Abstract
The aim of the study was to examine functional brain activity in response to unpleasant images in individuals with the 7-repeat (7R) allele compared to individuals with the 4-repeat (4R) allele of the dopamine receptor D4 (DRD4) gene (VNTR in exon 3). Based on the response ready hypothesis, individuals with the DRD4-4R/7R genotype were expected to show greater functional brain activity in response to unpleasant compared to neutral stimuli in specific regions of the frontal, temporal, parietal and limbic lobes, which form the networks involved in attentional, emotional, and preparatory responses. Functional Magnetic Resonance Imaging activity was studied in 26 young adults (13 with the DRD4-4R/7R genotype and 13 with the DRD4-4R/4R genotype). Participants were asked to look at and subjectively rate unpleasant and neutral images. Results showed increased brain activity in response to unpleasant images compared to neutral images in the right temporal lobe in participants with the DRD4-4R/7R genotype versus participants with the DRD4-4R/4R genotype. The increase in right temporal lobe activity in individuals with DRD4-4R/7R suggests greater involvement in processing negative emotional stimuli. Intriguingly, no differences were found between the two genotypes in the subjective ratings of the images. The findings corroborate the response ready hypothesis, which suggests that individuals with the 7R allele are more responsive to negative emotional stimuli compared to individuals with the 4R allele of the DRD4 gene.
Keywords: Magnetic Resonance Imaging, Emotion, Attention-Deficit/Hyperactivity Disorder, Risk taking
1. Introduction
Dopamine is a key neurotransmitter, which regulates cognition, attention, emotional processing, motor activation, short-term memory, behavioral inhibition and reward (Wickens, 1990; Nieoullon, 2002; Dreber et al., 2009; Eisenberg et al., 2010b). One of the most studied genes is the dopamine receptor D4 (DRD4), which is located near the telomere of chromosome 11p. The DRD4 gene has alleles due to variation in a 48 base pair tandem repeat (VNTR) in exon 3. The 48 base pair VNTR in exon 3 ranges from 2- repeat alleles (2R) to 11-repeat alleles (11R). The 4-repeat (4R) of the DRD4 gene is the most common allele with a global mean allele frequency of 64.3% (Chang et al., 1996). The 7-repeat (7R) of the DRD4 gene is the second most common allele with a global mean allele frequency of 20.6%, appearing with high frequency in the Americas (Chang et al., 1996). The 7R allele originated as a rare mutational event and was recently affected by positive selection (Ding et al., 2002). The 7R allele is estimated to have emerged 40,000 to 50,000 years ago, which is the same time that major human migration occurred (Wang et al., 2004). This genotype, including its homozygous and heterozygous variations, is found more frequently in populations who had to take great risks to travel long distances, such as early immigrants to the Americas (Chen et al., 1999; Eisenberg et al., 2010a). The association with risk taking was corroborated by research showing that individuals with the 7R allele of the DRD4 gene engage in more financial risk taking compared to those without this genotype (Dreber et al., 2009).
Humans with at least one 7R allele show increased levels of physical activity (Faraone et al., 2001; Kluger et al., 2002; Grady et al., 2003; Grady et al., 2005b; Li et al., 2006; Grady et al., 2013) and appear to be more reactive to environmental factors (Sheese at al., 2007; Belsky et al., 2009; Olsson et al., 2011; Grady et al., 2013). The 7R allele is over-represented in the phenotype of Attention-Deficit/Hyperactivity Disorder (ADHD) (LaHoste et al., 1996; Faraone et al., 2001; Grady et al., 2003; Grady et al., 2005a; Li et al., 2006). In addition, the 7R allele is associated with problematic behaviors including: alcoholism (MacKillop et al., 2007), financial risk-taking (Dreber et al., 2009), disinhibition (Congdon et al., 2008), increased sexual behavior, and infidelity (Zion et al., 2006; Eisenberg et al., 2007; Garcia et al., 2010). However, 7R carriers have an advantage in some tests of reaction time (Swanson et al., 2000a; Langley et al., 2004) and executive function (Swanson et al., 2000b; Gornick et al., 2007; Johnson et al., 2008), though these results have not been consistently replicated (Barkley et al., 2006; Konrad et al., 2010). Intriguingly, a study by Grady et al. (2013) has shown that the 7R allele contributes to longevity by moderating the beneficial effects of an enriched environment in increasing lifespan. The 7R allele of the DRD4 gene has been associated with the response ready hypothesis, which suggests that individuals with hypervigilance might be selected for by environments that are resource-depleted, time critical, or rapidly changing (Jensen et al., 1997; Wang et al., 2004). It is speculated that the 7R allele and its association with the response ready hypothesis might have played a role in its positive selection and human migration (Chen et al., 1999; Ding et al., 2002; Wang et al., 2004; Grady et al., 2013).
The DRD4 receptor protein is expressed in several brain regions, with high levels in the prefrontal cortex (PFC), middle temporal lobe, and limbic areas, i.e. hippocampus, amygdala, and hypothalamus (O’Malley et al., 1992; Meador-Woodruff et al., 1994; Oak et al., 2000). The human attentional network utilizes the superior parietal, inferior parietal, and superior temporal lobes for the orienting response, anterior cingulate for executive functioning, and lateral PFC for control (Fan and Posner, 2004). Previous research has shown that processing of negative emotional stimuli involves the inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus (Davidson and Irwin, 1999), right temporal lobe (Aldhafeeri et al., 2012), precuneus, inferior parietal lobules (Ferri et al., 2013), hippocampus, parahippocampus, and amygdala (Davidson and Irwin, 1999; Nolte, 2002; Aldhafeeri et al., 2012). In addition, the medial, dorsolateral, and ventrolateral PFC are involved in the appraisal process of emotional stimuli (Hariri et al., 2000; Goldin et al., 2008; Rosen and Levenson, 2009).
