Abstract
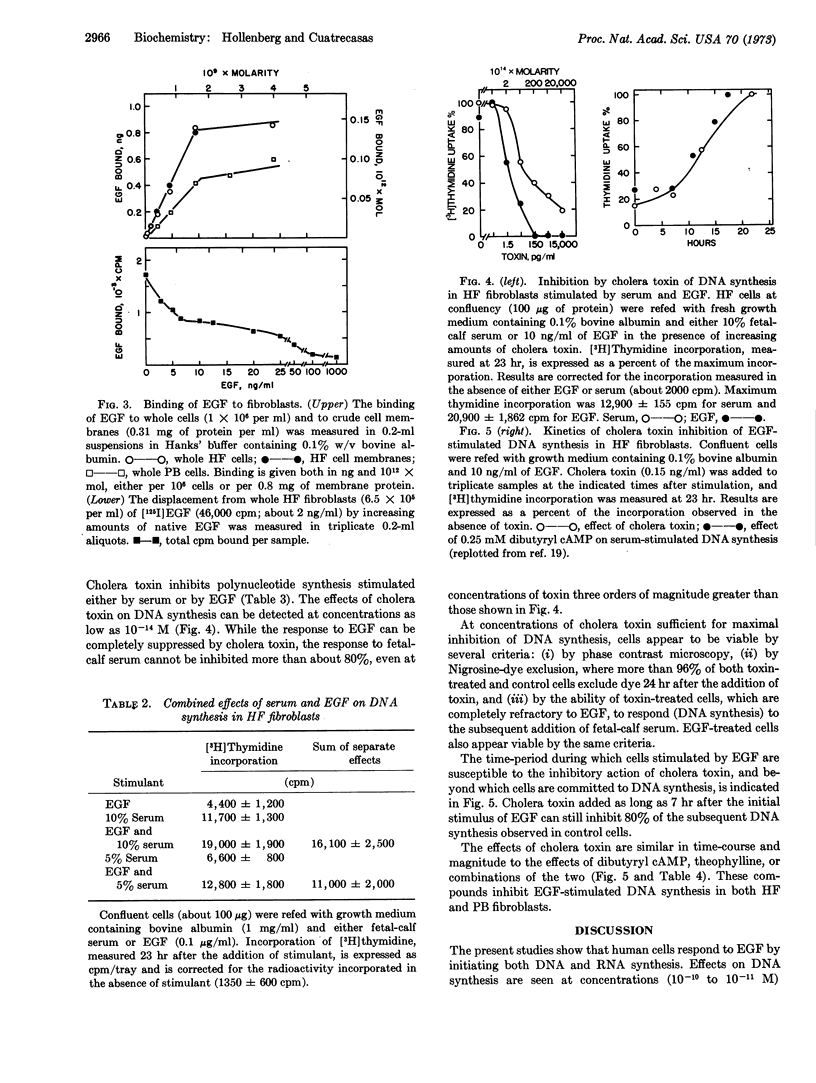
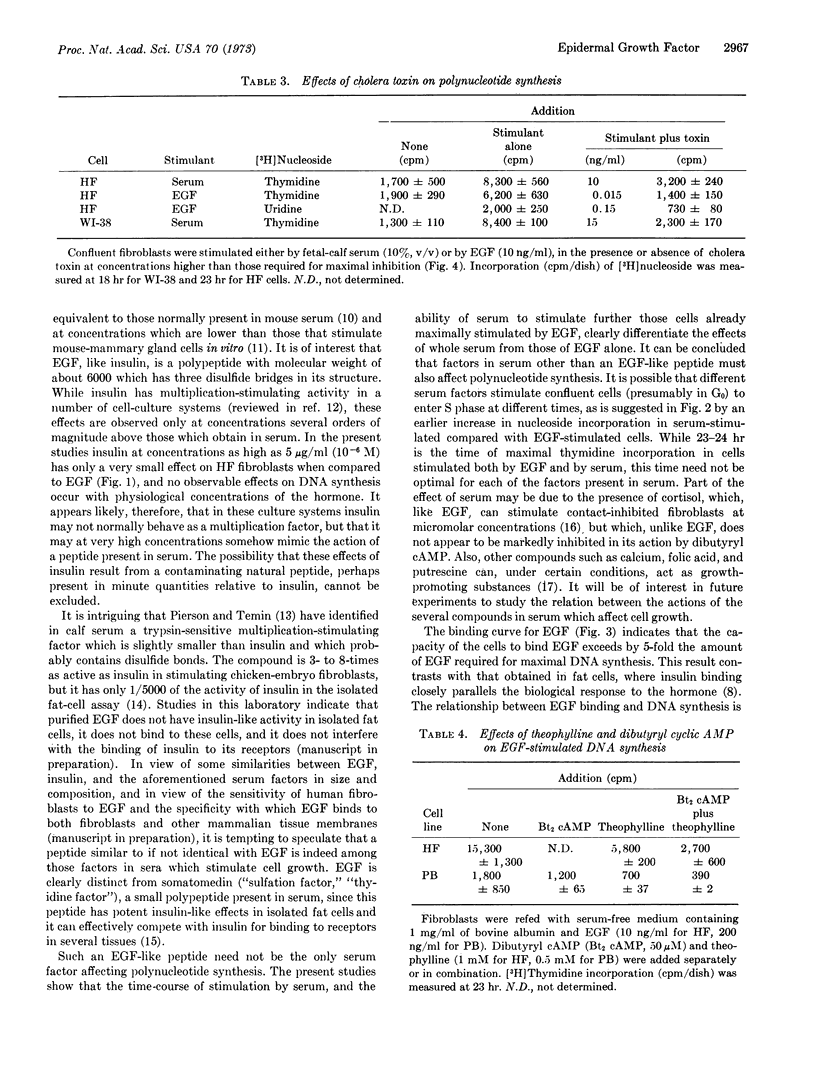
Epidermal growth factor (EGF) stimulates both DNA and RNA synthesis in contact-inhibited human fibroblasts. Stimulation of DNA synthesis is observed at concentrations as low as 3 pM, is half-maximal at 70 pM, and is maximal at 300 pM EGF. The action of EGF is similar to that of fetal-calf serum, but is distinguished by the time-course of stimulation and by the ability of serum to stimulate further those cells maximally stimulated by EGF. Cells that synthesize DNA in response to physiological concentrations of EGF (10-11 to 10-10 M) are insensitive to physiological concentrations of insulin (10-11 to 10-10 M) and respond only minimally to very high concentrations of this hormone (10-6 M). The biological activity of EGF is paralleled by binding of this peptide to fibroblasts in a specific and saturable manner; the dissociation constant is about 800 pM. The binding of EGF is unaffected by either insulin or cholera toxin. Cholera toxin inhibits the action of both EGF and serum. Suppression of DNA synthesis is observed at 0.02 pM toxin, and is maximal at about 2 pM. Cells treated with cholera toxin at these concentrations appear to be otherwise viable by several criteria. The stimulatory effects of EGF are also inhibited by theophylline and dibutyryl cyclic AMP separately or in combination. These observations indicate that fibroblasts possess receptors for EGF by biological and physicochemical criteria, and suggest that a similar if not identical peptide may be amongst those factors in sera which stimulate cell growth. The possibility is considered that EGF and cholera toxin modulate the ability of a cell to initiate polynucleotide synthesis by way of specific cell-surface interactions which in turn alter the levels of intracellular cyclic AMP.
