Abstract
Carbon–carbon (C–C) bonds form the backbone of many important molecules, including polymers, dyes, and pharmaceutical agents. The development of new methods to create these essential connections in a rapid and practical fashion has been the focus of numerous organic chemists. This endeavor heavily relies on the ability to form C–C bonds in the presence of sensitive functional groups and congested structural environments. Here we report a fundamentally new chemical transformation that allows for the facile construction of highly substituted and uniquely functionalized C–C bonds. Using a simple iron catalyst, an inexpensive silane, and a benign solvent under an ambient atmosphere, heteroatom-substituted olefins are easily merged with electron-deficient olefins to create molecular architectures that were previously difficult or impossible to access. More than sixty examples are presented with a wide array of substrates, demonstrating the unique chemoselectivity and mildness of this simple reaction.
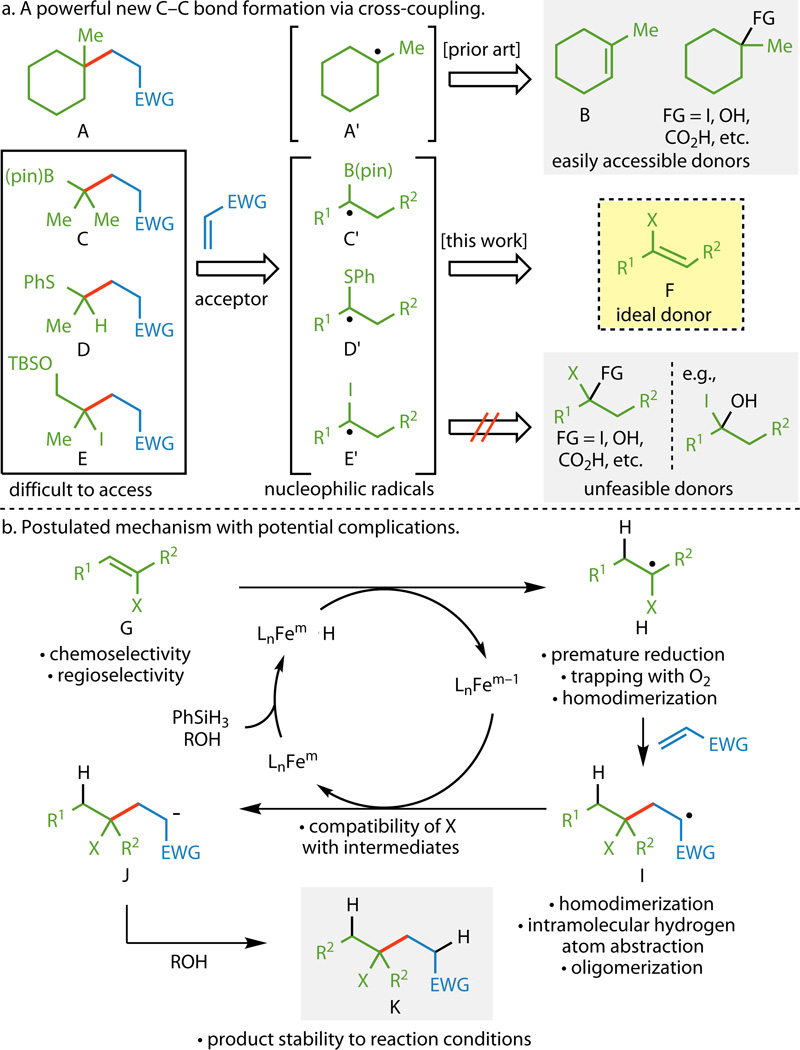
New methods for the construction of C–C bonds have the potential to shift paradigms in retrosynthetic analysis.1 Historically, those that have been most successful feature simple experimental procedures, exhibit broad scope, and allow access to chemical space previously deemed challenging or inaccessible. A recent exercise in total synthesis drew our attention to radical-based olefin hydrofunctionalizations of the sorts pioneered by Mukaiyama,2,3 Carreira,4 Boger,5 and others.6–9 Those illuminating studies led to the invention of a reductive coupling10–12 of simple olefins with electron-deficient olefins such as that depicted in Figure 1A.13 In that work, an adduct bearing an all-carbon quaternary center such as A could be easily accessed in minutes and in an open-flask from olefin B, presumably via the intermediacy of radical A′. Although a useful and practical method, the products it produced could already be obtained from readily accessible functionalized hydrocarbons such as alkyl halides,14 alcohols,15,16 and carboxylic acids17 via conventional radical-generating processes.
Figure 1. Functionalized olefin cross-coupling as a strategy for convergent chemical synthesis.
a, Functionalized olefin cross-coupling would allow for easy access to underexplored chemical space by using olefins as radical surrogates. Such a strategy would use easily accessible heteroatom-substituted olefins as donors, avoiding difficulties that could arise from the use of other radical precursors. b, Examination of the postulated mechanism for cross-coupling reveals several potential complications that could arise due to either the intermediacy of radicals or the heteroatom (X) present on the donor olefin. EWG, electron-withdrawing group; FG, functional group; (pin), pinacolato; TBS, tert-butyldimethylsilyl; X, heteroatom; L, ligand.
In contrast, the functionalized hydrocarbons required to access adducts such as C, D, and E either would require extensive functional group (FG) manipulations or are unfeasible donors owing to FG incompatibilities and chemoselectivity difficulties arising from the heteroatoms present (B, S, and I). By analogy to previous work, if olefins could be used as a surrogate for the intermediate radicals C′, D′, and E′, easily accessible compounds such as F could be employed directly, avoiding FG manipulations all together.