The aim of this study was to examine functional brain activity in response to unpleasant and neutral images in individuals with and without the 7R allele of the DRD4 gene. Based on the response ready hypothesis, we expected that individuals with a 7R allele would show increased brain activity compared to those without a 7R allele in response to unpleasant stimuli in the bilateral PFC (inferior, middle, and superior frontal gyri), limbic areas (hippocampus, parahippocampus, and amygdala), parietal lobe (inferior parietal lobule and superior parietal lobule), and the right temporal lobe, which are involved in attention, processing of sensory information, and emotions.
2. Methods
2.1 Participants
Twenty-six participants were recruited from the Multimodal Treatment Study of ADHD (MTA) at the University of California, Irvine (UCI). The MTA sample at UCI originally consisted of 144 children (96 with ADHD and 48 normative comparison children) who were seven to nine years old when they first enrolled in 1994 to 1996. After the treatment portion of the MTA study ended, the participants were followed on a regular basis for 16 years. At the time of the present study, there were 68 participants with a diagnosis of ADHD and 41 normative comparison participants (a total of 109) that were still participating in the follow-up protocols. The MTA sample was well-defined in terms of mental health status and dopamine genotypes were available for 86 participants (Swanson et al., 2000a). All 86 young adults were contacted regarding participation in the study. Forty participants were consented for the present study of which 13 had a heterozygous 7R allele (4R/7R). Participants with the DRD4-4R/7R genotype were age and gender matched with 13 participants with the DRD4-4R/4R genotype because the DRD4-4R/4R is the more common genotype. Eleven participants with the DRD4-4R/7R genotype and seven participants with the DRD4-4R/4R genotype had a childhood diagnosis of ADHD. All participants abstained from drugs and alcohol use before the functional Magnetic Resonance Imaging (fMRI) scan, which was verified with urinary and breath drug screens. Table 1 provides an overview of the participants’ characteristics.
Table 1.
Participant characteristics
|
DRD4-4R/4R (n = 13) M (S.D.) or % |
DRD4-4R/7R (n = 13) M (S.D.) or % |
Statistics | |
|---|---|---|---|
| Age, years | 23.23 (1.17) | 23.38 (1.66) | t(24) = −0.27, p = 0.78 |
| Male | 85% | 85% | χ2(1, N = 26) = 0.00, p = 1.00 |
| Female | 15% | 15% | χ2(1, N = 26) = 0.00, p = 1.00 |
| Right-handed | 85% | 92% | χ2(1, N = 26) = 0.38, p = 0.54 |
| Caucasian | 69% | 77% | χ2(4, N = 26) = 4.39, p = 0.36 |
| Employed | 69% | 85% | χ2(1, N = 26) = 0.87, p = 0.35 |
| Education, years | 12.62 (0.96) | 13.54 (1.45) | t(24) = −1.91, p = 0.68 |
| BMI | 27.06 (2.91) | 24.98 (6.16) | t(24) = 1.10, p = 0.28 |
| IQ | 104.92 (15.03) | 99.92 (13.82) | t(24) = 0.88, p = 0.39 |
| Birth weight, kg | 3.26 (0.54) | 3.55 (0.46) | t(24) = −1.48, p = 0.15 |
| Prematurity | 8% | 15% | χ2(2, N = 26) = 2.38, p = 0.30 |
| ADHD in childhood | 62% | 85% | χ2(1, N = 26) = 1.76, p = 0.19 |
| AHA1 Child Score | 8.92 (6.28) | 10.85 (6.45) | t(24) = 0.77, p = 0.45 |
| AHA1 Adult Score | 5.46 (4.81) | 5.85 (5.38) | t(24) = 0.44, p = 0.85 |
| Mothers smoked during pregnancy | 8% | 8% | χ2(2, N = 26) = 0.38, p = 0.83 |
| Emotional valence rating | |||
| Neutral Images | 1.23 (0.28) | 1.13 (0.18) | t(24) = 1.13, p = 0.27 |
| Unpleasant Images | 2.75 (0.69) | 2.69 (0.73) | t(24) = 0.21, p = 0.83 |
| Reaction times in seconds | |||
| Neutral Images | 1.98 (0.07) | 1.96 (0.05) | t(24) = 0.61, p = 0.55 |
| Unpleasant Images | 2.09 (0.08) | 2.09 (0.07) | t(24) = −1.00, p = 0.92 |
| CES-D2 Total scores | 7.31 (7.44) | 7.77 (7.27) | t(24) = −0.16, p = 0.87 |
Assessment for Hyperactivity and Attention,
Center for Epidemiologic Studies Depression Scale
2.2 Functional Magnetic Resonance Imaging (fMRI)
Each participant underwent one fMRI scan at the Center for Functional Onco-Imaging, which is equipped with a 3.0T Philips Achieva scanner and MRI compatible visual display, auditory stimuli, and response device systems. Structural and functional MRI acquisition, quality control, and calibration methods developed by the Center were utilized for the proposed experiments.