Keywords: cyclic AMP, insulin, DNA and RNA synthesis, cell surface
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter C. C., Jr Cholera enterotoxin--recent investigations yield insights into transport processes. Am J Med. 1971 Jan;50(1):1–7. doi: 10.1016/0002-9343(71)90198-7. [DOI] [PubMed] [Google Scholar]
- Covelli I., Mozzi R., Rossi R., Frati L. The mechanism of action of the epidermal growth factor. 3. Stimulation of the uptake of labeled precursors into RNA, DNA and proteins induced by EGF in isolated tumor cells. Hormones. 1972;3(3):183–191. [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Tell G. P. Insulin-like activity of concanavalin A and wheat germ agglutinin--direct interactions with insulin receptors. Proc Natl Acad Sci U S A. 1973 Feb;70(2):485–489. doi: 10.1073/pnas.70.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flawiá Mirtha M., Torres Héctor N. Insulin inhibition of membrane-bound adenylate cyclase in Neurospora crassa. FEBS Lett. 1973 Feb 15;30(1):74–78. doi: 10.1016/0014-5793(73)80622-2. [DOI] [PubMed] [Google Scholar]
- Frati L., Daniele S., Delogu A., Covelli I. Selective binding of the epidermal growth factor and its specific effects on the epithelial cells of the cornea. Exp Eye Res. 1972 Sep;14(2):135–141. doi: 10.1016/0014-4835(72)90059-0. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Rachmeler M. Effect of adenosine 3'-5'-cyclic monophosphate on cell proliferation. J Cell Biol. 1972 Oct;55(1):19–31. doi: 10.1083/jcb.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. II. The suppressible and non-suppressible insulin-like activity of serum. Diabetologia. 1967 Aug;3(4):389–394. doi: 10.1007/BF02342632. [DOI] [PubMed] [Google Scholar]
- Hepp K. D., Renner R. Insulin action on the adenyl cyclase system: Antagonism to activation by lipolytic hormones. FEBS Lett. 1972 Feb 1;20(2):191–194. doi: 10.1016/0014-5793(72)80791-9. [DOI] [PubMed] [Google Scholar]
- Hintz R. L., Clemmons D. R., Underwood L. E., Van Wyk J. J. Competitive binding of somatomedin to the insulin receptors of adipocytes, chondrocytes, and liver membranes. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2351–2353. doi: 10.1073/pnas.69.8.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Cohen S. Epidermal growth factor. I. The stimulation of protein and ribonucleic acid synthesis in chick embryo epidermis. Biochim Biophys Acta. 1967 Apr 18;138(2):347–356. [PubMed] [Google Scholar]
- Illiano G., Cuatrecasas P. Modulation of adenylate cyclase activity in liver and fat cell membranes by insulin. Science. 1972 Feb 25;175(4024):906–908. doi: 10.1126/science.175.4024.906. [DOI] [PubMed] [Google Scholar]
- Jiménez de Asúa L., Surian E. S., Flawia M. M., Torres H. N. Effect of insulin on the growth pattern and adenylate cyclase activity of BHK fibroblasts. Proc Natl Acad Sci U S A. 1973 May;70(5):1388–1392. doi: 10.1073/pnas.70.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Peery C. V., Johnson G. S., Pastan I. Adenyl cyclase in normal and transformed fibroblasts in tissue culture. Activation by prostaglandins. J Biol Chem. 1971 Sep 25;246(18):5785–5790. [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson R. W., Jr, Temin H. M. The partial purification from calf serum of a fraction with multiplication-stimulating activity for chicken fibroblasts in cell culture and with non-suppressible insulin-like activity. J Cell Physiol. 1972 Jun;79(3):319–330. doi: 10.1002/jcp.1040790302. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P., Raina A. Identification of a growth factor produced by human fibroblasts in vitro as putrescine. Nat New Biol. 1972 Feb 23;235(60):247–249. doi: 10.1038/newbio235247a0. [DOI] [PubMed] [Google Scholar]
- Richelson E. A microwell assay method for the biochemical study of cultured cells. Anal Biochem. 1973 Apr;52(2):563–573. doi: 10.1016/0003-2697(73)90062-6. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Tell G. P., Cuatrecasas P., Van Wyk J. J., Hintz R. L. Somatomedin: inhibiton of adenylate cyclase activity in subcellular membranes of various tissues. Science. 1973 Apr 20;180(4083):312–315. doi: 10.1126/science.180.4083.312. [DOI] [PubMed] [Google Scholar]
- Thrash C. R., Cunningham D. D. Stimulation of division of density inhibited fibroblasts by glucocorticoids. Nature. 1973 Apr 6;242(5397):399–401. doi: 10.1038/242399a0. [DOI] [PubMed] [Google Scholar]
- Turkington R. W. Stimulation of mammary carcinoma cell proliferation by epithelial growth factor in vitro. Cancer Res. 1969 Jul;29(7):1457–1458. [PubMed] [Google Scholar]