Development of the olefin cross-coupling
Although this idea is conceptually simple, examining the hypothetical mechanistic pathway revealed numerous obstacles that would need to be addressed, as shown in Figure 1B. The initiating step, radical formation from the donor olefin G by an in situ-generated Fe hydride, could be fraught with issues of both regioselectivity and chemoselectivity. Furthermore, depending on the nature of the X substituent, several competing pathways could arise with the Fe complexes in the catalytic cycle (e.g., transmetallation of a C–B bond, desulfurization of a C–S bond, and oxidative addition of a C–I bond). If the first step did occur as intended, the intermediate radical H could be prone to premature reduction,18–20 trapping with O2,2 or homodimerization. Provided that H undergoes the desired conjugate addition to the electron-deficient olefin coupling partner, the newly generated radical I could undergo homodimerization, intramolecular hydrogen atom abstraction, or consecutive conjugate additions leading to uncontrollable oligomerization. Formation of J from a single electron reduction of I would result in a substantially basic and nucleophilic site that could prove to be incompatible with the X group and its substituents. In order for the reaction to prove successful, the conditions must be mild enough to tolerate both the various intermediate species in the catalytic cycle, as well as the final coupled product K.
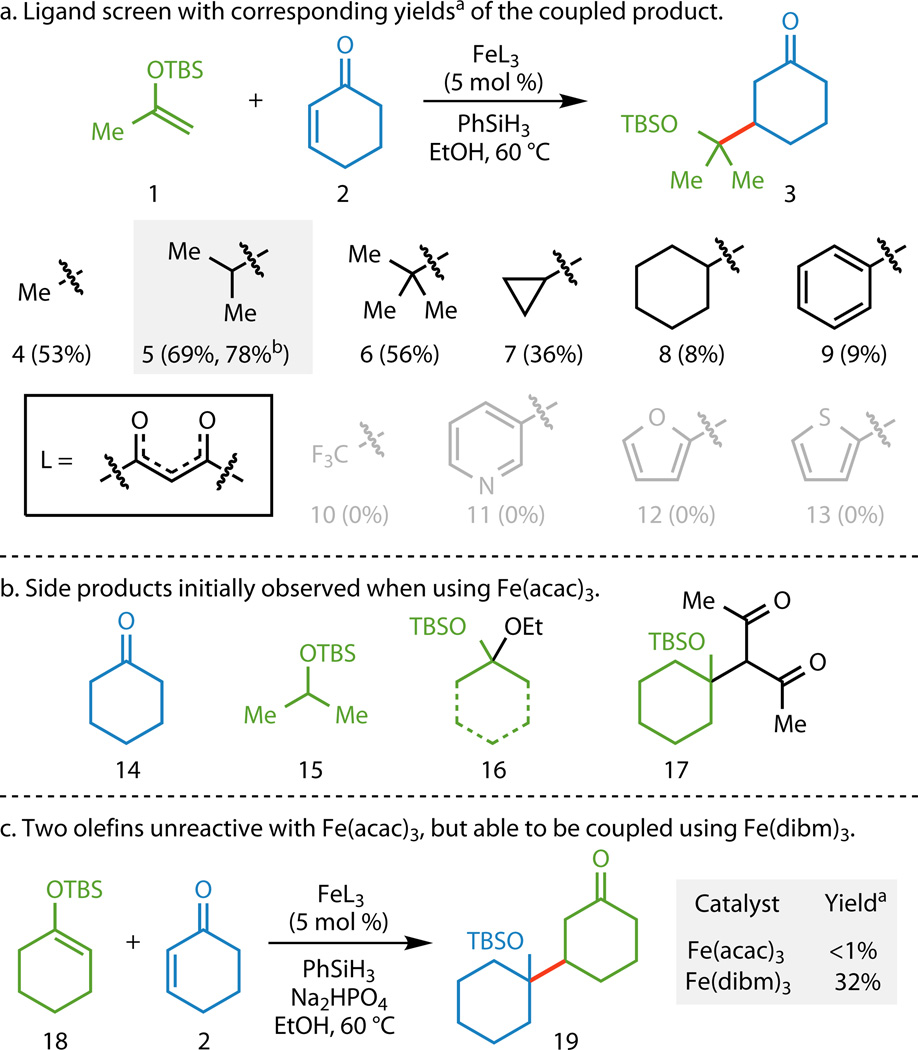
With these potential difficulties in mind, we used the model system depicted in Figure 2A, with silyl enol ether 1 serving as the donor and cyclohexenone (2) as the acceptor, to develop a functionalized olefin cross-coupling. Application of conditions similar to those previously developed, using Fe(acac)3 (4) as a catalyst and PhSiH3 as a stoichiometric reductant,3,13 formed the reductively coupled product 3 in 53% yield based on GC/MS (gas chromatography/mass spectrometry) using an internal standard. Analysis of the side products from the model system and related reactions led to the identification of compounds 14–17 (Figure 2B). As 16 and 17 presumably arise from pathways where Fe(acac)3 behaves as a Lewis acid,21 we hoped to attenuate the Lewis acidity of the catalyst by increasing the amount of steric shielding of the Fe center. Increasing the size of the substitution on the dione ligands (5–9) led to decreased amounts of 16, with Fe(dibm)3 (5, dibm = diisobutyrylmethane)22 providing the best balance between reactivity and steric shielding. Although attempts to alter the electronic structure of the ligand with electron-deficient (10 and 11) and electron-rich (12 and 13) substituents completely ablated reactivity, the addition of Na2HPO4 increased the yield of the desired product 3 from 69% to 78% when using Fe(dibm)3 as the catalyst. The use of ca. 45 other inorganic and amine bases as additives did not result in increased yields, suggesting that Na2HPO4 does not simply serve as a buffering agent. Additionally, Fe(dibm)3 enabled product formation with donors that were unreactive with Fe(acac)3 (18, Figure 2C), which instead provided significant quantities of byproducts 16 and 17. Over the course of the project, it was found that Fe(dibm)3 provided the highest yields when the heteroatom substitution on the donor olefin contained Lewis basic lone pairs, whereas Fe(acac)3 proved superior in the absence of such moieties (vide infra).
