Abstract
Active-β-galactosidase (EC 3.2.1.23) is often formed in rec- merozygotes containing a pair of mutations in the z gene of the lac operon. Contrary to expectation, with certain pairs of mutants this enzyme, upon dissociation, does not yield two independent polypeptides but only a single continuous protomer. Both genetic recombination and suppression have been ruled out as the source of this phenomenon. Therefore we believe we are observing the synthesis of one protein from two independently functioning genes.
Keywords: β-galactosidase, lactose operon
Full text
PDF
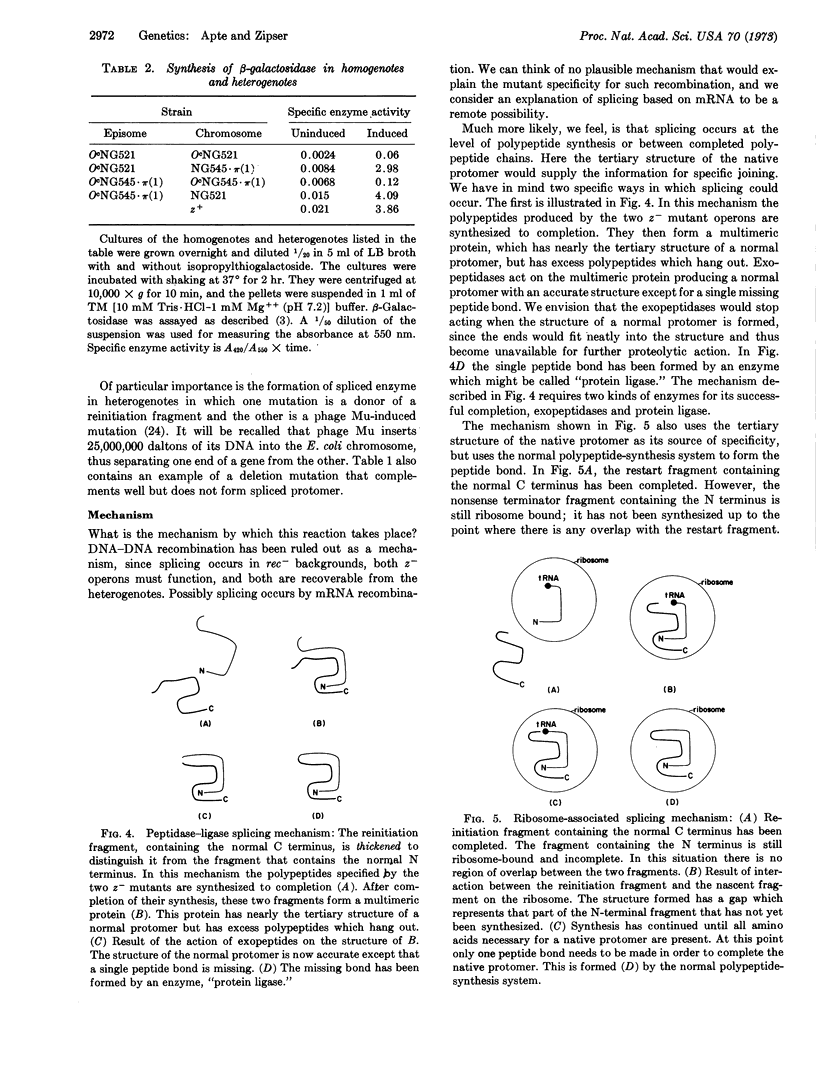

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee G. G., Cartwright E. M., Cowan N. J., Jarvis J. M., Milstein C. Purification and sequence of messenger RNA for immunoglobulin light chains. Nat New Biol. 1973 Aug 22;244(138):236–240. doi: 10.1038/newbio244236a0. [DOI] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Edelman G. M. Antibody structure and molecular immunology. Science. 1973 May 25;180(4088):830–840. doi: 10.1126/science.180.4088.830. [DOI] [PubMed] [Google Scholar]
- Frangione B., Lee L., Haber E., Bloch K. J. Protein Hal: partial deletion of a " " immunoglobulin gene(s) and apparent reinitiation at an internal AUG codon. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1073–1077. doi: 10.1073/pnas.70.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Zipser D. A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):305–314. doi: 10.1016/0022-2836(68)90388-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Zipser D. Mapping of polypeptide reinitiation sites within the beta-galactosidase structural gene. J Mol Biol. 1969 May 14;41(3):341–347. doi: 10.1016/0022-2836(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Beckwith J., Muller-Hill B. Direction of transcription of a regulatory gene in E. coli. Nature. 1968 Dec 28;220(5174):1287–1290. doi: 10.1038/2201287a0. [DOI] [PubMed] [Google Scholar]
- Newton A. Re-initiation of polypeptide synthesis and polarity in the lac operon of Escherichia coli. J Mol Biol. 1969 May 14;41(3):329–339. doi: 10.1016/0022-2836(69)90279-4. [DOI] [PubMed] [Google Scholar]
- PERRIN D., MONOD J. ON THE REVERSIBILITY BY TREATMENT WITH UREA OF THE THERMAL INACTIVATION OF E. COLI BETA-GALACTOSIDASE. Biochem Biophys Res Commun. 1963 Aug 14;12:425–428. doi: 10.1016/0006-291x(63)90118-9. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Steady-state measurement of the turnover of amino acid in the cellular proteins of growing Escherichia coli: existence of two kinetically distinct reactions. J Bacteriol. 1970 Jul;103(1):207–215. doi: 10.1128/jb.103.1.207-215.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Scaife J., Beckwith J. R. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. doi: 10.1101/sqb.1966.031.01.052. [DOI] [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Cohn M. Immunoglobulin biosynthesis. V. Light chain assembly. J Mol Biol. 1970 Nov 14;53(3):305–320. doi: 10.1016/0022-2836(70)90067-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Goldberg M. E., Perrin D., Monod J. On the determination of molecular weight of proteins and protein subunits in the presence of 6 M guanidine hydrochloride. Biochemistry. 1968 Jan;7(1):261–265. doi: 10.1021/bi00841a031. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. On the subunit structure of wild-type versus complemented beta-galactosidase of Escherichia coli. J Mol Biol. 1968 Feb 28;32(1):1–13. doi: 10.1016/0022-2836(68)90140-x. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., SUND H., WEBER K. DIE UNTEREINHEITEN DER BETA-GALAKTOSIDASE AUS E. COLI. Biochem Z. 1963;338:714–727. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- ZIPSER D. A STUDY OF THE UREA-PRODUCED SUBUNITS OF BETA-GALACTOSIDASE. J Mol Biol. 1963 Aug;7:113–121. doi: 10.1016/s0022-2836(63)80040-6. [DOI] [PubMed] [Google Scholar]
- Zabin I., Fowler A. V. The amino acid sequence of -galactosidase. 3. The sequences of NH 2 - and COOH-terminal tryptic peptides. J Biol Chem. 1972 Sep 10;247(17):5432–5435. [PubMed] [Google Scholar]
- Zipser D., Zabell S., Rothman J., Grodzicker T., Wenk M. Fine structure of the gradient of polarity in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):251–254. doi: 10.1016/0022-2836(70)90392-x. [DOI] [PubMed] [Google Scholar]