Abstract
Parkinson’s disease (PD) is not only characterized by motor disturbances but also, by cognitive, sensory, psychiatric and autonomic dysfunction. It has been proposed that some of these symptoms might be related to the widespread pathology of α-synuclein (α-syn) aggregation in different nuclei of the central and peripheral nervous system. However, the pathogenic formation of α-syn aggregates in different brain areas of PD patients is poorly understood. Most experimental models of PD are valuable to assess specific aspects of its pathogenesis, such as toxin-induced dopaminergic neurodegeneration. However, new models are required that reflect the widespread and progressive formation of α-syn aggregates in different brain areas. Such α-syn aggregation is induced in only a few animal models, for example perikaryon inclusions are found in rats administered rotenone, aggregates with a neuritic morphology develop in mice overexpressing either mutated or wild-type α-syn, and in Smad3 deficient mice, aggregates form extensively in the perikaryon and neurites of specific brain nuclei. In this review we focus on α-syn aggregation in the human disorder, its genetics and the availability of experimental models. Indeed, evidences show that dopamine (DA) metabolism may be related to α-syn and its conformational plasticity, suggesting an interesting link between the two pathological hallmarks of PD: dopaminergic neurodegeneration and Lewy body (LB) formation.
Keywords: α-synuclein, Parkinson’s disease, Lewy body, Animal models, Smad3, Rotenone
Introduction
While the first description of Parkinson’s disease (PD) may date back to ancient Indian and Chinese texts from 1000 BC, the first clear medical description of this disorder was presented by James Parkinson in 1817. Some years later, in the mid-1800s, Jean-Martin Charcot separated PD from multiple sclerosis and other disorders that are also characterized by tremor, and in 1895 Brissaud formulated the hypothesis that the substantia nigra (SN) is the main brain nucleus pathologically affected in PD [1]. Subsequently, it was Friedrich Lewy who first described the protein aggregates that form in different areas of the brain of PD patients, including the dorsal vagal nucleus, locus coeruleus and globus pallidus [2]. Not long after, Trétiakoff validated Brissaud’s hypothesis in 1919 and, by examining post-mortem tissue, he described the protein aggregates in the SN and called them Lewy bodies [3,4]. Despite this long history, even today the etiology of idiopathic PD remains unknown and given the diversity of the molecular mechanisms proposed, it has been suggested that multiple factors may cause the disease [5].
PD is the second most common neurodegenerative disorder that affects the human brain. It is primarily characterized by motor symptoms like akinesia, rigidity, resting tremor and postural instability, manifestations that are mainly derived from the progressive degeneration of dopaminergic neurons in the SN pars compacta. Non-motor symptoms also develop that are associated with cognitive deficits (ranging from memory impairment to dementia), emotional changes (depression, apathy and anxiety), sleep perturbations, autonomic dysfunction (bladder disturbances, orthostatic hypotension, sweating), sensory symptoms (pain, visual impairment, olfactory deficit, paresthesia, ageusia) and gastrointestinal symptoms (constipation, dribbling of saliva: [6].
Although the primary motor symptoms are shared by patients, both the full presentation of the disorder and the response to treatment are quite heterogeneous [7]. It must be borne in mind that non-dopaminergic neuronal loss is also detected in some areas of the brain, for example, that of monoaminergic cells in the locus coeruleus [8] and raphe nuclei, cholinergic cells in the nucleus basalis of Meynert [9] and in the pedunculopontine tegmental nucleus [10], as well as the loss of hypocretin cells in the hypothalamus [11]. Indeed, the other pathological changes observed are widespread, with the appearance of LB inclusions in different areas of the brain (mesostriatal system, cortex, thalamus, hypothalamus, olfactory bulb or brainstem), or alterations in the autonomic system (the spinal cord, sympathetic ganglia and myenteric plexus in the gastrointestinal tract). The widespread nature of this pathology is indicative that the disorder is not just a motor alteration but rather, a sensory, cognitive, psychiatric and autonomic disorder.
