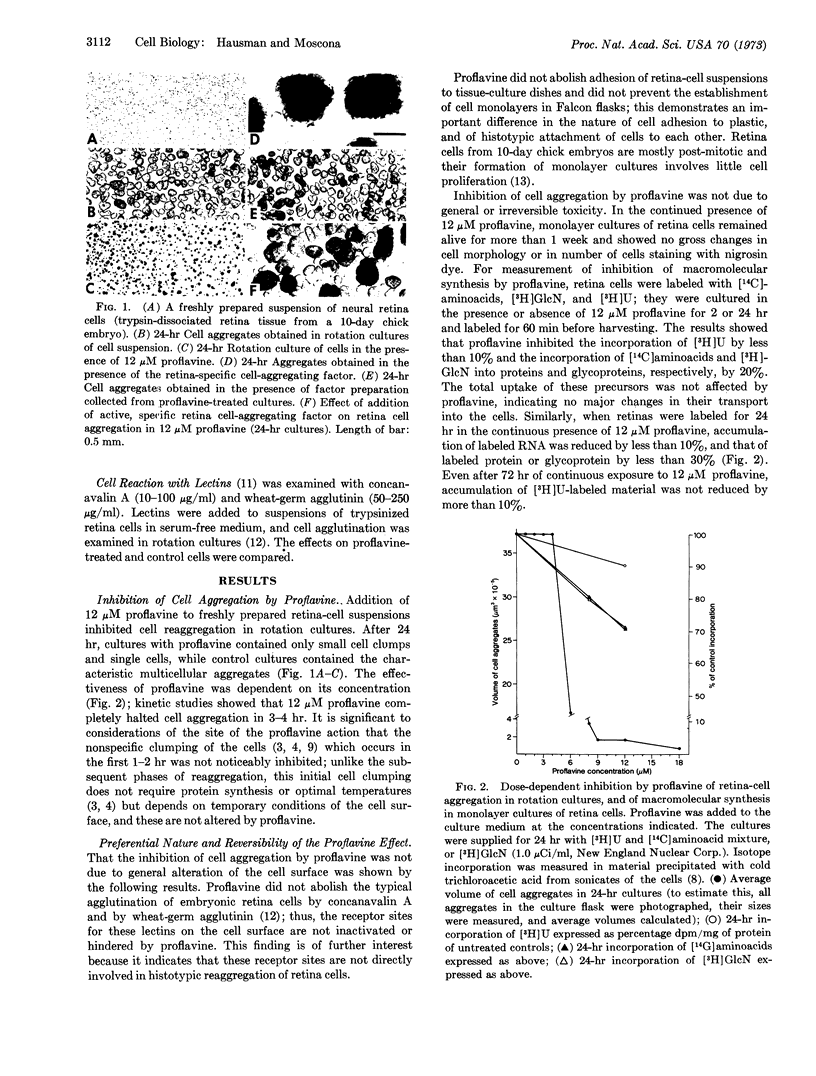
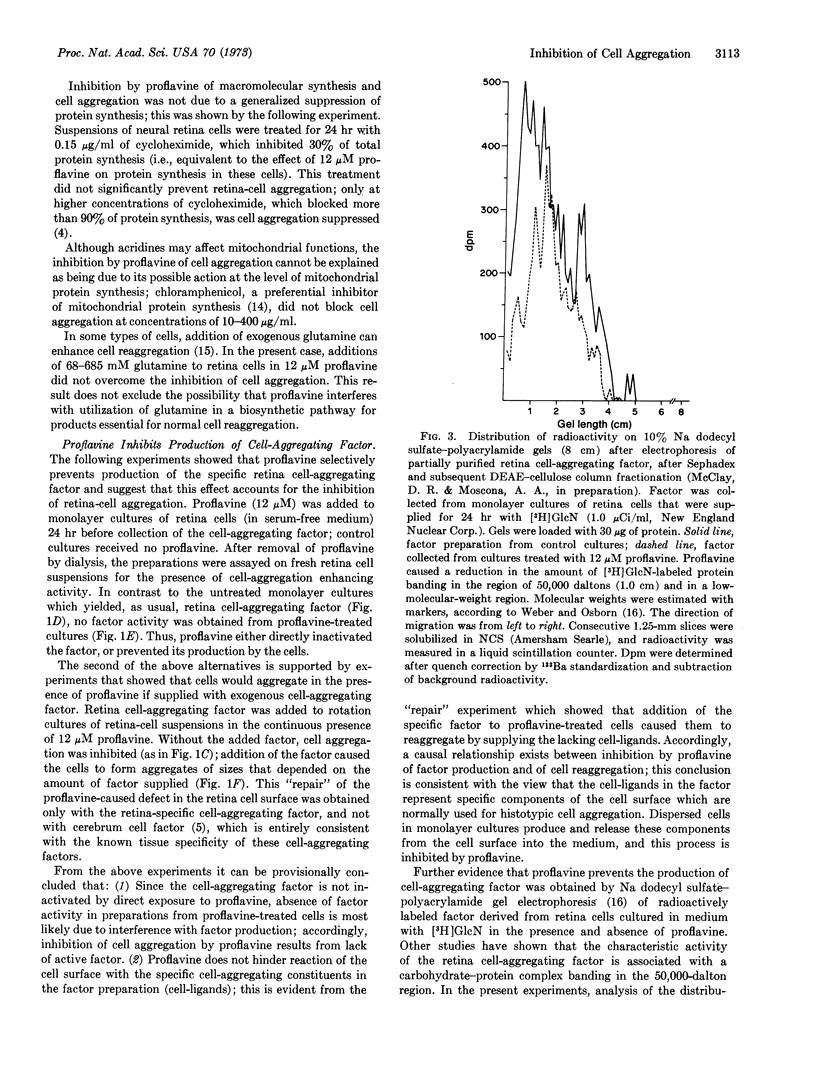
Abstract
Cell recognition and morphogenetic aggregation of embryonic cells into tissues are mediated by specific macromolecules in the cell surface (cell-aggregating factors). A factor specific for embryonic neural retina cells was demonstrated; its synthesis is required for histotypic reaggregation of retina cell suspensions. We show that proflavine (3,6-diaminoacridine) preferentially and reversibly suppresses production of the cell-aggregating factor and thereby inhibits normal cell reaggregation. If such proflavine-treated retina cells are exogenously supplied with the retina-specific factor, they reaggregate. The selectivity of these effects supports the postulated significance of specific cell-surface components in cell association into tissues; the results indicate that proflavine may be a useful molecular probe for studying formation of specific cell-surface components and their role in various cell interactions.
Keywords: cell ligands, cell recognition, differentiation, acridines
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber B. B., Moscona A. A. Reconstruction of brain tissue from cell suspensions. 2. Specific enhancement of aggregation of embryonic cerebral cells by supernatant from homologous cell cultures. Dev Biol. 1972 Feb;27(2):235–243. doi: 10.1016/0012-1606(72)90100-5. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Moscona A. A. Tissue-specific cell-surface antigens in embryonic cells. J Cell Biol. 1972 May;53(2):435–449. doi: 10.1083/jcb.53.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Sanford B., Jeanloz R. W. Regeneration of the surface glycoproteins of a transplantable mouse tumor cell after treatment with neuraminidase. Proc Natl Acad Sci U S A. 1972 Apr;69(4):942–945. doi: 10.1073/pnas.69.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschuster S. J., Moscona A. A. Interactions of embryonic and fetal neural retina cells with carbohydrate-binding phytoagglutinins: cell surface changes with dfferentiation. Exp Cell Res. 1972 Feb;70(2):397–410. doi: 10.1016/0014-4827(72)90152-8. [DOI] [PubMed] [Google Scholar]