The MRI operator ran quality assurance tests daily at the beginning of the day. Each session started with a high resolution (1 mm isotropic voxel size) T1 weighted anatomic scan using 3D MPRAGE pulse sequence with the following parameters: TR/TE = 11/3.3 ms, TI = 1100 ms, TFE = 192, number of slices = 150, SENSE factor = none, Flip angle = 180°, no SENSE acceleration was used and total acquisition duration was 7 minutes. For fMRI scans conventional T2*-weighted gradient echo planar sequences (GE-EPI) were used, with TR/TE = 2500 ms/30 ms, flip angle 90°. Fat-saturation prepulses were included to avoid fat signal overlapping the brain pixels. Slices were in the oblique-axial plane parallel with the AC-PC plane, with 4 mm thickness, and a 1 mm inter-slice gap, and acquired in ascending order. The acquisition matrix was 96×80, not interpolated, with 220 mm FOV. Typically, whole-brain coverage was achieved with 32 slices. The MRI acquisition was sequential. Responses to the task during scanning were monitored using a 4-button keypad (Current Designs, INC, Philadelphia, PA), and reaction times were recorded for subsequent psychophysical analysis of timing and accuracy of task performance. All stimuli were presented and responses recorded using Cogent 2000 software environment (Wellcome Department of Imaging Neuroscience, UK). Head movement in the scanner was restrained by vacuum pillows and tape across the subject’s forehead. A precautionary screening was used to exclude participants who may have contraindications to MRI.
2.3 Emotional stimuli
Participants were asked to look at unpleasant images (e.g., images of negative emotional faces, physical injuries, and insects) and neutral images (e.g., images of neutral faces, landscapes and household items) from the International Affective Picture System (IAPS) (Lang et al., 2008) during the scan. More specifically, participants were asked to look at 24 neutral and 24 unpleasant images1, which were presented in 8 blocks of 3 neutral images followed by 3 unpleasant images. Each image was presented for 5 seconds and was rated within those 5 seconds by participants according to neutrality and unpleasantness. The participants used a keypad with 4 buttons to indicate their rating on a scale from 1 (neutral) to 4 (extremely unpleasant). There were no rest periods between images during these relatively brief sessions. Prior to the scan, participants were familiarized with sample images from the IAPS without labeling or rating them.
2.4 Image processing
Brain images were processed and analyzed with the Statistical Parametric Mapping software, SPM8 (Wellcome Department of Imaging Neuroscience, UK) in MATLAB 7.9 (MathWorks, Natick, MA, USA). Six motion parameters (three rotational and three translational) were used for image realignment and motion correction. Brain volume images with more than 2 mm displacement or 2° rotation were excluded. As a result, 13 out of 240 brain volumes were excluded from one male participant with the DRD4-4R/4R genotype. All images were motion corrected by realignment to the first image, co-registration with the anatomical scan, normalized using the Montreal Neurological Institute (MNI) brain template, and spatially smoothed with an isotropic 5 mm full-width-half-maximum Gaussian kernel.
A first-level general linear model analysis (GLM) was used to generate Blood Oxygen Level Dependent (BOLD) contrasts between unpleasant and neutral images for each participant at p = 0.001 and a voxel threshold of 20. A second-level analysis used the contrast images from the first-level GLM analyses in an analysis of variance (ANOVA) to compare BOLD contrasts of brain activation during unpleasant versus neutral images between the two genotypes at p ≤ 0.05 (False Discovery Rate-corrected) and a voxel threshold of 20.
To provide additional information about brain activation patterns in response to unpleasant compared to neutral images for each genotype, BOLD contrasts from the first-level GLM analyses were examined with a second-level random effects model analysis at p = 0.001 (uncorrected) and a voxel threshold of 20. Clusters were identified by SPM8 and translated into Talairach space with Brett transformations using the MNI-Talairach Viewer (UCI - Institute for Clinical and Translational Science).
2.5 Genotyping
DNA isolation from blood samples and genotyping/sequencing of the DRD4 VNTR was conducted as described previously (Ding et al., 2002; Grady et al., 2003; Wang et al., 2004; Grady et al., 2013).
All experiments were carried out in accordance with the Institutional Review Board at the University of California, Irvine, and were consistent with Federal Guidelines.
3. Results
3.1 Emotional ratings
A significant main effect revealed that unpleasant images were rated significantly more unpleasant than neutral images (M = 2.72, S.D. = 0.69 versus M = 1.19, S.D. = 0.24, F(1,24) = 148.25, p < 0.001). No group differences were found in the image ratings between the two genotypes (see Table 1). Therefore, the choice of stimuli was appropriate and confirmed by this main effect.