Lewy bodies in PD
A common neuropathological feature of some neurodegenerative diseases is the presence of proteinaceous inclusion bodies caused by misfolded and intracellular aggregation of proteins in many brain regions. These abnormal protein deposits may provoke LB pathologies that involve the deposition of LBs in cell bodies, or the formation of Lewy neurites (LNs) and Papp-Lantos inclusions. While the presence of LBs is a histological hallmark of PD, they are also associated with disorders such as dementia with LBs, multiple system atrophy, Alzheimer’s disease, Down’s syndrome, neurodegeneration with brain iron accumulation type I (Hallervorden-Spatz disease), progressive autonomic failure, rapid eye movement sleep disorder, parkinsonism-dementia complex of Guam, Gaucher’s disease or Pick’s disease [12].
LB morphology
LBs are morphologically heterogeneous, with classic LB arising in the brainstem as cytoplasmic inclusions of 8–30 μm in diameter, with a dense eosinophilic core and a narrow pale stained rim. On haematoxylin/eosin staining, classic LBs are observed as a spherical body with a dense core surrounded by a halo [13], whereas the cortical LBs present in layers V-VI of the temporal, insular and cingulate cortex have no obvious halo [14-16]. A third type of LBs are known as pale bodies, that are rounded, pale, eosinophilic granules which lack the eosinophilic core of classic LBs, and that are only weakly and diffusely stained with eosin. These pale bodies are thought to be precursor LBs [17]. In addition, dystrophic LNs are present in axonal processes with both thread-like and spheroid forms [13,18,19].
In dementia with LB disease, inclusions seem to be morphologically homogeneous in the neocortical and paralimbic regions of the brain. In the SN, the LBs immunolabeled with α-syn and ubiquitin fall within the spectrum from diffuse to pale bodies, and to classic LBs. Indeed, this morphological diversity in the SN may represent different stages in the formation of LBs [14].
Electron microscopy has shown that the pale rim and dense core of classic LBs correspond to zones of radially-oriented straight filaments, and zones of circular profiles, respectively. Cortical LBs and LNs also contain filaments [20,21]. Three-dimensional reconstruction from serial confocal images reflects that the LB core, immunolabeled with α-syn and ubiquitin, has a concentric layered structure, with neurofilament encircling these inner layers. Indeed, a frequent continuity between LBs and LNs has been detected, indicating that LNs may evolve into LBs [22].
LB composition
LBs are thought to be mainly composed of α-syn [23] and the morphological characterization of LBs is mostly based on immunochemistry for α-syn, ubiquitin and neurofilament. Furthermore, the α-syn in LBs undergoes post-translational modifications, such as phosphorylation, ubiquitination and oxidative nitration [24]. However, LBs also contain many different proteins [12], such as the Leucine-rich repeat kinase 2 (LRRK2: [25], histone deacetylase 6 (HDAC6: [26] and charged multivesicular body protein 2B (CHMP2B: [27], all of which are found in the core. Indeed, LRRK2 is associated with the endoplasmic reticulum of dopaminergic neurons, HDAC6 is considered a sensor of proteasomal inhibition that plays a central role in autophagy, and CHMP2B is a component of the endosomal sorting complex involved in protein degradation. The identification of proteins present in LBs may offer molecular clues to the processes that may participate in LBs formation.
Nevertheless, the comprehensive molecular composition of LBs is still unclear, although new proteomics approaches based on mass spectrometry provide an interesting approach to discover proteins not yet described in aggregates. However, the success of such approaches will depend on the techniques used to isolate LBs. One study used cortical LB-enriched fractions derived from the LB variant of Alzheimer’s disease to identify 40 LB proteins involved in phosphorylation, ubiquitination, oxidative stress or protein trafficking [28]. Laser capture microdissection of cortical LBs from neurons located in the temporal cortex of dementia with LB disease patients, allowed 296 proteins to be detected, including the chaperone HSC71, confirming the presence of chaperone molecules in LBs [29].
