Abstract
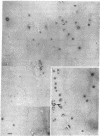
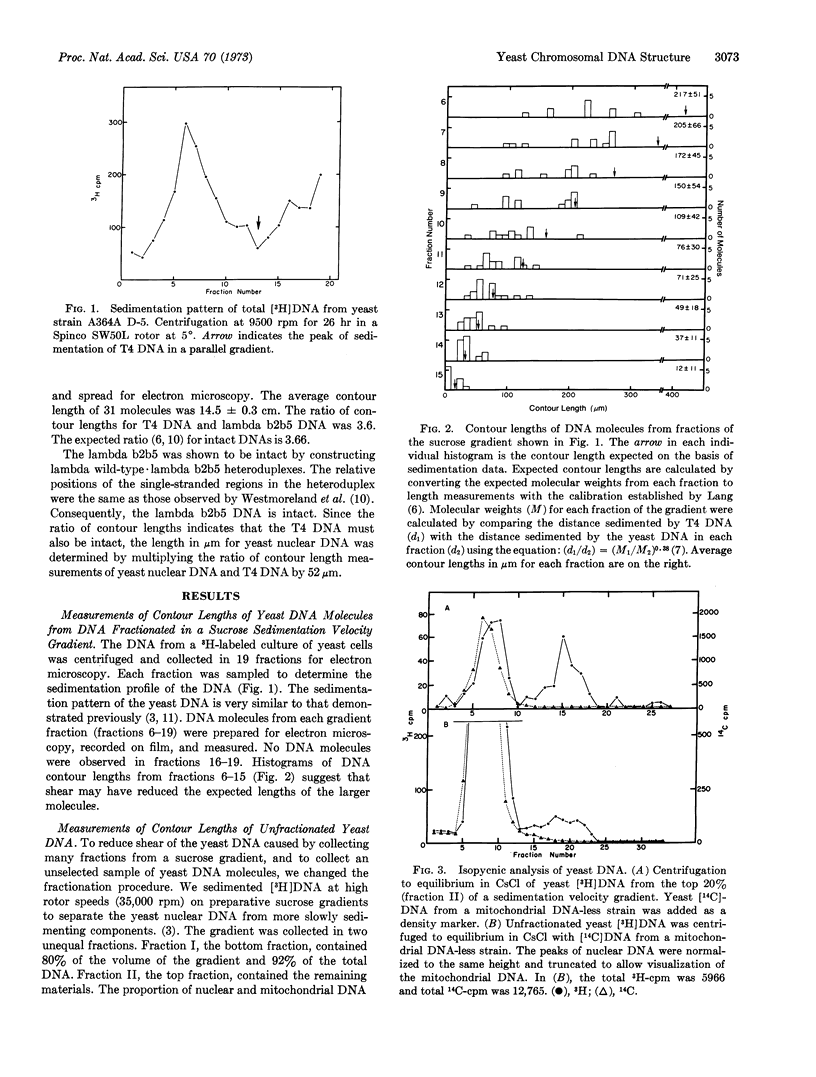
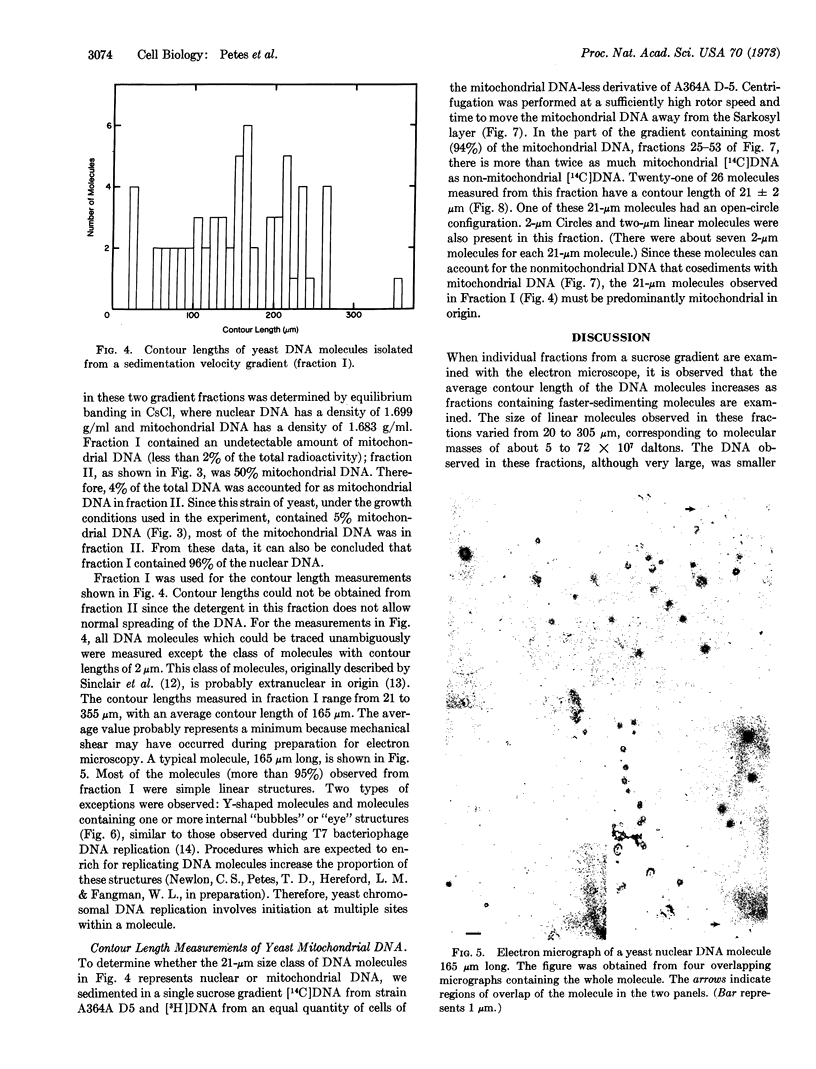
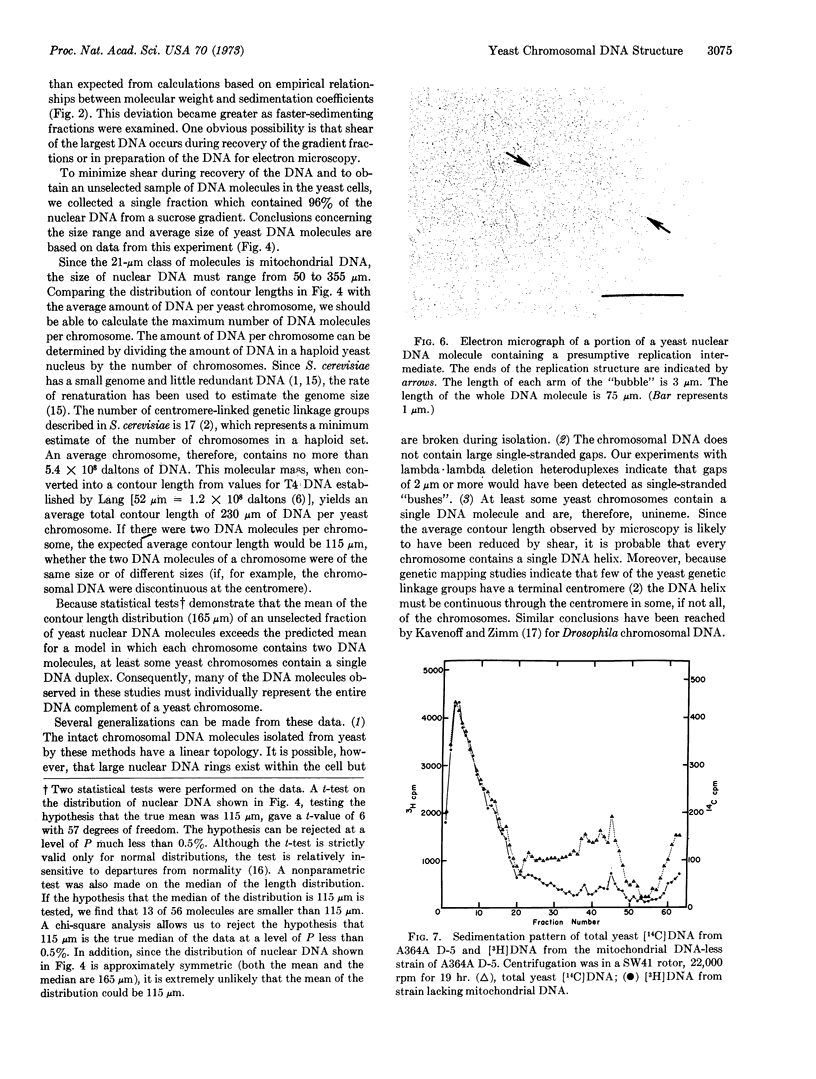
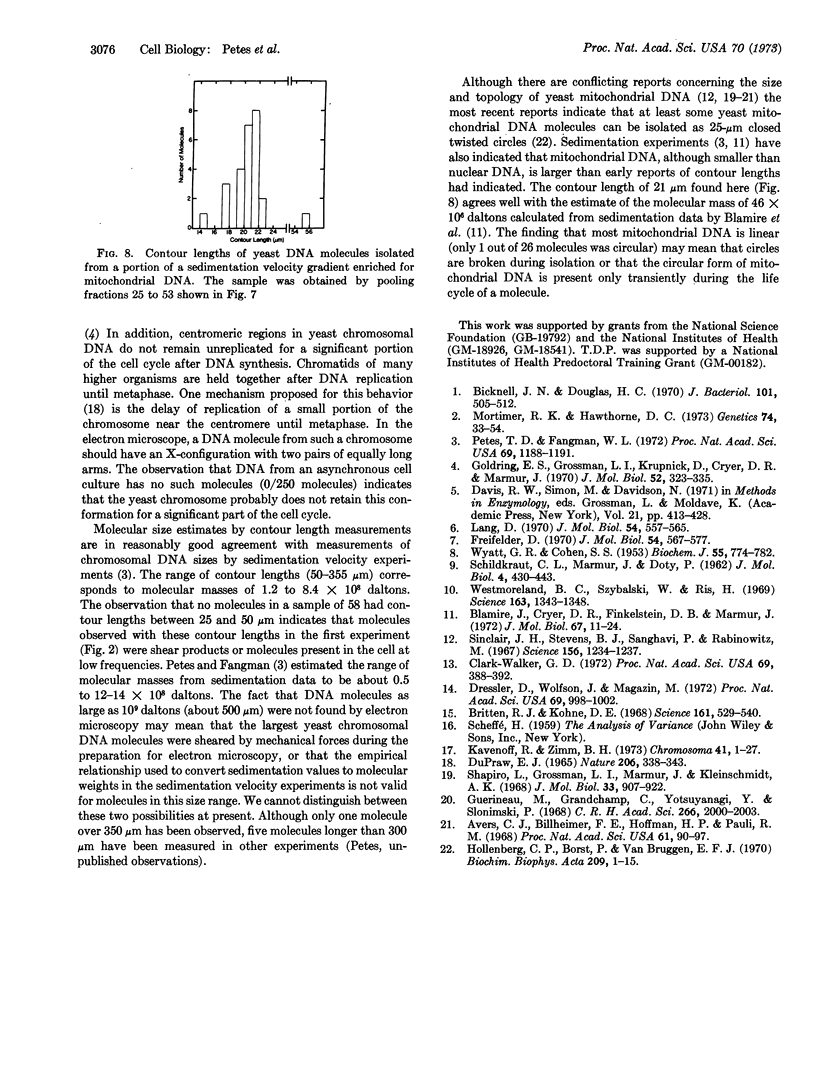
Electron microscopic analysis indicates that yeast nuclear DNA can be isolated as linear molecules ranging in size from 50 μm (1.2 × 108 daltons) to 355 μm (8.4 × 108 daltons). Analysis indicates the data is consistent with the hypothesis that each yeast chromosome contains a single, linear DNA duplex. Mitochondrial DNA molecules have a contour length of 21 ± 2 μm and are mostly linear.
Keywords: electron microscopy, mitochondrial DNA, linear DNA
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avers C. J., Billheimer F. E., Hoffmann H. P., Pauli R. M. Circularity of yeast mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):90–97. doi: 10.1073/pnas.61.