Abstract
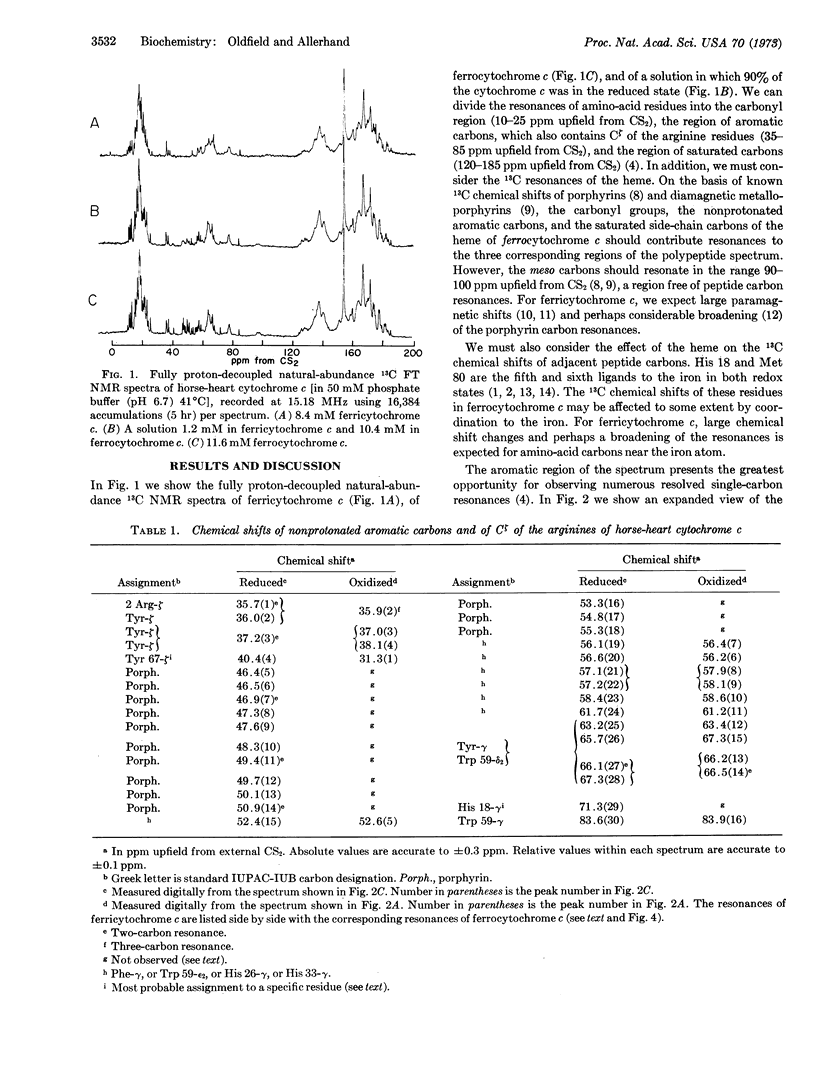
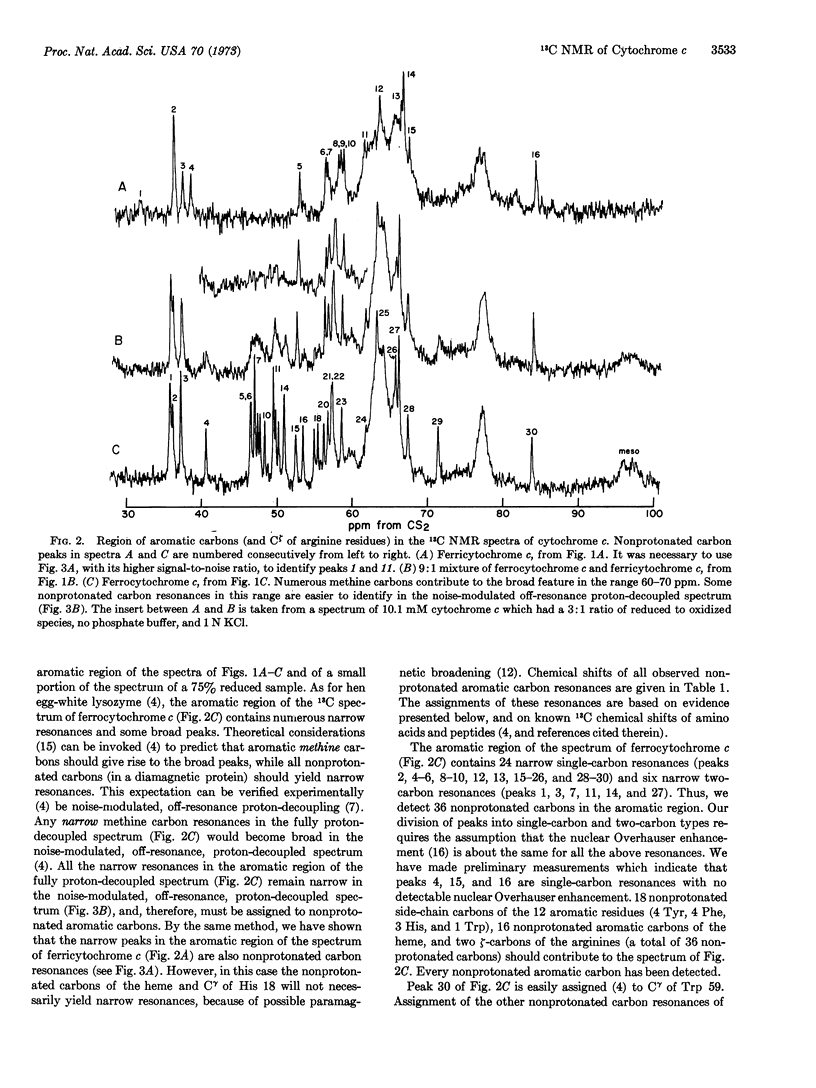
Proton-decoupled, natural-abundance 13C nuclear magnetic resonance spectra (obtained at 15.18 MHz by the Fourier transform method) of aqueous ferrocytochrome c, ferricytochrome c, and mixtures of both species were recorded. The 18 nonprotonated aromatic carbons of amino-acid residues and the 16 nonprotonated aromatic carbons of the heme yielded 22 narrow single-carbon resonances and 6 narrow two-carbon resonances in the spectrum of ferrocytochrome c. Only some of these resonances were detected in the spectrum of ferricytochrome c.
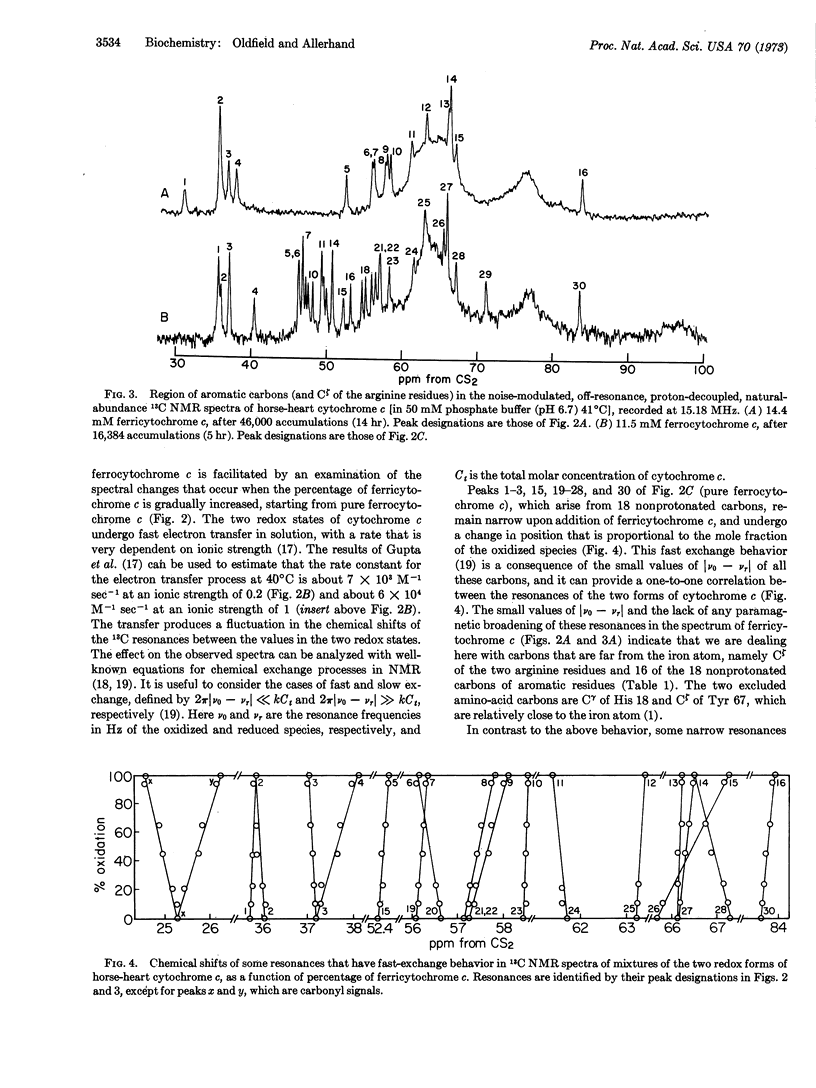
Fast electron transfer between ferrocytochrome c and ferricytochrome c produced chemical exchange effects in spectra of mixtures of the two species: 16 nonprotonated aromatic carbons yielded narrow exchange-averaged resonances as a consequence of their small natural linewidths in both redox states and the small changes in their chemical shifts (relative to the reciprocal of the lifetime between electron exchange) when going from the reduced to the oxidized species. These peaks were assigned to carbons situated far from