Abstract
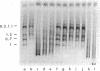
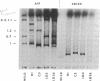
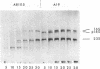
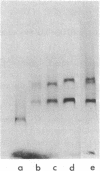
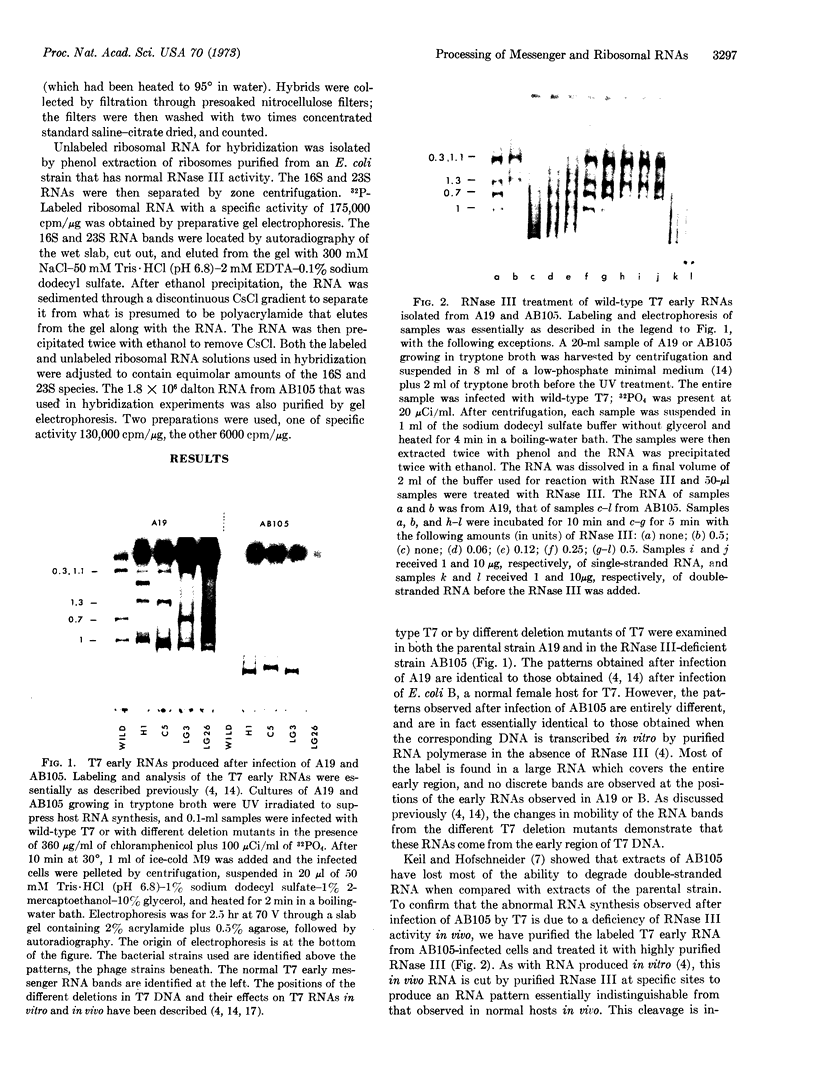
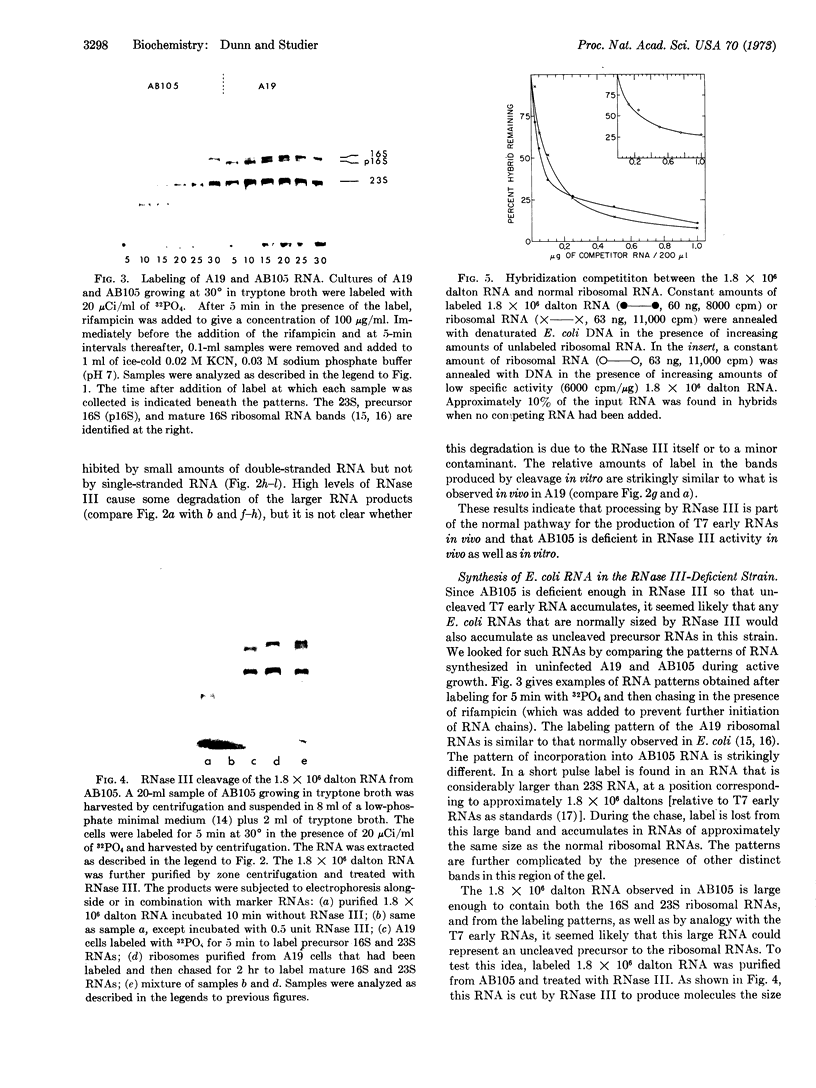
The early region of T7 DNA is transcribed as a single unit in a Ribonuclease III-deficient E. coli strain to produce large molecules essentially identical to those produced in vitro by E. coli RNA polymerase. As with the in vitro RNAs, these molecules are cut by purified RNase III in vitro to produce the messenger RNAs normally observed in vivo. Thus, the normal pathway for producing the T7 early messenger RNAs in vivo appears to involve endonucleolytic cleavage by RNase III. The uninfected RNase III-deficient strain contains several RNAs not observed in the parent strain. Patterns of labeling in vivo suggest that the largest of these RNAs, about 1.8 × 106 daltons, may be a precursor to the 16S and 23S ribosomal RNAs. When this large molecule is treated in vitro with purified RNase III, molecules the size of precursor 16S and 23S ribosomal RNAs are released; hybridization competition experiments also indicate that the 1.8 × 106 dalton RNA does indeed represent ribosomal RNA. Thus, RNase III cleavage seems to be part of the normal pathway for producing at least the 16S and 23S ribosomal RNAs in vivo. Several smaller molecules are also released from the 1.8 × 106 dalton RNA by RNase III, but it is not yet established whether any of these contain 5S RNA sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- Buck K. W., Chain E. B., Himmelweit F. Comparison of interferon induction in mice by purified Penicillium chrysogenum virus and derived double-stranded RNA. J Gen Virol. 1971 Aug;12(2):131–139. doi: 10.1099/0022-1317-12-2-131. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Peacock A. C. Studies of 16 and 23 S ribosomal RNA of Escherichia coli using composite gel electrophoresis. J Mol Biol. 1971 Jan 14;55(1):61–74. doi: 10.1016/0022-2836(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil T. U., Hofschneider P. H. Secondary structure of RNA phage M12 replicative intermediates in vivo. Biochim Biophys Acta. 1973 Jun 23;312(2):297–310. doi: 10.1016/0005-2787(73)90375-4. [DOI] [PubMed] [Google Scholar]
- Kossman C. R., Stamato T. D., Pettijohn D. E. Tandem synthesis of the 16S and 23S ribosomal RNA sequences of Escherichia coli. Nat New Biol. 1971 Nov 24;234(47):102–104. doi: 10.1038/newbio234102a0. [DOI] [PubMed] [Google Scholar]
- MAEKELAE O., MAEKELAE P. H., SOIKKELI S. SEX-SPECIFICITY OF THE BACTERIOPHAGE T7. Ann Med Exp Biol Fenn. 1964;42:188–195. [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Stonington O. G., Kossman C. R. Chain termination of ribosomal RNA synthesis in vitro. Nature. 1970 Oct 17;228(5268):235–239. doi: 10.1038/228235a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Snyder A. L., Kann H. E., Jr, Kohn K. W. Inhibition of the processing of ribosomal precursor RNA by intercalating agents. J Mol Biol. 1971 Jun 14;58(2):555–565. doi: 10.1016/0022-2836(71)90371-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Brunovskis I., Hyman R. W. The process of infection with coliphage T7. VII. Characterization and mapping of the major in vivo transcription products of the early region. J Mol Biol. 1973 Mar 5;74(3):291–300. doi: 10.1016/0022-2836(73)90374-4. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Control of template specificity of E. coli RNA polymerase by a phage-coded protein. Nature. 1969 Sep 13;223(5211):1111–1113. doi: 10.1038/2231111a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]