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell J. N., Douglas H. C. Nucleic acid homologies among species of Saccharomyces. J Bacteriol. 1970 Feb;101(2):505–512. doi: 10.1128/jb.101.2.505-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamire J., Cryer D. R., Finkelstein D. B., Marmur J. Sedimentation properties of yeast nuclear and mitochondrial DNA. J Mol Biol. 1972 Jun 14;67(1):11–24. doi: 10.1016/0022-2836(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPraw E. J. Macromolecular organization of nuclei and chromosomes: a folded fibre model based on whole-mount electron microscopy. Nature. 1965 Apr 24;206(982):338–343. doi: 10.1038/206338a0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Yotsuyanagi Y., Slonimski P. P. Examen au microscope électronique, du DNA mitochondrial de la levure: molécules circulaires. C R Acad Sci Hebd Seances Acad Sci D. 1968 May 6;266(19):2000–2003. [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Kavenoff R., Zimm B. H. Chromosome-sized DNA molecules from Drosophila. Chromosoma. 1973;41(1):1–27. doi: 10.1007/BF00284071. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic Mapping in Saccharomyces IV. Mapping of Temperature-Sensitive Genes and Use of Disomic Strains in Localizing Genes. Genetics. 1973 May;74(1):33–54. doi: 10.1093/genetics/74.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Sedimentation properties of yeast chromosomal DNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1188–1191. doi: 10.1073/pnas.69.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Grossman L. I., Marmur J., Kleinschmidt A. K. Physical studies on the structure of yeast mitochondrial DNA. J Mol Biol. 1968 May 14;33(3):907–922. doi: 10.1016/0022-2836(68)90327-6. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]