Histological localization of LBs
Studies of the topographic distribution of LBs during the course of sporadic PD has enabled a classification of the stages of disease progression to be drawn up. Neurons susceptible to contain LBs have long, thin, unmyelinated or poorly myelinated axons [30] and the parkinsonian brains analyzed can nearly all be categorized into one of six different stages, based on the location of the inclusion bodies [31]:
Stage 1: At the earliest stage, only the dorsal motor nucleus of the vagus nerve (in the lower medulla oblongata) and the anterior olfactory nucleus are affected, suggesting that the brain pathology originates in these structures.
Stage 2: LB inclusions appear in the raphe nuclei, magnocellular reticular nuclei and locus coeruleus.
Stage 3: The pathology occurs in the basal portions of the midbrain and forebrain, with the SNpc, amygdala, pedunculopontine tegmental nucleus, magnocellular basal forebrain nuclei, tuberomammillary nucleus and spinal cord being affected.
Stage 4: Cortical LBs appear in the temporal mesocortex, in the allocortical CA2 of the hippocampus and in thalamic structures.
Stage 5: The anterior cingulate, insular and subgenual mesocortex is affected, and the neocortex appears to be affected for the first time, with LBs in high order sensory association structures and in the prefrontal neocortex. The hippocampus is clearly affected in the CA1, CA3 and entorhinal cortex.
Stage 6: LBs are present in the primary sensory areas (the auditory field), primary motor field and premotor neocortex.
This model reflects the pathological progression in PD patients (Braak PD stage 4–6), with a caudo-rostral gradient in LB deposition from the lower brainstem to the neocortex, and with both dopaminergic and non-dopaminergic areas being affected (Table 1). Some patients do not have clinical PD symptoms, but display α-syn deposition at autopsy in areas according to Braak PD stage 1–3. They are referred to as showing incidental LB disease (iLBD), considered a prodromal state of PD [32].
Table 1.
Overview of LB-like formation in rodent models. Brain areas with the presence of DA receptors and LB-like and/or LN-like aggregates may suggest an interaction between the dopaminergic system and α-syn (see text)
| Brain Nucleus | Braak’s stage | PrP-A53T-α-syn mice | Rotenone in rats | Smad3 null mice | DA system | |||
|---|---|---|---|---|---|---|---|---|
| X-XII | 1 | +++ | LB- LN | Yes | ||||
| OB | 1 | ++ | LB-LN | Yes | ||||
| sp5 | 2 | ++ | LN | ++ | LN | Yes | ||
| ll | 2 | ++ | LN | Yes | ||||
| Sc | 2 | ++++ | LB-LN | ++ | LB | Yes | ||
| RF/LC | 2 | +++ | LB- LN | ++ | LN | Yes | ||
| RN | 2, 3 | +++ | LB-LN | Yes | ||||
| VTA | 3 | + | LB- LN | ++ | LN | Yes | ||
| CPu | 3 | ++ | LB- LN | +++++ | LB- LN | ++++ | LN | Yes |
| SN | 3 | + | LB- LN | +++++ | LB- LN | +++++ | LN | Yes |
| Hp | 3,4 | ++++ | LB-LN | Yes | ||||
| Th | 4 | +++ | LB-LN | Yes | ||||
| M1-M2 | 5,6 | ++++ | LB-LN | Yes | ||||
| Cg | 5,6 | +++ | LN | Yes | ||||
| cc-ic-cp | -- | +++ | LN | |||||
| Pn | -- | ++ | LB | Yes | ||||
| CB | -- | ++++ | LB-LN | ++ | LN | Yes | ||
| Pir | -- | +++ | LN | Yes | ||||
| IC | -- | +++ | LB-LN | |||||
| SC | -- | +++ | LB-LN | Yes | ||||
Besides neuronal α-syn deposition in LBs and LNs, the presence of inclusions in astrocytes has been detected by silver staining. Glial inclusions are widespread in PD, both in areas with neuronal loss and gliosis (substantia nigra, locus coeruleus and dorsal vagal nucleus), and in areas with no clear neurodegeneration or gliosis (cerebral cortex, cerebral white matter, striatum, globus pallidus, thalamus, cerebellum and spinal cord: [33]. Indeed, α-syn-immunoreactive (−ir) astrocytes adopt a topographical distribution that closely parallels that of the cortical LBs [34].
