Abstract
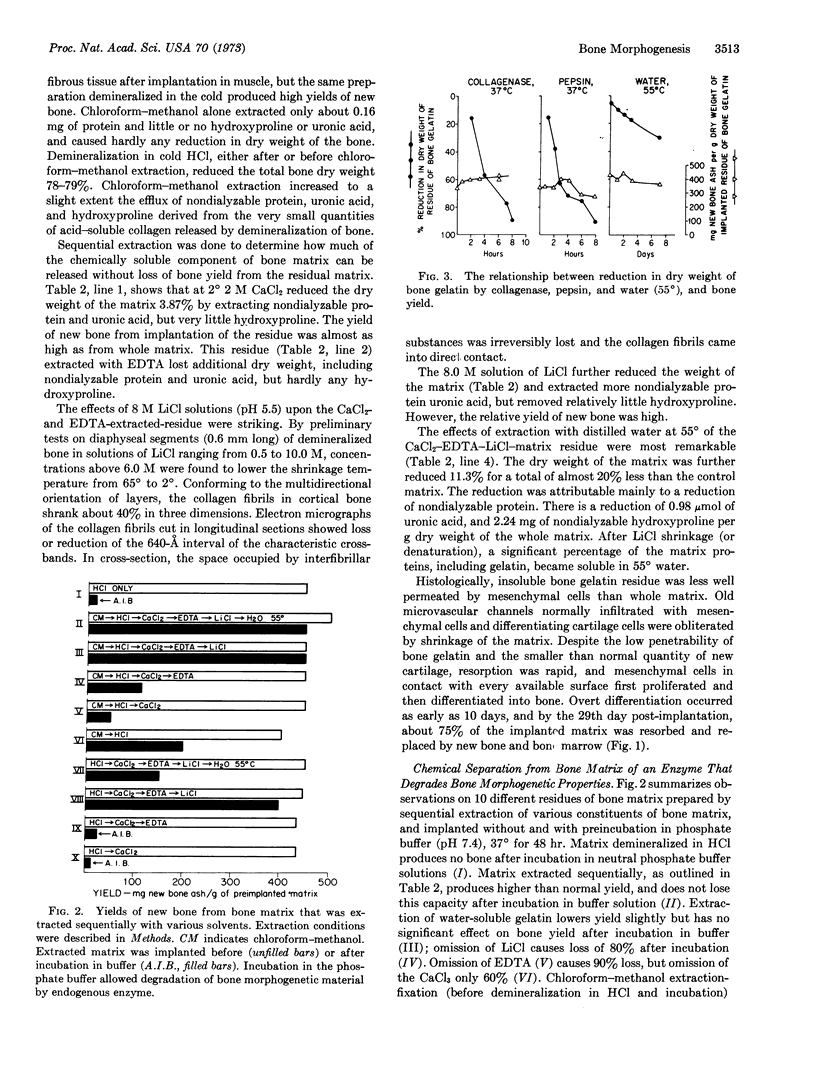
Insoluble bone gelatin with inclusions of insoluble noncollagenous protein produces new bone when implanted in muscle in allogeneic rats. The implanted residue provides the milieu for expression of bone morphogenetic potential of migratory mesenchymal cells. Neutral buffer solutions activate endogenous enzymes that degrade components essential for cell interactions and differentiation of bone. Chloroform-methanol either denatures or extracts constituents responsible for degradation. Insoluble bone gelatin produces new bone after extraction at 2° with neutral salts, 0.5 M EDTA, 0.1 M Tris·HCl, 4 M urea, 0.5 M hydroxylamine, and 10 M KCNS, as well as after limited digestion with pepsin or collagenase, but not after extraction with 5 M guanidine, 7 M urea, water saturated with phenol, or after alkali hydrolysis with 0.1 N NaOH. The specific activity of cell populations interacting with insoluble bone gelatin suggests that a chemical bond between collagen and a noncollagenous protein or part of a protein, cleaved by a neutral proteinase, controls the bone morphogenetic reaction.