- Lilien J. E., Moscona A. A. Cell aggregation: its enhancement by a supernatant from cultures of homologous cells. Science. 1967 Jul 7;157(3784):70–72. doi: 10.1126/science.157.3784.70. [DOI] [PubMed] [Google Scholar]
- Lilien J. E. Specific enhancement of cell aggregation in vitro. Dev Biol. 1968 Jun;17(6):657–678. doi: 10.1016/0012-1606(68)90012-2. [DOI] [PubMed] [Google Scholar]
- Lilien J. E. Toward a molecular explanation for specific cell adhesion. Curr Top Dev Biol. 1969;4:169–195. doi: 10.1016/s0070-2153(08)60484-6. [DOI] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- MOSCONA M. H., MOSCONA A. A. INHIBITION OF ADHESIVENESS AND AGGREGATION OF DISSOCIATED CELLS BY INHIBITORS OF PROTEIN AND RNA SYNTHESIS. Science. 1963 Nov 22;142(3595):1070–1071. doi: 10.1126/science.142.3595.1070. [DOI] [PubMed] [Google Scholar]
- Moscona A. A. Embryonic and neoplastic cell surfaces: availability of receptors for concanavalin A and wheat germ agglutinin. Science. 1971 Mar 5;171(3974):905–907. doi: 10.1126/science.171.3974.905. [DOI] [PubMed] [Google Scholar]
- Moscona M. H., Moscona A. A. Inhibition of cell aggregation in vitro by puromycin. Exp Cell Res. 1966 Mar;41(3):703–706. doi: 10.1016/s0014-4827(66)80127-1. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. B., Edidin M., Orr C. W., Roseman S. An L-glutamine requirement for intercellular adhesion. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1395–1402. doi: 10.1073/pnas.63.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S. B., Humphreys T. Isolation of specific macromolecules required for adhesion of mouse tumour cells. Nature. 1971 Jul 9;232(5306):125–127. doi: 10.1038/232125a0. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A. 1973 Jan;70(1):240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J. E., Glaeser R. M., Todd P. Protein synthesis and aggregation of embryonic cells. Exp Cell Res. 1968 Sep;52(1):43–58. doi: 10.1016/0014-4827(68)90546-6. [DOI] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. An assay for intercellular adhesive specificity. J Cell Biol. 1971 Nov;51(21):525–535. doi: 10.1083/jcb.51.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Studies on intercellular adhesive selectivity. Dev Biol. 1968 Dec;18(6):602–631. doi: 10.1016/0012-1606(68)90029-8. [DOI] [PubMed] [Google Scholar]
- Sheffield J. B., Moscona A. A. Electron microscopic analysis of aggregation of embryonic cells: the structure and differentiation of aggregates of neural retina cells. Dev Biol. 1970 Sep;23(1):36–61. doi: 10.1016/s0012-1606(70)80006-9. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Onodera K. Studies of the plasma membrane of normal and virus-transformed 3 T 3 mouse cells. Biochim Biophys Acta. 1972 Jul 3;274(1):49–63. doi: 10.1016/0005-2736(72)90279-9. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinburg M. S., Gepner I. A. Are concanavalin A receptor sites mediators of cell-cell adhesion? Nat New Biol. 1973 Feb 21;241(112):249–251. doi: 10.1038/newbio241249a0. [DOI] [PubMed] [Google Scholar]
- Storrie B., Attardi G. Expression of the mitochondrial genome in HeLa cells. 13. Effect of selective inhibition of cytoplasmic or mitochondrial protein synthesis on mitochondrial nucleic acid synthesis. J Mol Biol. 1972 Nov 14;71(2):177–199. doi: 10.1016/0022-2836(72)90345-2. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiens A. W., Moscona A. A. Preferential inhibition by proflavine of the hormonal induction of glutamine synthetase in embryonic neural retina. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1504–1507. doi: 10.1073/pnas.69.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler R. J. Carbohydrates in cell surfaces. Int Rev Cytol. 1970;29:77–125. doi: 10.1016/s0074-7696(08)60033-9. [DOI] [PubMed] [Google Scholar]