Figure 2. Functionalized olefin cross-coupling optimization studies.
a, Altering the ligands on the Fe center had the greatest influence on the outcome of the reaction with Fe(dibm)3 (5) giving the highest yields. The addition of Na2HPO4 further increased the yield. b, Side products that were observed when Fe(acac)3 (4) was used as the catalyst. The formation of compounds 16 and 17 could be attributed to the Lewis acidity of 4. The use of 5 as the catalyst reduced the formation of compounds 16 and 17. c, An example where the use of 5 instead of 4 was essential in obtaining the desired functionalized olefin cross-coupling reactivity. aYields based on GC/MS analysis using 1,3,5-trimethoxybenzene as an internal standard. bUsing 1 equiv Na2HPO4 as an additive. TBS, tert-butyldimethylsilyl; L, ligand; acac, acetylacetonate; dibm, diisobutyrylmethane; GC/MS, gas chromatography/mass spectrometry.
Scope and functional group tolerance
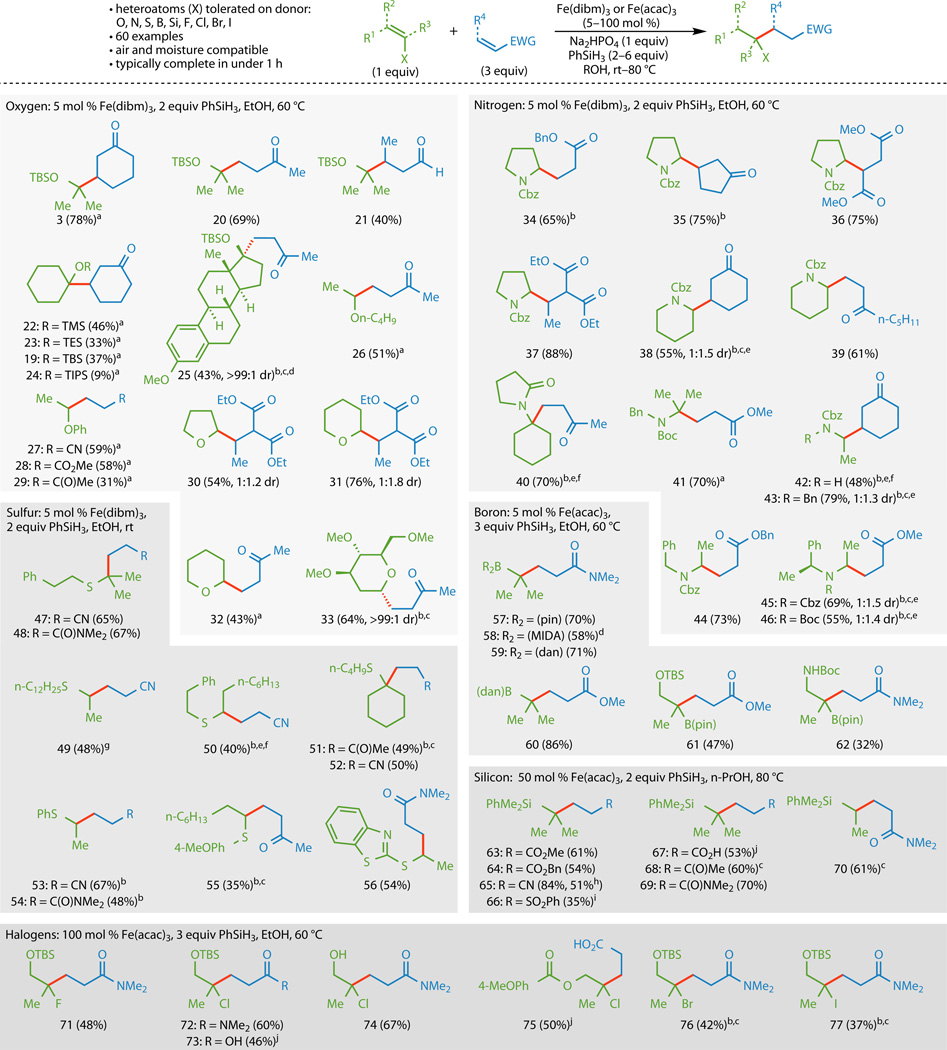
The optimized conditions were then applied to a wider variety of donor and acceptor olefins (Figure 3), initially focusing on enol ethers. Utilizing Fe(dibm)3 (5 mol %), silyl enol ethers could be coupled to cyclic and acyclic enones, an enal, and an acrylamide to generate adducts 3 and 20–25 with yields that generally increased with decreasing substitution on the silicon atom (19 and 22–24). Remarkably, even a severely congested estrone derivative could undergo addition to methyl vinyl ketone to generate steroidal adduct 25 with the stereochemistry of the newly formed neopentyl quaternary stereocenter corresponding to that obtained through a conventional organometallic addition of an alkyl group to estrone.23 Alkyl and aryl vinyl ethers could also be used, although higher yields were generally obtained by using the donor olefin in excess (26–33). Endocyclic enol ethers were also tolerated, as shown by the formation of 30–33.