3.2 fMRI group differences
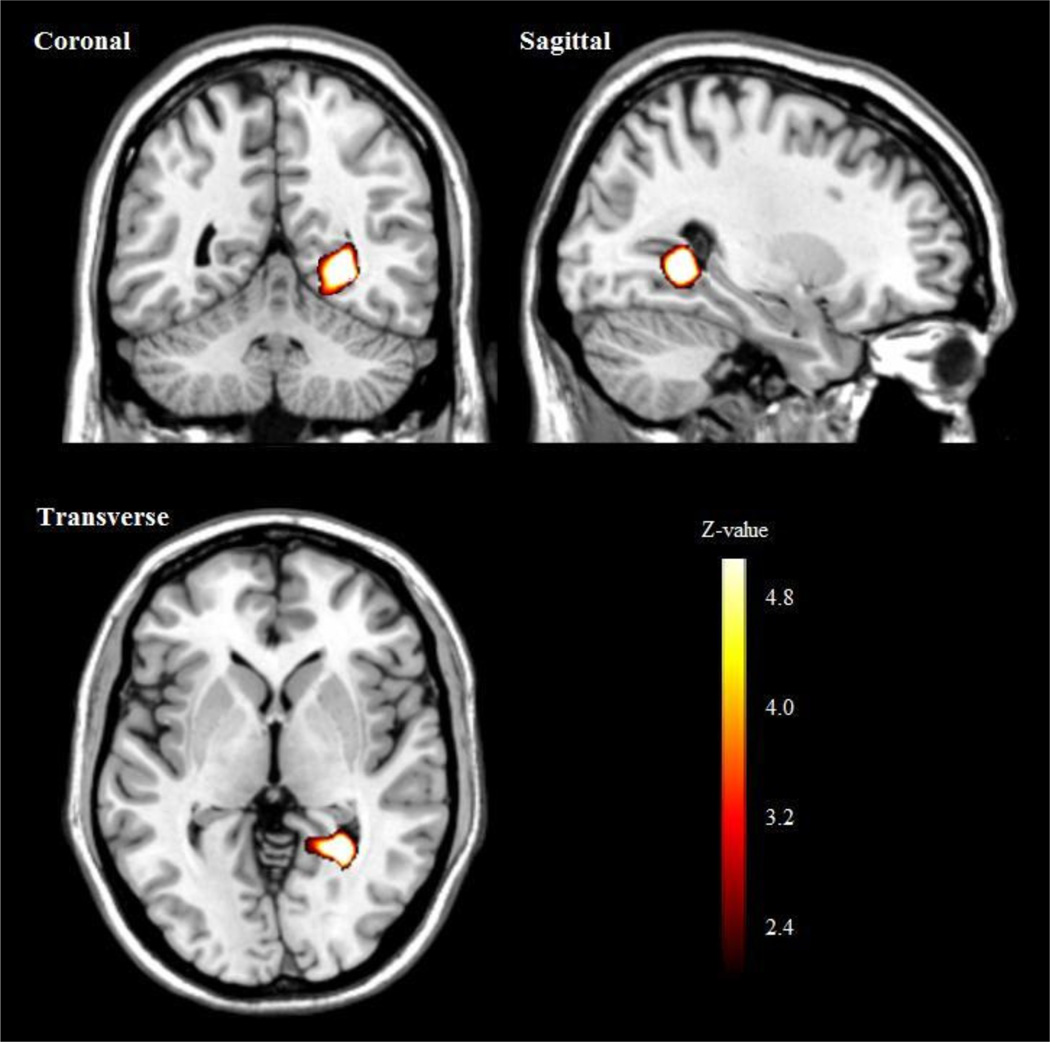
Individuals with the DRD4-4R/7R genotype, compared to the DRD4-4R/4R genotype, showed significant increased BOLD activity in the right temporal lobe in response to unpleasant versus neutral images (see Fig. 1, Table 2).
Fig. 1.
Right temporal lobe activation in response to unpleasant versus neutral images in the DRD4-4R/7R compared to the DRD4-4R/4R genotype.
Table 2.
DRD4-4R/7R genotype activation versus DRD4-4R/4R genotype activation
| Region | Side | Cluster size |
BA | MNI Coordinates | Fmax | P | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Temporal Lobe | ||||||||
| Sub-gyral | R | 30 | 28 | −52 | 0 | 4.51 | 0.0001 | |
3.3 fMRI activity in the DRD4-4R/7R genotype
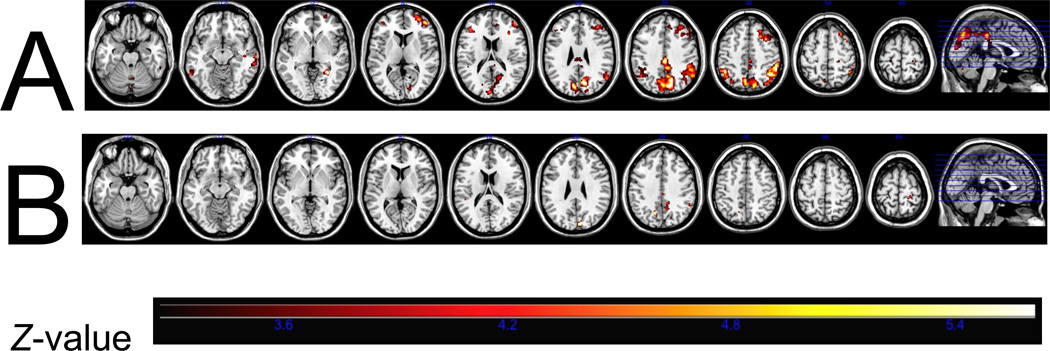
Participants with the DRD4-4R/7R genotype showed increased BOLD activity in response to unpleasant images compared to neutral images in the right superior frontal gyrus, right middle frontal gyrus, right middle temporal gyrus, left parahippocampal gyrus, left posterior cingulate, right cingulate, right precuneus, left cuneus, and the bilateral inferior parietal lobules, which are involved in paying attention, showing empathy, and preparing for action (see Fig. 2, Table 3).
