1016/s0006-291x(72)80155-4. [DOI] [PubMed] [Google Scholar]