Figure 3. Adducts synthesized by functionalized olefin cross-coupling.
The donor component is shown in green and the acceptor component is shown in blue. Couplings using donor olefins with heteroatom substitution containing Lewis basic lone pairs proceeded in higher yields with Fe(dibm)3 whereas couplings without such moieties proceeded in higher yields with Fe(acac)3. a3 equiv donor and 1 equiv acceptor used. b6 equiv PhSiH3 used. c6 equiv acceptor used. dTHF used as a cosolvent. e15 mol % [Fe] used. fSecond portion of [Fe], acceptor, and PhSiH3 added after 1 h. gHeated at 60 °C. hRun on gram-scale. i100 mol % [Fe] used. jNa2HPO4 omitted. TBS, tert-butyldimethylsilyl; TMS, trimethylsilyl; TES, triethylsilyl; TIPS, triisopropylsilyl; Bn, benzyl; Cbz, benzyloxycarbonyl; Boc, tert-butyloxycarbonyl; (pin), pinacolato; (MIDA), N-methyliminodiacetate; (dan), 1,8-diaminonaphthyl.
Additionally, enecarbamates and enamides could undergo cross-coupling under the reaction conditions. Adducts 34 and 35 were formed by the coupling of a Cbz-protected dihydropyrrole with benzyl acrylate and cyclopent-2-enone, respectively, although these couplings necessitated larger amounts of PhSiH3 than the enol ethers. The amount of PhSiH3 used could be decreased with the use of more electronically activated acceptors, as the formation of 36 and 37 demonstrated. Cyclic enecarbamates could also be employed and added to cyclic and acyclic acceptor olefins (38, 39, and 41–46), although higher loadings (15 mol %) of Fe(dibm)3 were required for useful yields. The formation of 40 also demonstrated that the nitrogen atom present on the donor olefin could be protected as an amide instead of a carbamate. Mono- and 1,1-disubstituted acyclic donor olefins were competent donors (41–46), although attempts to control the stereochemistry of the cross-coupling by using α-phenylethylamine as a chiral auxiliary24 provided only modest amounts of diastereoselectivity (45 and 46).
Vinyl thioethers proved to be unique donor olefins, with the cross-couplings of those surveyed taking place at ambient temperature to generate adducts 47–56. Although the cross-coupling to form 49 proceeded in a higher yield when the reaction was heated at 60 °C, the yields of the other vinyl thioether cross-couplings did not benefit from elevated temperatures. With the exception of 50, the coupling of the alkenyl thioether donors proceeded with 5 mol % of Fe(dibm)3, however, increased amounts of PhSiH3 and acceptor olefin were required for certain recalcitrant substrates (50, 51, 53, and 54). Syringe pump addition of the acceptor and PhSiH3 to the reaction mixture could also improve yields in certain cases (51 and 55).
Boron substitution on the donor olefin could also be tolerated, with the use of 5 mol % Fe(acac)3 providing slightly higher yields than Fe(dibm)3. An isopropenyl pinacolato (pin) boronic ester, N-methyliminodiacetate (MIDA) boronate,25,26 and a 1,8-diaminonaphthyl (dan) boronamide27 could all be coupled to N,N-dimethyl acrylamide (57–59), although the use of THF as a cosolvent was required to solubilize the MIDA boronate. Additionally, methyl acrylate could be used as an acceptor (60 and 61), and oxygen- and nitrogen-containing functionalities could be tolerated at allylic positions (61 and 62).
Vinyl silanes could also be used as donor olefins, although highest yields were obtained using a substoichiometric amount (50 mol %) of Fe(acac)3. Additionally, switching the solvent from EtOH to n-PrOH and heating the reactions to 80 °C instead of 60 °C resulted in higher yields. With these slight modifications, an isopropenyl and vinyl silane could be coupled to a wide variety of acceptor olefins to form 63–70, although the coupling to obtain the phenyl vinyl sulfone adduct 66 required a stoichiometric amount of Fe(acac)3. With the omission of Na2HPO4, unprotected acrylic acid could be used as an acceptor to provide the coupled product 67 in a transformation difficult to achieve using conventional conjugate addition techniques.28,29
As a final testament to the mildness of this C–C bond forming reaction, alkenyl halides were found to partake in the cross-coupling in reasonable yields using stoichiometric amounts of Fe(acac)3. Alkenyl fluorides, chlorides, bromides, and even iodides could all be used as donors, with the 2-haloallyl alcohol derivatives delivering products 71, 72, 76, and 77, where the halogen atom remained intact. Interestingly, acrylic acid could once again be used as an acceptor (73, 75), and the reaction proceeded readily with a free alcohol (74), demonstrating the superb chemoselectivity of this method.