Fig. 2.
Brain activation in response to unpleasant versus neutral images in the DRD4- 4R/7R genotype (Panel A) and the DRD4-4R/4R genotype (Panel B).
Table 3.
DRD4-4R/7R genotype activation
| Region | Side | Cluster size |
BA | MNI Coordinates | Tmax | P | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Frontal Lobe | ||||||||
| Superior Frontal Gyrus | R | 920 | 30 | 40 | 34 | 5.37 | 0.0001 | |
| Superior Frontal Gyrus | R | 8 | 24 | 24 | 56 | 5.31 | 0.0001 | |
| Middle Frontal Gyrus | R | 462 | 46 | 46 | 46 | 12 | 5.81 | 0.0001 |
| Middle Frontal Gyrus | R | 32 | 52 | 8 | 4.92 | 0.0001 | ||
| Superior Frontal Gyrus | R | 10 | 26 | 58 | 4 | 4.79 | 0.0001 | |
| Temporal Lobe | ||||||||
| Sub-Gyral | R | 128 | 28 | −52 | 0 | 8.19 | 0.0001 | |
| Middle Temporal Gyrus | R | 125 | 21 | 66 | −20 | −14 | 5.16 | 0.0001 |
| Middle Temporal Gyrus | R | 62 | −34 | −14 | 4.99 | 0.0001 | ||
| Limbic Lobe | ||||||||
| Parahippocampal Gyrus | L | 24 | −34 | −48 | −10 | 6.46 | 0.0001 | |
| Sub-Gyral | L | −30 | −54 | −6 | 5.39 | 0.0001 | ||
| Posterior Cingulate | L | 24 | 31 | −18 | −66 | 12 | 5.81 | 0.0001 |
| Lingual Gyrus | L | −18 | −64 | 2 | 4.72 | 0.0001 | ||
| Parietal Lobe | ||||||||
| Precuneus | R | 3335 | 12 | −72 | 34 | 7.5 | 0.0001 | |
| Cuneus | L | −10 | −78 | 30 | 6.09 | 0.0001 | ||
| Cingulate | R | 2 | −28 | 40 | 6.01 | 0.0001 | ||
| Inferior Parietal Lobule | R | 1653 | 40 | 54 | −44 | 50 | 6.3 | 0.0001 |
| Inferior Parietal Lobule | R | 40 | 42 | −54 | 48 | 6.1 | 0.0001 | |
| Inferior Parietal Lobule | R | 56 | −32 | 46 | 5.11 | 0.0001 | ||
| Inferior Parietal Lobule | L | 549 | 40 | −46 | −50 | 50 | 6.01 | 0.0001 |
| Inferior Parietal Lobule | L | 40 | −44 | −60 | 52 | 4.97 | 0.0001 | |
| Inferior Parietal Lobule | L | −54 | −48 | 48 | 4.62 | 0.0001 | ||
3.4 fMRI activity in the DRD4-4R/4R genotype
Participants with the DRD4-4R/4R genotype showed increased BOLD activity in response to unpleasant images compared to neutral images, which was limited to the precuneus and cingulate gyrus in the right parietal lobe (see Fig. 2, Table 4).
Table 4.
DRD4-4R/4R genotype activation
| Region | Side | Cluster size |
BA | MNI Coordinates | Tmax | P | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Parietal Lobe | ||||||||
| Precuneus | R | 121 | 10 | −52 | 34 | 4.97 | 0.0001 | |
| Cingulate Gyrus | R | 7 | 14 | −44 | 40 | 4.11 | 0.0001 | |
| Precuneus | R | 12 | −58 | 42 | 3.75 | 0.0001 | ||
4. Discussion
The findings corroborate the response ready hypothesis for the DRD4-4R/7R genotype at the neurobehavioral level. Individuals with the DRD4-4R/7R genotype showed significantly more right temporal lobe activity while viewing unpleasant images compared to those with the DRD4-4R/4R genotype. The right temporal lobe is primarily associated with processing of sensory and emotional information (Rosen et al., 2002; Aldhafeeri et al., 2012). Thus, the greater activation of the right temporal lobe suggests a greater involvement in processing emotional stimuli in individuals with the DRD4-4R/7R compared to those with the DRD4-4R/4R genotype.
Additional findings for each genotype revealed that individuals with the DRD4-4R/7R genotype showed increased brain activity in a complex network including the frontal, temporal, limbic, and parietal lobes when viewing unpleasant compared to neutral images. More specifically, participants with the DRD4-4R/7R genotype showed activation in the parietal cortex, cingulate, and PFC (Fan and Posner, 2004), which are associated with attention to and appraisal of emotional stimuli (Hariri et al., 2000; Goldin et al., 2008; Rosen and Levenson, 2009). As expected, both DRD4-4R/7R and DRD4-4R/4R genotypes were associated with activation of inferior parietal regions, which are involved in passive observation (Cunnington et al., 2006), but only participants with the 7R allele showed activation of DLPFC (Brodmann areas 8, 10, and 46), which reflects attention and preparation for action (Cunnington et al., 2006; Herwig et al., 2007). In addition, the activation of frontal and temporal regions in participants with the 7R allele indicates that the stimuli were perceived as meaningful (Decety et al., 1997). Furthermore, those with the 7R allele showed activation in the right middle temporal gyrus and right cingulate gyrus, which have been associated with empathy during perception of unpleasant or painful stimuli in others (Gu et al., 2010; Lang et al., 2011; Azevedo et al., 2012). In contrast, participants with the DRD4-4R/4R genotype showed brain activation in response to unpleasant compared to neutral images, which was limited to the cingulate gyrus and precuneus in the right parietal lobe without involvement of limbic, frontal or temporal brain areas, suggesting a more passive processing of information.