the iron atom. Their fast exchange behavior was used to establish a one-to-one correspondence between resonances in spectra of the two redox states. The other 18 nonprotonated aromatic carbons yielded exchange-broadened resonances as a consequence of large chemical-shift differences between the diamagnetic and paramagnetic species, and/or large paramagnetic broadening of the resonances of ferricytochrome c. We assigned these resonances (only one of which was identified in the spectrum of ferricytochrome c alone) to carbons that are near the iron atom: Cζ of Tyr 67, Cγ of His 18, and the 16 nonprotonated carbons of the porphyrin ring. Tentative specific assignments for Cζ of Tyr 67 (in the spectra of both redox forms) and for Cζ of His 18 (in the spectrum of ferrocytochrome c) are also presented.
Keywords: horse heart, protein, heme, electron transfer, nonprotonated aromatic carbons
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allerhand A., Childers R. F., Oldfield E. Natrual-abundance carbon-13 nuclear magnetic resonance studies in 20-mm sample tubes. Observation of numerous single-carbon resonances of hen egg-white lysozyme. Biochemistry. 1973 Mar 27;12(7):1335–1341. doi: 10.1021/bi00731a013. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Doddrell D., Caughey W. S. Carbon-13 Fourier transform nuclear magnetic resonance study of some porphyrins. Evidence for a preferred delocalization pathway. J Am Chem Soc. 1972 Apr 5;94(7):2510–2512. doi: 10.1021/ja00762a055. [DOI] [PubMed] [Google Scholar]
- Margalit R., Schejter A. Thermodynamics of the redox reaction of cytochromes c of five different species. FEBS Lett. 1970 Feb 16;6(3):278–280. doi: 10.1016/0014-5793(70)80077-1. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D., Vinogradov S. N. Proton magnetic resonance evidence for methionine-iron coordination in mammalian-type ferrocytochrome c. Biochem Biophys Res Commun. 1969 Aug 7;36(3):442–449. doi: 10.1016/0006-291x(69)90584-1. [DOI] [PubMed] [Google Scholar]
- Redfield A. G., Gupta R. K. Pulsed NMR study of the structure of cytochrome c. Cold Spring Harb Symp Quant Biol. 1972;36:405–411. doi: 10.1101/sqb.1972.036.01.052. [DOI] [PubMed] [Google Scholar]
- Takano T., Kallai O. B., Swanson R., Dickerson R. E. The structure of ferrocytochrome c at 2.45 A resolution. J Biol Chem. 1973 Aug 10;248(15):5234–5255. [PubMed] [Google Scholar]