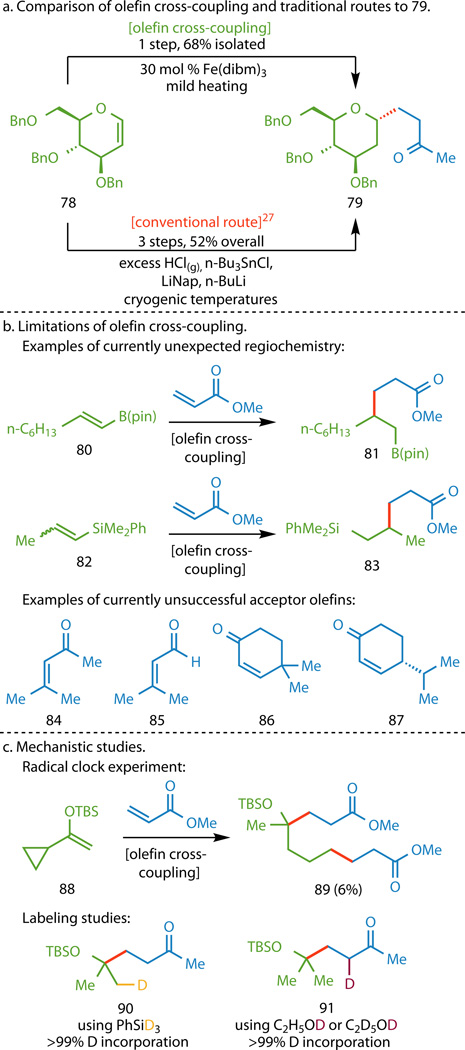
To highlight the efficiency of the newly developed coupling reaction, we chose to target glucal derivative 79 (Figure 4A). Hutchinson and Fuchs were able to prepare the compound in three steps from readily available 78 in 52% yield, although their route required the use of excess gaseous HCl, toxic and harsh organometallic reagents, and cryogenic temperatures.30 By contrast, olefin cross-coupling allowed for the desired product 79 to be synthesized directly from 78 in a single step over two hours in 68% isolated yield, although it did require the slow addition of a large excess (12 equiv) of both methyl vinyl ketone and PhSiH3.
Figure 4. Additional functionalized olefin cross-coupling studies.
a, Functionalized olefin cross-coupling offers a direct route to glucal derivative 79 that circumvents the use of harsh reagents, superstoichiometric organometallic reagents, and cryogenic temperatures in traditional approaches. b, The use of certain 1,2-disubstituted donor olefins (80 and 82) gave adducts where the C–C bond formed distal instead of adjacent to the heteroatom (81 and 83). Additionally, the use of acceptors with excessive aliphatic substitution (84–87) gave trace or no product. c, The use of vinyl cyclopropane 88 resulted in the isolation of 89, where the fragmentation of the cyclopropane ring supports the formation of a radical adjacent to the heteroatom in the donor. Isolation of compounds 90 and 91 from deuterium labeling studies further support the mechanism depicted in Figure 1B.
Finally, the resilience of the functionalized olefin cross-coupling to adverse conditions was evaluated by performing the reaction in a variety of unconventional solvents. As indicated by GC/MS, the coupling to form silyl ether 20 proved to be successful in a selection of beer, wine, and various spirits (see Supporting Information). In addition to showcasing the ability of the reaction to proceed under aqueous conditions, these results demonstrate the reaction’s tolerance of a host of organic compounds31 and microorganisms, suggesting possible downstream applications to the area of bioconjugation.32
Discussion and limitations of the method
From a strategic perspective, this methodology grants access to areas of chemical space that, in most cases, were previously inaccessible. Historically, heteroatom-substituted quaternary centers are synthesized with multiple FG manipulations and rarely, if ever, through a direct C–C disconnection enabled here. Thus, ca. 90% of the compounds listed in Figure 3 are new chemical entities despite their simplicity. In the case of 30, 31, and 34–37, where a comparison to contemporary reactivity modes could be made, it was found that the olefin cross-coupling route offers a complementary approach to the recently reported decarboxylative method.33 Furthermore, the olefin cross-coupling reaction setup was operationally simple as no precautions were made with regards to moisture or air exclusion, and reactions were typically done within a few minutes to an hour. The reaction is also readily scalable with the coupling to form 65 being conducted on gram-scale (51% yield).
However, no reaction is without limitations. Although nearly all of the substrate classes tested delivered the expected product, the 1,2-disubstituted vinyl boronic ester 80 and vinyl silane 82 exclusively provided adducts 81 and 83, respectively, where bond formation occurred distal to the heteroatom (Figure 4B). Additionally, excessive alkyl substitution on the acceptor olefin was not well tolerated, with trisubstituted acceptors (e.g., 84 and 85) and disubstituted acceptors (e.g., 86 and 87) containing aliphatic β branching generally giving little or no product. Cases where the isolated yield was ca. 50% and below could be attributed to incomplete conversion, premature reduction, or substrate dimerization. It is finally worth noting that as Figure 3 demonstrates, the stereochemical outcomes of this reaction are all currently substrate-controlled.
Although a thorough mechanistic investigation has not been pursued, several observations are consistent with the mechanism depicted in Figure 1B. Subjecting a donor olefin bearing a vinylcyclopropane (88, Figure 4C) to the reaction conditions led to the isolation of adduct 89, arising from cleavage of the cyclopropane ring. Furthermore, the utilization of PhSiD3 instead of PhSiH3 resulted in the isolation of C6 deuterated adduct 90. These two observations support the notion that a hydrogen atom originating from PhSiH3 becomes incorporated into donor olefin G (Figure 1B) through a radical-based process. Boger has previously proposed a similar initiating step in his Fe-mediated oxidation of anhydrovinblastine to vinblastine and originated the idea that Fe-mediated Mukaiyama-type hydrofunctionalizations may not occur via hydrometallation.34 In recent work developing a mild thermodynamic olefin reduction applicable to haloalkenes, Shenvi has suggested hydrogen atom transfer (HAT) to be the initial step of these hydrofunctionalizations.18 Taken together, these observations support the initiation of the functionalized olefin cross-coupling by HAT from an Fe hydride35 generated in situ to the donor olefin G to form radical intermediate H. The protonation of intermediate J to the final coupled product K is supported by the isolation of adduct 91 when using either ethanol-d1 or ethanol-d6 as the solvent. Submitting undeuterated analog 20 to the reaction conditions using deuterated ethanol did not lead to any deuterium incorporation, demonstrating the deuterium incorporation observed in the labeling studies occurred during the course of the reaction.