Intriguingly, participants with the DRD4-4R/4R genotype rated unpleasant stimuli as unpleasant as those with the DRD4-4R/7R genotype although their brain activation pattern indicates less of a response. Thus, the two groups differ in their right temporal brain activity but not in their self-reported emotion. This suggests a mismatch between brain activity and subjective emotional experience. Participants with the DRD4-4R/4R genotype may be over-reporting their emotional experience or participants with the DRD4-4R/7R may be under-reporting their emotional experience.
Taken together, participants with the 7R allele showed a more global and intense activation pattern in response to unpleasant images, which may be indicating increased attention, showing empathy, and preparing for action during unpleasant stimuli. Given higher propensity for risk taking with the 7R allele of the DRD4 gene, the results support the notion that part of risk taking is an appropriate risk assessment that includes paying close attention to unpleasant or threatening stimuli. Furthermore, risky decision-making behaviors have been associated with activation in regions of the temporal lobe including the temporoparietal junction, temporal pole, and the middle and superior temporal gyri (Rodrigo et al., 2014).
However, there are some limitations that require interpreting the findings of the present study with caution. Although not unusual for brain imaging studies, the present study had a small sample size, which may have compromised statistical power, replicability, and stability of the findings. Since pleasant stimuli were not included in this study, it is not possible to determine whether the increased brain activations reflect response readiness rather than an overwhelmed brain experiencing overload from unpleasant stimuli. Similarly, the present findings cannot distinguish between appraisal and response processes. In addition, risk-taking, attention, and empathy measures were not administered, so that the link between brain activity and risky behaviors, attention and empathy was not directly examined. However, by only investigating DRD4-4R homozygous individuals in comparison to DRD4-7R heterozygous individuals, genetic heterogeneity was minimized and our ability to detect differences in brain activity was potentially maximized. Clearly, further studies are warranted.
Despite these limitations, the present findings contribute to the concept of the DRD4-4R/7R genotype as a "plasticity gene" (Belsky et al., 2009). Grady et al. (2013) proposed that the 7R allele of the DRD4 gene may mediate reactivity to environmental stimuli via a dopamine signaling deficit. The present study corroborates this hypothesis by showing that individuals with the DRD4-4R/7R genotype are less likely to ignore negative emotional stimuli. Such mechanisms underlying the 7R allele of the DRD4 gene associated with a response ready hypothesis (Jensen et al., 1997) may have played a role in its positive selection and human migration (Chen et al., 1999; Ding et al., 2002; Wang et al., 2004; Grady et al., 2013).
Forty thousand to 50,000 years ago, humans may have encountered conditions leading to large migrations. Migration into new territories requires a mindset that is dominated by both risk-taking and appropriate response readiness to increase survival. Ding et al. (2002) found that the 7R allele of the DRD4 gene is under positive selective pressure, which raises questions about its adaptability to modern culture. According to Sheese et al. (2007) the 7R allele is associated with a greater influence of parenting, which allows the caregiver more influence on the behavior of the child and increases the child’s adaptation to the social environment. The 7R allele may predispose an individual to be more reactive to the environment, hence faster reaction times (Swanson et al., 2000a) and negative behavioral outcomes in an unfavorable environment (Sheese et al., 2007; Olsson et al., 2011). In particular, modern industrialized environments may increase the risk for negative behaviors in individuals with a 7R allele of the DRD4 gene. Negative behaviors in response to modern environments in individuals with the 7R allele may include ADHD and associated conditions such as drug abuse, financial risk taking, disinhibition, and sexual infidelity (Zion et al., 2006; Eisenberg et al., 2007; MacKillop et al., 2007; Congdon et al., 2008; Dreber et al., 2009; Garcia et al., 2010; Olsson et al., 2011). Such negative behaviors may be the result of dopamine dysregulation, which is at the core of ADHD and associated conditions (Spencer et al., 2007; Swanson et al., 2007; Volkow et al., 2009; Volkow et al., 2011).
Given the documented link between the 7R allele of the DRD4 gene and health risk behaviors, future studies should focus on the effects of health messages (e.g., health warning labels) in this genotype. Within this context, the results of the present study suggest that individuals with a DRD4-4R/7R genotype might be more responsive to unpleasant visual health warning labels about risky drug and sexual behaviors compared to individuals with the more common DRD4-4R/4R genotype. Providing health information and health warning labels in a visual fashion may be a tailored intervention to prevent and reduce health risk behaviors in individuals with the 7R allele of the DRD4 gene.