Keywords: cell differentiation, osteogenesis, noncollagenous proteins
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Burckard J., Degand P. Isolement et étude des mucopolysaccharides sulfates de l'os compact de Lapin. C R Seances Soc Biol Fil. 1970 Sep 25;164(2):432–438. [PubMed] [Google Scholar]
- Cohen A. M., Hay E. D. Secretion of collagen by embryonic neuroepithelium at the time of spinal cord--somite interaction. Dev Biol. 1971 Dec;26(4):578–605. doi: 10.1016/0012-1606(71)90142-4. [DOI] [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagenous stroma by isolated epithelium grown in vitro. Exp Cell Res. 1971 Mar;65(1):215–220. doi: 10.1016/s0014-4827(71)80069-1. [DOI] [PubMed] [Google Scholar]
- Enlow D. H., Conklin J. L., Bang S. Observations on the occurrence and the distribution of lipids in compact bone. Clin Orthop Relat Res. 1965 Jan-Feb;38:157–169. doi: 10.1097/00003086-196500380-00022. [DOI] [PubMed] [Google Scholar]
- Firschein H. E., Shill J. P. The determination of total hydroxyproline in urine and bone extracts. Anal Biochem. 1966 Feb;14(2):296–304. doi: 10.1016/0003-2697(66)90140-0. [DOI] [PubMed] [Google Scholar]
- Grobstein C., Cohen J. Collagenase: effect on the morphogenesis of embryonic salivary epithelium in vitro. Science. 1965 Oct 29;150(3696):626–628. doi: 10.1126/science.150.3696.626. [DOI] [PubMed] [Google Scholar]
- Huggins C. B., Reddi A. H. Coagulation of blood plasma of guinea pig by the bone matrix. Proc Natl Acad Sci U S A. 1973 Mar;70(3):929–933. doi: 10.1073/pnas.70.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J. T., Wuthier R. E. Histochemistry and biochemistry of calcification with special reference to the role of lipids. Clin Orthop Relat Res. 1968 Jan-Feb;56:237–260. [PubMed] [Google Scholar]
- Iwata H., Urist M. R. Protein polysaccharide of bone morphogenetic matrix. Clin Orthop Relat Res. 1972 Sep;87:257–274. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaver A. G., Shuttleworth C. A. Fractionation of the acid-soluble nitrogen of bone and dentine. Arch Oral Biol. 1967 Aug;12(8):947–958. doi: 10.1016/0003-9969(67)90089-1. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Martin G. R. The collagen of bone. Clin Orthop Relat Res. 1968 Jul-Aug;59:195–232. [PubMed] [Google Scholar]
- Nogami H., Urist M. R. A substratum of bone matrix for differentiation of mesenchymal cells into chondro-osseous tissues in vitro. Exp Cell Res. 1970 Dec;63(2):404–410. doi: 10.1016/0014-4827(70)90229-6. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro I. M. The phospholipids of mineralized tissues. I. Mammalian compact bone. Calcif Tissue Res. 1970;5(1):21–29. doi: 10.1007/BF02017530. [DOI] [PubMed] [Google Scholar]
- Steven F. S. The effect of chelating agents on collagen interfibrillar matrix interactions in connective tissue. Biochim Biophys Acta. 1967 Aug 15;140(3):522–528. doi: 10.1016/0005-2795(67)90526-0. [DOI] [PubMed] [Google Scholar]
- Strates B. S., Urist M. R. Origin of the indutive signal in implants of normal and lathyritic bone matrix. Clin Orthop Relat Res. 1969 Sep-Oct;66:226–240. [PubMed] [Google Scholar]
- Urist M. R., Dowell T. A., Hay P. H., Strates B. S. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968 Jul-Aug;59:59–96. [PubMed] [Google Scholar]
- Urist M. R., Hay P. H., Dubuc F., Buring K. Osteogenetic competence. Clin Orthop Relat Res. 1969 May-Jun;64:194–220. [PubMed] [Google Scholar]
- Urist M. R., Iwata H. Preservation and biodegradation of the morphogenetic property of bone matrix. J Theor Biol. 1973 Jan;38(1):155–167. doi: 10.1016/0022-5193(73)90231-2. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Iwata H., Strates B. S. Bone morphogenetic protein and proteinase in the guinea pig. Clin Orthop Relat Res. 1972;85:275–290. doi: 10.1097/00003086-197206000-00039. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Jurist J. M., Jr, Dubuc F. L., Strates B. S. Quantitation of new bone formation in intramuscular implants of bone matrix in rabbits. Clin Orthop Relat Res. 1970 Jan-Feb;68:279–293. [PubMed] [Google Scholar]
- Urist M. R., Silverman B. F., Büring K., Dubuc F. L., Rosenberg J. M. The bone induction principle. Clin Orthop Relat Res. 1967 Jul-Aug;53:243–283. [PubMed] [Google Scholar]
- Urist M. R., Strates B. S. Bone morphogenetic protein. J Dent Res. 1971 Nov-Dec;50(6):1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- Urist M. R. The substratum for bone morphogenesis. Symp Soc Dev Biol. 1970;29:125–163. [PubMed] [Google Scholar]
- Veis A., Spector A. R., Zamoscianyk H. The isolation of an EDTA-soluble phosphoprotein from mineralizing bovine dentin. Biochim Biophys Acta. 1972 Feb 29;257(2):404–413. doi: 10.1016/0005-2795(72)90293-0. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Cohen J. H. Effects of collagenase on developing epithelia in vitro: lung, ureteric bud, and pancreas. Dev Biol. 1968 Sep;18(3):294–309. doi: 10.1016/0012-1606(68)90037-7. [DOI] [PubMed] [Google Scholar]