Conclusion
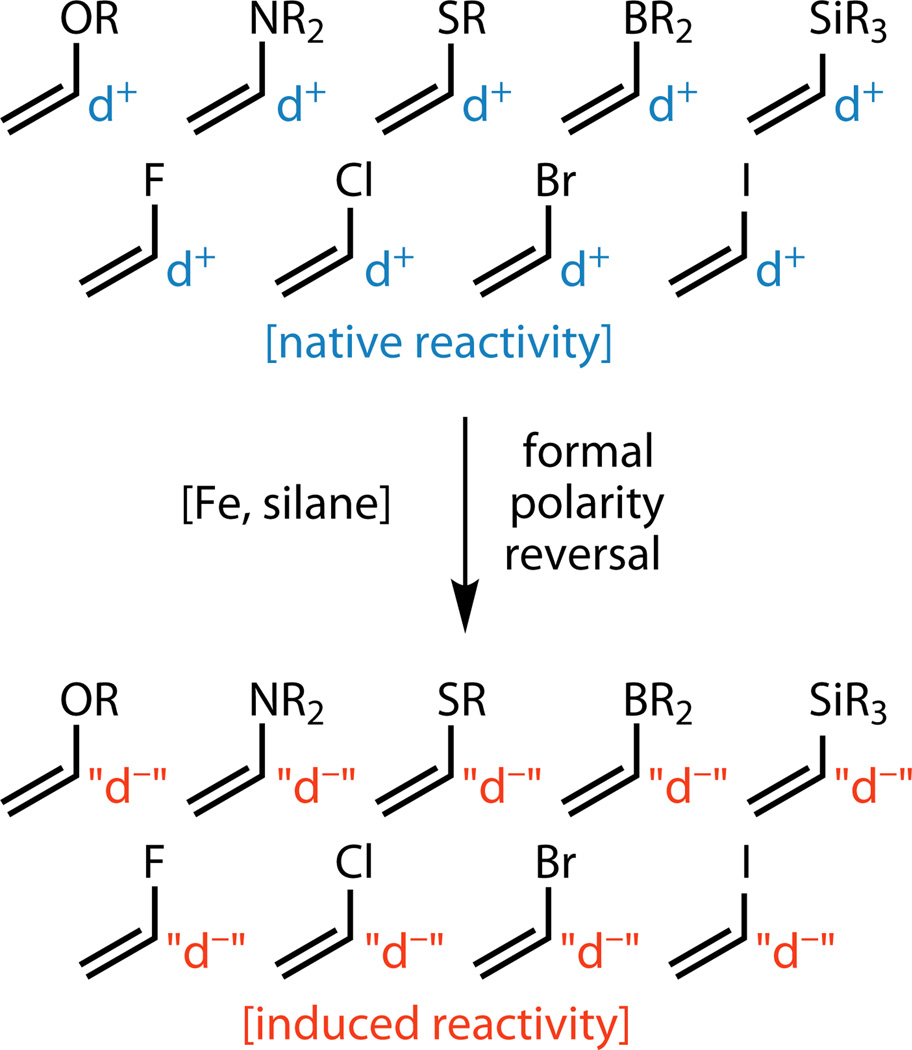
In summary, a new method for forming unique C–C bonds in a rapid, scalable, and practical fashion has been described using an inexpensive iron catalyst and a simple reaction setup. From a retrosynthetic perspective, this method requires one to rethink the classic roles of some common building blocks in organic synthesis. For example, enol ethers and enamides need not be viewed as reacting as nucleophiles solely at their β position.36,37 Vinyl boronates, normally used to fashion new C(sp2) centers,38 can now be viewed as potential progenitors to tertiary boronates for a variety of Ni- and Pd-based C(sp3) couplings.39 Vinyl thioethers, rarely employed in molecule construction,40 can now be viewed in a different light. Vinyl silanes have been employed in cyclizations41 and C(sp2) cross coupling chemistry42 but never as precursors to silyl-substituted quaternary centers. In the case of vinyl halides, the halide (F, Cl, Br, and even I) no longer needs to be viewed as a disposable functionality for conventional transition metal mediated cross-coupling,43 but rather as a spectator FG that can be incorporated into a final product. Functionalized olefin cross-coupling ultimately represents a method of reversing the native reactivity44 of heteroatom-substituted olefins (Figure 5), thus permitting the facile exploration of underdeveloped chemical space and serving as an alternative to other powerful retrosynthetic C–C bond disconnections.45–47 Although achieving ligand control of stereo- and regiochemical outcomes and a deeper understanding of the mechanism are prominent future goals, potential applications of this method, even in its current form, to virtually all areas of chemical science can be envisioned.
Figure 5. Functionalized olefin cross-coupling reverses conventional reactivity expectations.
The substrates employed as donors in this study typically are electrophilic at the position adjacent to the heteroatom. Functionalized olefin cross-coupling reverses this reactivity via the intermediacy of radicals, resulting in those same positions bearing nucleophilic properties.