Acknowledgements
This investigation was supported by Public Health Service research grants DA25131 and RR00827 to Jean-G. Gehricke. In addition, the project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000153. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors gratefully acknowledge Dr. L. Eugene Arnold for his insightful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Neutral Images: 1900,2240, 2280, 2381, 2393, 2745, 2840, 2980, 5030, 5200, 5201, 5600, 5731, 5750, 5760, 5780, 5800, 5890, 5891, 5982, 6150, 7035, 7550, 9070
Unpleasant Images: 1050, 1111, 1200, 1275,1932, 2095, 2110, 2276, 2375.1, 2688, 2700, 3181, 3030, 3301, 3550, 7380,8480, 8485, 9050, 9410, 9421, 9570, 9585, 9810
References
- Aldhafeeri FM, Mackenzie I, Kay T, Alghamdi J, Sluming V. Regional brain responses to pleasant and unpleasant IAPS pictures: different networks. Neuroscience Letters. 2012;512:94–98. doi: 10.1016/j.neulet.2012.01.064. [DOI] [PubMed] [Google Scholar]
- Azevedo RT, Macaluso E, Avenanti A, Santangelo V, Cazzato V, Aglioti SM. Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Human Brain Mapping. 2012;34(12):3168–3181. doi: 10.1002/hbm.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Smith KM, Fischer M, Navia B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2006;141B:487–498. doi: 10.1002/ajmg.b.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human Genetics. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- Chen CS, Burton M, Greenberger E, Dmitrieva J. Population migration and the variation of dopamine D4 receptor (DRD4) allele frequencies around the globe. Evolution and Human Behavior. 1999;20:309–324. [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implication for impulsivity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Robinson S, Moser E. The selection of intended actions and the observation of others’ actions: a time-resolved fMRI study. NeuroImage. 2006;29:1294–1302. doi: 10.1016/j.neuroimage.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin I. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes N, Costes N, Perani D, Jeannerod M, Proyck E, Grassi F, Fazio F. Brain activity during observation of actions: influence of action content and subject’s strategy. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Flodman P, Spence MA, Schuck S, Swanson JM, Zhang YP, Moyzis RK. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreber A, Apicella CL, Eisenberg DT, Garcia JR, Zamore RS, Lum JK, Campbell B. The 7R polymorphism in the dopamine receptor D4 gene (DRD4) is associated with financial risk taking in men. Evolution and Human Behavior. 2009;30:85–92. [Google Scholar]
- Eisenberg DT, Apicella CL, Campbell BC, Dreber A, Garcia JG, Lum JK. Assortative human pair-bonding for partner ancestry and allelic variation of the dopamine receptor D4 (DRD4) gene. Social Cognitive and Affective Neuroscience. 2010a;5:194–202. doi: 10.1093/scan/nsp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Campbell B, MacKillop J, Modi M, Dang D, Lum JK, Wilson DS. Polymorphisms in the dopamine D4 and D2 receptor genes and reproductive and sexual behaviors. Evolutionary Psychology. 2007;5:696–715. [Google Scholar]
- Eisenberg DT, Kohn PD, Baller EB, Bronstein JA, Masdeu JC, Berman KF. Seasonal effects on human striatal presynaptic dopamine synthesis. The Journal of Neuroscience. 2010b;30:14691–14694. doi: 10.1523/JNEUROSCI.1953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Posner M. Human attentional networks. Psychiatrische Praxis. 2004;31:S210–S214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D4 receptor gene and attention deficit hyperactivity disorder. American Journal of Psychiatry. 2001;158:1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Ferri J, Schmidt J, Hajcak G, Canli T. Neural correlates of attentional deployment within unpleasant pictures. NeuroImage. 2013;70:268–277. doi: 10.1016/j.neuroimage.2012.12.030. [DOI] [PubMed] [Google Scholar]
- Garcia JR, MacKillop J, Aller EL, Merriwether AM, Wilson DS, Lum JK. Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. Public Library of Science One. 2010;5:e14162, 1–6. doi: 10.1371/journal.pone.0014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornick MC, Addington A, Shaw P, Bobb AJ, Sharp W, Greenstein D, Arepalli S, Castellanos FX, Rapoport JL. Association of the dopamine receptor D4 (DRD4) gene 7-repeat allele with children with attention-deficit/hyperactivity disorder (ADHD): an update. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;144B:379–382. doi: 10.1002/ajmg.b.30460. [DOI] [PubMed] [Google Scholar]
- Grady DL, Chi HC, Ding YC, Smith M, Wang E, Schuck S, Flodman P, Spence MA, Swanson JM, Moyzis RK. High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Molecular Psychiatry. 2003;8:536–545. doi: 10.1038/sj.mp.4001350. [DOI] [PubMed] [Google Scholar]
- Grady DL, Harxhi A, Smith M, Flodman P, Spence MA, Swanson JM, Moyzis RK. Sequence variants of the DRD4 gene in autism: further evidence that rare DRD4 7R haplotypes are ADHD specific. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2005a;136B:33–35. doi: 10.1002/ajmg.b.30182. [DOI] [PubMed] [Google Scholar]
- Grady D, Moyzis R, Swanson JM. Molecular genetics and attention in ADHD. Clinical Neuroscience Research. 2005b;5:265–272. [Google Scholar]