However, we should consider that this disorder has a very heterogeneous clinical manifestation [35] and as yet, there is no clear significance attributed to the presence of LBs and LNs, as direct determinants of the clinical manifestations and symptoms evident in patients with PD [36,37].
LB genetics
It is considered that most patients with PD develop the idiopathic form of the disease as opposed to that which is genetically inherited. Although, 10-30% of PD patients report a first-degree relative with the disorder, this does not necessarily reflect genetic inheritance as it may correspond with exposure to a common environmental factor [38,39]. However, it is increasingly clear that genetic factors contribute to the pathogenesis of PD and it is currently considered a multifactorial complex disorder, caused by interactions between genetic and environmental risk factors. Alternatively, only 10% of cases develop the rarer form of PD that follows Mendelian inheritance, representing 2% of late-onset and 50% of early-onset familial PD [40,41]. Studies into these familial forms have identified several causative genes related to mitochondrial or lysosomal dysfunction, protein aggregation, the proteasome system and kinase signaling [42].
LB deposition is associated with specific mutations in some of these already known genes, including α-syn (SNCA: [4,43] and LRRK2 [44,45]. Moreover, the distinction between the familial and idiopathic form of PD is currently not clear in all cases, as some evidence suggests that SNCA or LRKK2 mutations also participate in the sporadic disease.
SNCA
Several dominant mutations have been described in the gene encoding α-syn, SNCA, with varying penetrance. In 1997, the A53T missense mutation was reported in a large Italian family associated with familial PD [4]. Subsequently, two additional missense mutations in SNCA were also seen to be associated to familial PD, A30P [46] and E46K [47], although these point mutations are extremely rare. Duplicate and triplicate loci of SNCA of different sizes (from 0.4 to 4.5 Mb) have been seen to give rise to PD, which has been related to the overexpression of the wild-type protein [43,48], influencing the age of onset and severity of the disorder [49]. Indeed, SNCA duplications have also been reported in apparently sporadic PD patients [50] and interestingly, patients carrying these mutations present a broad clinical phenotype, even within a given family, suggesting an influence of genetic modifiers [25,51,52]. In addition, a dinucleotide repeat polymorphism (Rep1) has been described in the promoter region, 10 Kb upstream of the start codon, which induces SNCA overexpression and that may account for increased risk in 3% of non-familiar PD [53,54]. Moreover, specific haplotypes, primarily in the 3’ UTR region, may also be associated to sporadic PD [55,56].
Although SNCA mutations are very rare, their identification led to the association of α-syn with LBs. This protein is predominantly localized in presynaptic nerve terminals [57,58] and missense mutations may reduce its affinity for lipids, enhancing its propensity to adopt a β-sheet conformation and promoting self-assemble into oligomers and fibril formation [59]. In this sense, the A53T mutation of SNCA is associated with the predominantly neuritic aggregation of α-syn in the human brain [60,61] and in a mouse model [62], as well as with severe motor impairments. Alternatively, SNCA overexpression may decrease the density of dopaminergic vesicles and of synaptic contacts [63]. Neuropathological diagnosis of PD requires both dopaminergic neurodegeneration in the SNpc and the presence of LBs, and as described previously, the severity of the clinical manifestation has been correlated with the broad distribution of LBs [31], which may be related to the number of alleles. Both triplication and point mutations in SNCA are associated with the formation of cortical and subcortical LBs, and a clinical diagnosis of PD with dementia [47,64].