Supplementary Material
Acknowledgments
Financial support for this work was provided by NIH/NIGMS (GM-097444). The National Science Foundation supported a predoctoral fellowship to J. C. L., the Shanghai Institute of Organic Chemistry, Zhejiang Medicine Co., and Pharmaron supported a postdoctoral fellowship to J. G., and the Japan Society for the Promotion of Science supported a postdoctoral fellowship to Y. Y. We are grateful to Dr. D.-H. Huang and Dr. L. Pasternack (TSRI) for assistance with NMR spectroscopy and Professor A. L. Rheingold and Dr. C. E. Moore (UCSD) for X-ray crystallographic analysis. We thank Professor R. A. Shenvi (TSRI) and Dr. Y. Ji (TSRI) for valuable discussions.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions J. C. L. and P. S. B. conceived the work; J. C. L., J. G., Y. Y., C.-M. P., and P. S. B. designed the experiments and analyzed the data; J. C. L., J. G., Y. Y., and C.-M. P. performed the experiments; and J. C. L. and P. S. B. wrote the manuscript.
Crystallographic data for the structure of Fe(dibm)3 (5) is available free of charge from the Cambridge Crystallographic Data Centre under deposition number CCDC 1022625.
The authors declare no competing financial interests.
References
- 1.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. Wiley; 1995. [Google Scholar]
- 2.Isayama S, Mukaiyama T. A new method for the preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(II) Chem. Lett. 1989;18:1071–1074. [Google Scholar]
- 3.Kato K, Mukaiyama T. Iron(III) complex catalyzed nitrosation of terminal and 1,2-disubstituted olefins with butyl nitrite and phenylsilane. Chem. Lett. 1992;21:1137–1140. [Google Scholar]
- 4.Waser J, Gaspar B, Nambu H, Carreira EM. Hydrazines and azides via the metal-catalyzed hydrohydrazination and hydroazidation of olefins. J. Am. Chem. Soc. 2006;128:11693–11712. doi: 10.1021/ja062355+. [DOI] [PubMed] [Google Scholar]
- 5.Leggans EK, Barker TJ, Duncan KK, Boger DL. Iron(III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20′-vinblastine analogues. Org. Lett. 2012;14:1428–1431. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shigehisa H, Aoki T, Yamaguchi S, Shimizu N, Hiroya K. Hydroalkoxylation of unactivated olefins with carbon radicals and carbocation species as key intermediates. J. Am. Chem. Soc. 2013;135:10306–10309. doi: 10.1021/ja405219f. [DOI] [PubMed] [Google Scholar]
- 7.Shigehisa H, Nishi E, Fujisawa M, Hiroya K. Cobalt-catalyzed hydrofluorination of unactivated olefins: a radical approach of fluorine transfer. Org. Lett. 2013;15:5158–5161. doi: 10.1021/ol402696h. [DOI] [PubMed] [Google Scholar]
- 8.Girijavallabhan V, Alvarez C, Njoroge FG. Regioselective cobalt-catalyzed addition of sulfides to unactivated alkenes. J. Org. Chem. 2011;76:6442–6446. doi: 10.1021/jo201016z. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi T, Goto N, Nishibata A, Ishibashi H. Iron-catalyzed redox radical cyclizations of 1,6-dienes and enynes. Org. Lett. 2010;12:112–115. doi: 10.1021/ol902562j. [DOI] [PubMed] [Google Scholar]
- 10.Wang LC, et al. Diastereoselective cycloreductions and cycloadditions catalyzed by Co(dpm)2-silane (dpm) 2,2,6,6-tetramethylheptane-3,5-dionate): mechanism and partitioning of hydrometallative versus anion radical pathways. J. Am. Chem. Soc. 2002;124:9448–9453. doi: 10.1021/ja020223k. [DOI] [PubMed] [Google Scholar]
- 11.Streuff J. The electron-way: metal-catalyzed reductive umpolung reactions of saturated and α,β-unsaturated carbonyl derivatives. Synthesis. 2013;45:281–307. [Google Scholar]
- 12.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Enantioselective C–H crotylation of primary alcohols via hydrohydroxyalkylation of butadiene. Science. 2012;336:324–327. doi: 10.1126/science.1219274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo JC, Yabe Y, Baran PS. A practical and catalytic reductive olefin coupling. J. Am. Chem. Soc. 2014;136:1304–1307. doi: 10.1021/ja4117632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikanth GSC, Castle SL. Advances in radical conjugate additions. Tetrahedron. 2005;61:10377–10441. [Google Scholar]
- 15.Lackner GL, Quasdorf KW, Overman LE. Direct construction of quaternary carbons from tertiary alcohols via photoredox-catalyzed fragmentation of tert-alkyl N-phthalimidoyl oxalates. J. Am. Chem. Soc. 2013;135:15342–15345. doi: 10.1021/ja408971t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton DHR, Crich D. Formation of quaternary carbon centres from tertiary alcohols by free radical methods. Tetrahedron Lett. 1985;26:757–760. [Google Scholar]
- 17.Barton DHR, Crich D, Kretzchmar G. Formation of carbon-carbon bonds with radicals derived from the esters of thiohydroxamic acids. Tetrahedron Lett. 1984;25:1055–1058. [Google Scholar]
- 18.Iwasaki K, Wan KW, Oppedisano A, Crossley SWM, Shenvi RA. Simple, chemoselective hydrogenation with thermodynamic stereocontrol. J. Am. Chem. Soc. 2014;136:1300–1303. doi: 10.1021/ja412342g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King SM, Ma X, Herzon SBA. A method for the selective hydrogenation of alkenyl halides to alkyl halides. J. Am. Chem. Soc. 2014;136:6884–6887. doi: 10.1021/ja502885c. [DOI] [PubMed] [Google Scholar]