- Grady DL, Thanos PK, Corrada MM, Barnett JC, Jr, Ciobanu V, Shustarovich D, Napoli A, Moyzis AG, Grandy D, Rubinstein M, Wang GJ, Kawas CH, Chen C, Dong Q, Wang E, Volkow ND, Moyzis RK. DRD4 genotype predicts longevity in mouse and human. The Journal of Neuroscience. 2013;33:286–291. doi: 10.1523/JNEUROSCI.3515-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. The Journal of Neuroscience. 2010;30(10):3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli—an fMRI study. Psychiatry Research. 2007;154:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Mrazek D, Knapp PK, Steinberg L, Pfeffer C, Schowalter J, Shapiro T. Evolution and revolution in child psychiatry: ADHD as a disorder of adaptation. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1672–1679. doi: 10.1097/00004583-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Robertson IH, Barry E, Mulligan A, Daly M, Lambert D, McDonnell C, Connor TJ, Hawi Z, Gill M, Bellgrove MA. Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:927–937. doi: 10.1002/ajmg.b.30718. [DOI] [PubMed] [Google Scholar]
- Kluger AN, Siegfried Z, Ebstein RP. A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Molecular Psychiatry. 2002;7(7):712–717. doi: 10.1038/sj.mp.4001082. [DOI] [PubMed] [Google Scholar]
- Konrad K, Dempfle A, Friedel S, Heiser P, Holtkamp K, Walitza S, Sauer S, Warnke A, Remschmidt H, Gilsbach S, Shafer H, Hinney A, Herebrand J, Herpetz-Dahlmann B. Familiality and molecular genetics of attention networks in ADHD. American Journal of Medical Genetics Part B. 2010;153B:148–158. doi: 10.1002/ajmg.b.30967. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Swanson JM, Glabe C, Wigal T, King N, Kennedy JL. Dopamine D4 receptor gene polymorphism is associated with attention deficit disorder. Molecular Psychiatry. 1996;1:121–124. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A. Vol. 8. Gainesville, FL: University of Florida; 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lang S, Yu T, Markl A, Muller F, Kotchoubey B. Hearing others’ pain: neural activity related to empathy. Cognitive Affective and Behavioral Neuroscience. 2011;11:386–395. doi: 10.3758/s13415-011-0035-0. [DOI] [PubMed] [Google Scholar]
- Langley K, Marshall L, Van den Bree M, Thomas H, Owen M, O’Donovan M, Thapar A. Association of the dopamine D4 receptor gene 7-repeat allele with neuropsychological test performance of children with ADHD. American Journal of Psychiatry. 2004;161:133–138. doi: 10.1176/appi.ajp.161.1.133. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity (ADHD) Human Molecular Genetics. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Menges DP, McGeary JE, Lisman SA. Effects of craving and DRD4 VNTR genotype on the relative value of alcohol: an initial human laboratory study. Behavioral and Brain Functions. 2007;3:1–12. doi: 10.1186/1744-9081-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ., Jr Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10(4):239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Progress in Neurobiology. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nolte J. The Human Brain: An Introduction to its Functional Anatomy. 5th ed. Mosby, St: Louis; 2002. [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HHM. The dopamine D4 receptor: one decade of research. European Journal of Pharmacology. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Moyzis RK, Williamson E, Ellis JE, Parkinson-Bates M, Patton GC, Dwyer T, Romaniuk H, Moore EE. Gene-environment interaction in problematic substance use: interaction between DRD4 and insecure attachments. Addiction Biology. 2011 Nov;29:1355–6215. doi: 10.1111/j.1369-1600.2011.00413.x. [DOI] [PubMed] [Google Scholar]
- O’Malley KL, Harmon S, Tang L, Todd RD. The rat dopamine D4 receptor: sequence, gene structure, and demonstration of expression in the cardiovascular system. The New Biologist. 1992;4:137–146. [PubMed] [Google Scholar]
- Rodrigo MJ, Padrón I, Vega M, de, Ferstl EC. Adolescents’ risky decision-making activates neural networks related to social cognition and cognitive control processes. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Levenson RW. The emotional brain: combing insights from patients and basic science. Neurocase: The Neural Basis of Cognition. 2009;15(3):173–181. doi: 10.1080/13554790902796787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, Weiner M, Levenson RW, Miller BL. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125(10):2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biological Psychiatry. 2007;62:1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, Murias M, Moriarity J, Barr C, Smith M, Posner M. Dopamine genes and ADHD. Neuroscience and Biobehavioral Reviews. 2000a;24:21–25. doi: 10.1016/s0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention deficit/hyperactivity disorder: Brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Review. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Swanson J, Oosterlaan J, Murias M, Schuck S, Flodman P, Spence MA, Wasdell M, Ding Y, Chi HC, Smith M, Mann M, Carlson C, Kennedy JL, Sergeant JA, Leung P, Zhang YP, Sadeh A, Chen C, Whalen CK, Babb KA, Moyzis R, Posner MI. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proceedings of the National Academy of Sciences of the United States of America. 2000b;97:4754–4759. doi: 10.1073/pnas.080070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD. The Journal of the American Medical Association. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ding YC, Flodman P, Kidd JR, Kidd KK, Grady DL, Ryder OA, Spence MA, Swanson JM, Moyzis RK. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. American Journal of Human Genetics. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens J. Striatal dopamine in motor activation and reward-mediated learning: steps toward a unifying model. Journal of Neural Transmission. 1990;80:9–31. doi: 10.1007/BF01245020. [DOI] [PubMed] [Google Scholar]
- Zion B, Tessler R, Cohen L, Lerer E, Raz Y, Bachner-Melman R, Gritsenko I, Nemanov L, Zohar AH, Belmaker RH, Benjamin J, Ebstein RP. Polymorphisms in the dopamine D4 receptor gene (DRD4) contribute to individual differences in human sexual behavior: desire, arousal and sexual function. Molecular Psychiatry. 2006;11:782–786. doi: 10.1038/sj.mp.4001832. [DOI] [PubMed] [Google Scholar]