Abstract
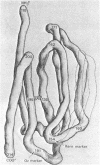
The structure of the Fab′ fragment of a human myeloma immunoglobulin was determined by x-ray crystallographic analysis at 2.8-Å resolution. The Fourier map of the electron density was correlated with the aminoacid sequence to obtain a three-dimensional model. Four globular subunits, which correspond to the homology regions of the light and heavy chains, are arranged in a tetrahedral configuration. These subunits closely resemble each other, sharing a basic pattern of polypeptide chain folding. In each subunit, long sequences of tightly packed, hydrogen bonded polypeptide chain run parallel to the major axis of the subunit. No helical conformation can be seen. Different patterns of interchain disulfide linkage and unusual intrachain disulfide bonds that have been observed in other immunoglobulins can be explained with this model. The regions of hypervariable sequences in the light and heavy chains occur at one end of the molecule, in close spatial proximity.
Keywords: subunit arrangement, disulfide bonds, hypervariable regions, x-ray crystallography
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birshtein B. K., Hussain Q. Z., Cebra J. J. Structure of heavy chain from strain 13 guinea pig immunoglobulin-G(2). 3. Amino acid sequence of the region around the half-cystine joining heavy and light chains. Biochemistry. 1971 Jan 5;10(1):18–25. doi: 10.1021/bi00777a003. [DOI] [PubMed] [Google Scholar]
- Brient B. W., Nisonoff A. Quantitative investigations of idiotypic antibodies. IV. Inhibition by specific haptens of the reaction of anti-hapten antibody with its anti-idiotypic antibody. J Exp Med. 1970 Nov;132(5):951–962. doi: 10.1084/jem.132.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D., Weigert M. Immunochemical analysis of the cross-reacting idiotypes of mouse myeloma proteins with anti-dextran activity and normal anti-dextran antibody. Proc Natl Acad Sci U S A. 1973 Jan;70(1):235–239. doi: 10.1073/pnas.70.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Préval C., Pink J. R., Milstein C. Variability of interchain binding of immunoglobulins. Interchain bridges of mouse IgG2a and IgG2b. Nature. 1970 Dec 5;228(5275):930–932. doi: 10.1038/228930a0. [DOI] [PubMed] [Google Scholar]
- Fleet G. W., Knowles J. R., Porter R. R. The antibody binding site. Labelling of a specific antibody against the photo-precursor of an aryl nitrene. Biochem J. 1972 Jul;128(3):499–508. doi: 10.1042/bj1280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver F. A., Hilschmann N. The primary structure of a monoclonal human lambda-type immunoglobulin L-chain of subgroup II (Bence-Jones protein NEI). Eur J Biochem. 1972 Mar 15;26(1):10–32. doi: 10.1111/j.1432-1033.1972.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Ghose A. C., Jirgensons B. Circular dichroism studies on the variable and constant halves of kappa-type Bence-Jones proteins. Biochim Biophys Acta. 1971 Oct;251(1):14–20. doi: 10.1016/0005-2795(71)90053-5. [DOI] [PubMed] [Google Scholar]
- Givol D., Strausbauch P. H., Hurwitz E., Wilchek M., Haimovich J., Eisen H. N. Affinity labeling and cross-linking of the heavy and light chains of a myeloma protein with anti-2,4-dinitrophenyl activity. Biochemistry. 1971 Aug 31;10(18):3461–3466. doi: 10.1021/bi00794a023. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Eanes E. D., Bladen H. A., Terry W., Page D. L. Creation of "amyloid" fibrils from Bence Jones proteins in vitro. Science. 1971 Nov 12;174(4010):712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Sequence and position of a labeled tryptic peptide. Biochemistry. 1970 Sep 29;9(20):3862–3871. doi: 10.1021/bi00822a003. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. M., Capra J. D. Localization of two additional hypervariable regions in immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2019–2021. doi: 10.1073/pnas.68.9.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell I. J., Frangione B., Porter R. R. The disulphide bonds of the heavy chain of rabbit immunoglobulin G. Biochem J. 1970 Jan;116(2):261–268. doi: 10.1042/bj1160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N., Goldstein D. J., Humphrey R. L. X-ray crystallographic studies of the Fab and Fc fragments of human myeloma immunoglobulins. Cold Spring Harb Symp Quant Biol. 1972;36:421–425. doi: 10.1101/sqb.1972.036.01.054. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N. Structure of Fab' New at 6 A resolution. Nat New Biol. 1972 Feb 2;235(57):137–140. doi: 10.1038/newbio235137a0. [DOI] [PubMed] [Google Scholar]
- Poulsen K., Fraser K. J., Haber E. An active derivative of rabbit antibody light chain composed of the constant and the variable domains held together only by a native disulfide bond. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2495–2499. doi: 10.1073/pnas.69.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Hogg N. M. The amino acid sequences of the Fd fragments of two human gamma-1 heavy chains. Biochem J. 1970 May;117(4):641–660. doi: 10.1042/bj1170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Shimizu A., Paul C., Shinoda T., Köhler H. The amino acid sequence of human macroglobulins. Ann N Y Acad Sci. 1971 Dec 31;190:83–103. doi: 10.1111/j.1749-6632.1971.tb13525.x. [DOI] [PubMed] [Google Scholar]
- Ray A., Cebra J. J. Localization of affinity-labeled residues in the primary structure of anti-dinitrophenyl antibody raised in strain 13 guinea pigs. Biochemistry. 1972 Sep 12;11(19):3647–3657. doi: 10.1021/bi00769a024. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Rossi G., Choi T. K., Nisonoff A. Crystals of fragment Fab': preparation from pepsin digests of human IgG myeloma proteins. Nature. 1969 Aug 23;223(5208):837–838. doi: 10.1038/223837a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Martin N., Thorpe N. O. Affinity labeling of the active sites of antibodies and myeloma proteins. Ann N Y Acad Sci. 1971 Dec 31;190:342–351. doi: 10.1111/j.1749-6632.1971.tb13546.x. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Hood L., Fitch W. M. Antibody diversity. Annu Rev Biochem. 1971;40:969–1012. doi: 10.1146/annurev.bi.40.070171.004541. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]