- 20.Magnus P, Waring MJ, Scott DA. Conjugate reduction of α,β-unsaturated ketones using an MnIII catalyst, phenylsilane and isopropyl alcohol. Tetrahedron Lett. 2000;41:9731–9733. [Google Scholar]
- 21.Zotto CD, et al. FeCl3-catalyzed addition of nitrogen and 1,3-dicarbonyl nucleophiles to olefins. J. Organomet. Chem. 2011;696:296–304. [Google Scholar]
- 22.Shigematsu T, Matsui M, Utsunomiya K. Gas chromatography of diisobutyrylmethane metal chelates. Bull. Inst. Chem. Res. Kyoto Univ. 1968;46:256–261. [Google Scholar]
- 23.Djerassi C, Miramontes L, Rosenkranz G, Sondheimer F. Steroids. LIV. Synthesis of 19-nor-17α-ethynyltestosterone and 19-nor-17α-methyltestosterone. J. Am. Chem. Soc. 1954;76:4092–4094. doi: 10.1016/j.ajog.2005.02.118. [DOI] [PubMed] [Google Scholar]
- 24.Juaristi E, León-Romo JL, Reyes A, Escalante J. Recent applications of α-phenylethylamine (α-PEA) in the preparation of enantiopure compounds. Part 3: α-PEA as chiral auxiliary. Part 4: α-PEA as chiral reagent in the stereodifferentiation of prochiral substrates. Tetrahedron: Asymmetry. 1999;10:2441–2495. [Google Scholar]
- 25.Mancilla T, Contreras R. New bicyclic organylboronic esters derived from iminodiacetic acids. J. Organomet. Chem. 1986;307:1–6. [Google Scholar]
- 26.Gillis EP, Burke MD. A simple and modular strategy for small molecule synthesis: iterative Suzuki−Miyaura coupling of B-protected haloboronic acid building blocks. J. Am. Chem. Soc. 2007;129:6716–6717. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi H, Hojo K, Suginome M. Boron-masking strategy for the synthesis of oligioarenes via iterative Suzuki–Miyaura coupling. J. Am. Chem. Soc. 2007;129:758–759. doi: 10.1021/ja067975p. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Maruyama K. RCu•BF3. 3. Conjugate addition to previously unreactive substituted enoate esters and enoic acids. J. Am. Chem. Soc. 1978;100:3240–3241. [Google Scholar]
- 29.Aurell MJ, Domingo LR, Mestres R, Muñoz E, Zaragová RJ. Conjugate addition of organolithium reagents to α,β-unsaturated carboxylic acids. Tetrahedron. 1999;55:815–830. [Google Scholar]
- 30.Hutchinson DK, Fuchs PL. Amelioration of the conjugate addition chemistry of α-alkoxycopper reagents: application to the stereospecific synthesis of C-glycosides. J. Am. Chem. Soc. 1987;109:4930–4939. [Google Scholar]
- 31.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 44. World Health Organization; 1988. pp. 71–99. [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Disc. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 33.Chu L, Ohta C, Zuo Z, MacMillan DWC. Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-pregabalin. J. Am. Chem. Soc. 2014;136:10886–10889. doi: 10.1021/ja505964r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa H, et al. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogs. J. Am. Chem. Soc. 2009;131:4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullock RM, Samsel EG. Hydrogen atom transfer reactions of transition-metal hydrides. Kinetics and mechanism of the hydrogenation of α-cyclopropylstyrene by metal carbonyl hydrides. J. Am. Chem. Soc. 1990;112:6886–6898. [Google Scholar]
- 36.Matsuo J, Murakami M. The Mukaiyama aldol raction: 40 years of continuous development. Angew. Chem. Int. Ed. 2013;52:9109–9118. doi: 10.1002/anie.201303192. [DOI] [PubMed] [Google Scholar]
- 37.Stork G, Brizzolara A, Landesman H, Szmuszkovicz J, Terrell R. The enamine alkylation and acylation of carbonyl compounds. J. Am. Chem. Soc. 1963;85:207–222. [Google Scholar]
- 38.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. [Google Scholar]
- 39.Jana R, Pathak TP, Sigman MS. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011;111:1417–1492. doi: 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubbaka SR, Vogel P. Organosulfur compounds: electrophilic reagents in transition-metal-catalyzed carbon–carbon bond-forming reactions. Angew. Chem. Int. Ed. 2005;44:7674–7684. doi: 10.1002/anie.200463007. [DOI] [PubMed] [Google Scholar]
- 41.Blumenkopf TA, Overman LE. Vinylsilane- and alkynylsilane-terminated cyclization reactions. Chem. Rev. 1986;86:857–873. [Google Scholar]
- 42.Nakao Y, Hiyama T. Silicon-based cross-coupling reaction: an environmentally benign version. Chem. Soc. Rev. 2011;40:4893–4901. doi: 10.1039/c1cs15122c. [DOI] [PubMed] [Google Scholar]
- 43.Diederich F, Stang PJ, editors. Metal-catalyzed Cross-coupling Reactions. Wiley-VCH; 1998. [Google Scholar]
- 44.Seebach D. Methods of reactivity umpolung. Angew. Chem. Int. Ed. Engl. 1979;18:239–258. [Google Scholar]
- 45.Gao X, Soo SK, Krische MJ. Total synthesis of 6-deoxyerythronolide B via C−C bond-forming transfer hydrogenation. J. Am. Chem. Soc. 2013;135:4223–4226. doi: 10.1021/ja4008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner EW, Mei T-S, Burckle AJ, Sigman MS. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meek SJ, O’Brien RV, Llaveria J, Schrock RJ, Hoveyda AH. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.