Abstract
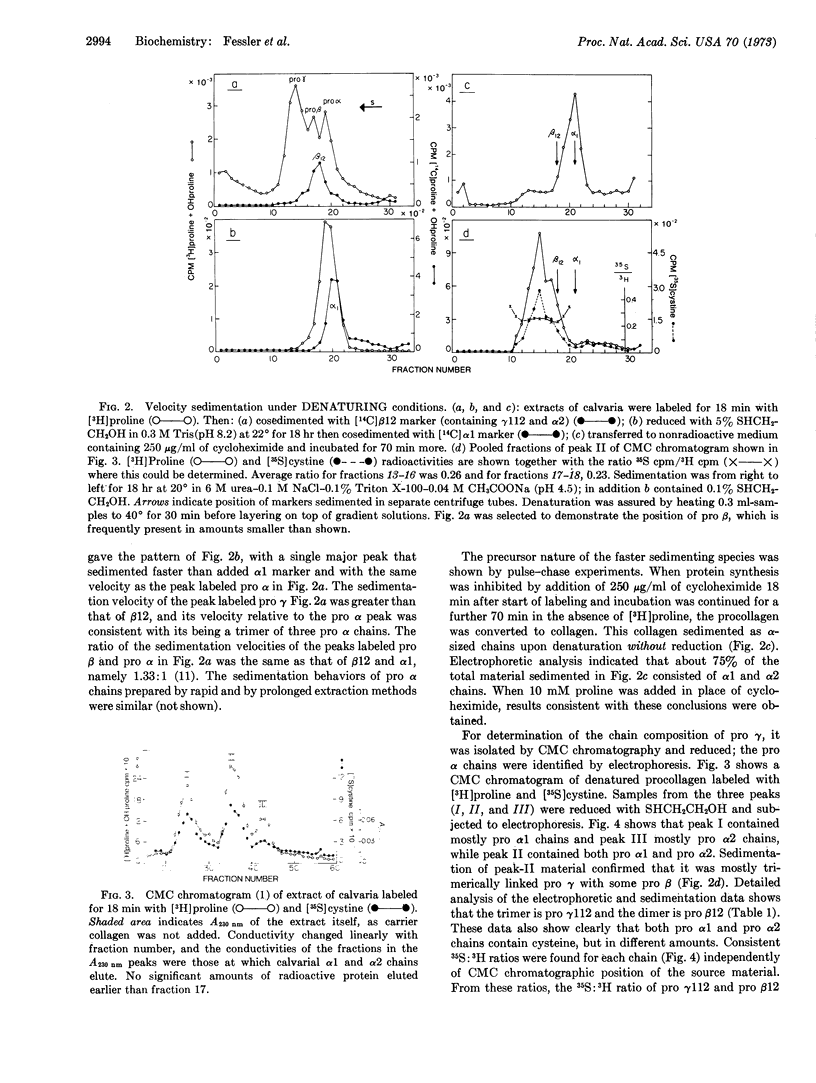
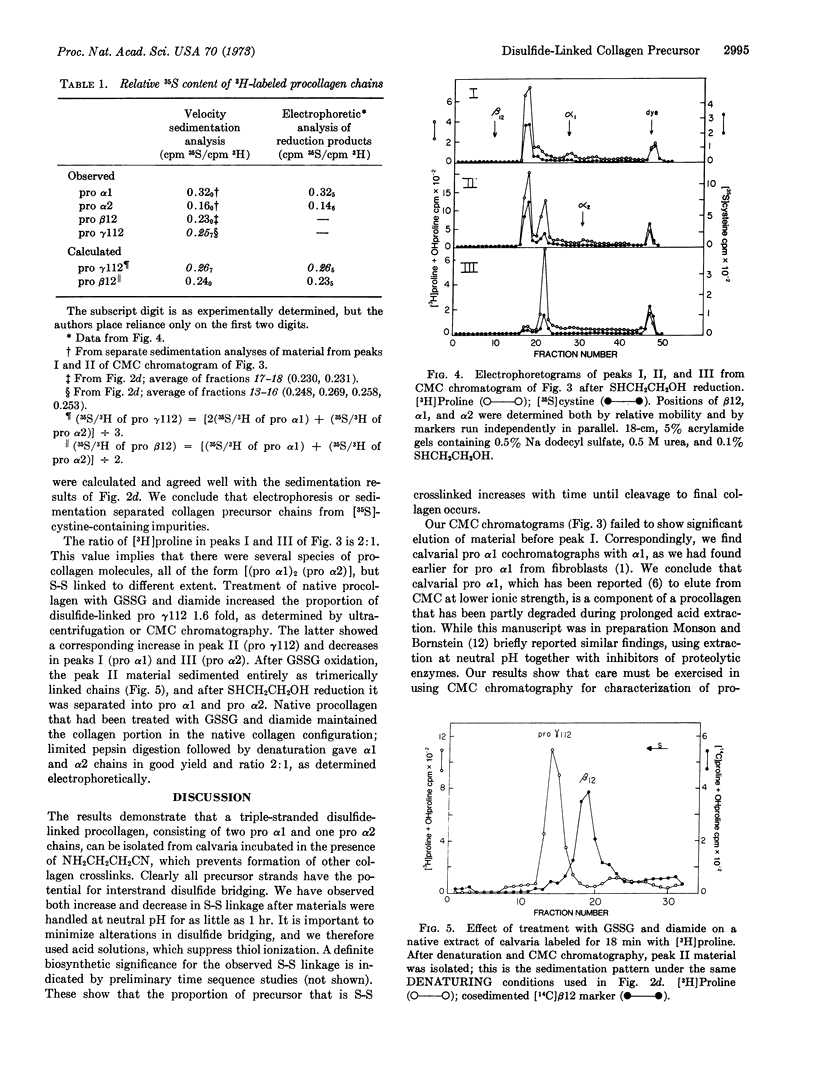
Chick calvaria labeled in vitro contain a triple-stranded collagen precursor that contains two pro α1 and one pro α2 chains. All chains contain cystine and are linked by S-S bonds. Ultracentrifugal analysis was used to measure the relative size of the disulfide-linked units. It is proposed that the S-S links help to determine the correct alignment of the triple-stranded structure.
Keywords: calvaria, ultracentrifugation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTGELT K., HODGE A. J., SCHMITT F. O. Gamma tropocollagen: a reversibly denaturable collagen macromolecule. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1914–1924. doi: 10.1073/pnas.47.12.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Burgeson R. E., Wyke A. W., Fessler J. H. Collagen synthesis by cells II: secretion of a disulfide linked material. Biochem Biophys Res Commun. 1972 Aug 21;48(4):892–897. doi: 10.1016/0006-291x(72)90692-4. [DOI] [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Sherr C. J. Secretion and extracellular processing of procollagen by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1973 Feb;70(2):361–365. doi: 10.1073/pnas.70.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Veis A., Anesey J. R., Garvin J. E., Dimuzio M. T. High molecular weight collagen: a long-lived intermediate in the biogenesis of collagen fibrils. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1404–1411. doi: 10.1016/0006-291x(72)90869-8. [DOI] [PubMed] [Google Scholar]