The SNCA gene and that encoding the microtubule-associated protein tau (MAPT) have consistently been associated in different populations of sporadic PD [65], mainly those of European origin [66]. However, such an associated was not identified in Japanese population [67], suggesting that population-specific differences may exist in the genetics of PD.
Leucine-rich repeat kinase 2 (LRRK2)
Point mutations in the LRRK2 gene are frequently associated to PD, and they are found in both late onset familial and sporadic PD. LRRK2 mutations have the highest prevalence rate in PD patients discovered to date, having been found in 10% of cases with autosomal dominant familial PD, in 3.6% of sporadic PD cases and even in 1.8% of healthy controls [44,45,68]. The presence of mutations in healthy controls may suggest a reduced and incomplete age-dependent penetrance. However, while over 80 missense variants have been identified, only seven mutations are considered pathogenic (N1437H, R1441G/C/H, Y1699C, G2019S and I2020T), most of them lying in the C-terminal half of the protein, while two others are considered as risk factors (G2385R and R1628P: [69]. Of these, G2019S is the most frequent mutation in the Caucasian population, which explains 1% of PD cases [70]. The prevalence of this mutation is strongly influenced by ethnicity and as such, it is more frequent in patients of Southern European or North African origin, and in Ashkenazi Jews (18-40% PD cases), yet it is very rare in Asians or Northern Europeans [40,69]. The risk of PD for a person who inherits this mutation is 28% at age 59 years, 51% at 69 years and 74% at 79 years [71]. The G2019S mutation gives rise to a uniform clinical phenotype that resembles sporadic PD, both in homozygous and heterozygous carriers [71].
LRRK2 is a gene with 51 exons and it encodes a large protein with two different enzymatic activities in the same molecule: that of a kinase and a GTPase. LRRK2 also has multiple protein interaction domains suggesting that it may serve as a scaffold for the assembly of other proteins. The G2019S mutation affects the kinase domain of the protein, with other common mutations lying in the GTPase domain or in the protein interaction domains. The function of LRRK2 remains largely elusive but G2019S mutations enhance its kinase activity, which may mediate neural toxicity [72]. LRRK2 is widely expressed in the healthy adult brain, mainly in the endoplasmic reticulum. Interestingly, in sporadic PD patients LRRK2 is found in the core of LBs in the SN and locus coeruleus, suggesting it may contribute to LB formation [25]. Indeed, some PD patients with LRRK2 mutations have LB pathology [73] but this is not always the case, even within a family [40]. How LRRK2 might participate in LB formation remains unknown, although dysfunctional autophagy has been proposed [74], as well as altered solubility and aggregation of α-syn [75].
Recessive mutations
Homozygous or compound heterozygous mutations in the recessive genes Parkin, PINK and DJ-1 are associated to relatively rare forms of familial PD, and these result in early onset PD and nigral dopaminergic neuronal loss. Parkin mutations account for around 50% of familial juvenile and early onset PD, reaching 80% in patients with onset before the age of 20 and decreasing with increasing age at onset, becoming very rare when onset occurs after the age of 50 [42]. At least 170 mutations have been described in Parkin, including point mutations, exon rearrangements, “indels” and duplications. Homozygous mutations with loss-of-function predominantly induce neuronal loss in the SN and locus coeruleus, in the absence of LBs. However, compound heterozygous mutations with LB pathology have been described in a minority of patients [76]. This variability may indicate some mutation-specific effect, as it has been described loss-of-function, inactivating and activating mutations [77]. Parkin is a cytosolic E3 ubiquitin ligase that transfers ubiquitin to specific protein substrates for proteasomal and autophagic degradation. Mutations may impair Parkin activity, which might reduce the clearance and aggregation of proteins. As such, these mutations could participate in decreased α-synuclein clearance [78] or alternatively, Parkin mutations might decrease its solubility and lead to aggregate formation [79].
Other recessive mutations are less common, such as PINK-1 [80] and DJ-1 [81], producing clinical manifestations broadly similar to Parkin mutations and in the absence of LBs.
GBA mutations
Glucocerebrosidase (GBA) mutations were first described in Gaucher’s disease, a recessive lysosomal storage disorder that may develop into parkinsonism, and PD patients may have an increased frequency of GBA mutations [82]. Indeed, carriers of GBA mutations exhibit clinical features related to early-onset, levodopa-responsive PD, experiencing hallucinations and symptoms of cognitive decline or dementia, with the abundant presence of LBs in the neocortex [83]. GBA mutations in PD patients confer increased susceptibility to an earlier disease onset, to have affected relatives and to develop atypical clinical manifestations [84].
Animal models of α-syn aggregation
Animal models are used in order to study the neurobiological basis of human disorders and to develop new treatments. When examining the validity of an animal model we can consider face validity (similarity between neurological and behavioral phenotypes seen in an animal model and the human patient), construct validity (consistent with a theoretical rational, such that the molecular and cellular changes that result from genetic manipulations should be the same as those that occur in humans), and predictive validity (i.e. it should respond to pharmacological treatment as in humans) [85,86]. Face validity is a major criterion for model evaluation. One can argue that modeling of LB deposition and dopaminergic neurodegeneration may correlate to the human pathology. However, the behavioral phenotypes differ considerably between animals such as mice and humans. Thus, face validity may prove to be an unrealistic criterion for some symptoms of the disease such as motor and cognitive deficits or emotional changes. Although some researchers advocate the primacy of one of these approaches, in practice, the validity of a model should consider all three sources of evidence [87].
Considering the histopathological hallmarks of PD, models of the disorder should address the progressive loss of dopaminergic neurons in the SNpc, reduced striatal dopamine content and altered catabolism, and it should be age dependent and with progressive alteration of the animal’s movement. Furthermore, the Braak staging scheme has strongly influenced what we think would be a good animal model for PD. Indeed, the presence of LBs and LNs, the post-translational modifications observed in the human disorder, and the composition and histological localization of LBs are all central features that an animal model of PD must address. In this review we will focus on those models related to α-syn aggregation and LB-like formation.
Classical neurotoxin-induced rodent models of PD have been studied in depth and they involve treatment with 6-hydroxydopamine (oxidative stress), MPTP, rotenone and paraquat (mitochondrial complex I inhibition), PSI and epoxomicin (proteasomal inhibition), and lipopolysaccharide (glial activation). Some of these models address the toxic insult hypothesis, whereby pesticide and herbicide use can increase the risk of PD. Most of these chemical models induce degeneration of the dopaminergic neurons in the rodent and primate SN, inducing a motor syndrome that can be modulated by anti-parkinsonian medication. Indeed, the MPTP-treated primate is still the animal model used to test drugs during their selection for clinical trials in humans [88]. These models have been useful to propose pathogenic events that occur in the disease and each mechanism (oxidative stress, mitochondrial complex I inhibition, etc.) is thought to contribute to the pathogenesis of the disorder. While all these models usually display robust nigro-striatal degeneration, the formation of LB inclusions is not a common feature. In this sense, MPTP and 6-OHDA neurotoxic models have face validity related to motor features, although construct (also known as aetiological) validity are limited [89]. Indeed, the poor predictive validity of these models seems to be related to the high failure rate of new treatments in clinical trials [90].
The discovery of mutations in patients has led to the generation of different genetic models, which may prove to be a more realistic approach to study PD. Many different species and cell models are useful for genetic manipulation, including mice, Drosophila melanogaster and Caenorhabditis elegans. In this sense, several transgenic models have been described that carry SNCA or LRRK2 mutations, and while many of these develop inclusions they fail to display robust neurodegeneration. Recessive models using knockout mice of PINK-1, Parkin or DJ-1 similarly fail to exhibit a nigro-striatal pathology [88]. Animal models carrying mutations in GBA have recently been described in the context of PD [91-93], while other animal models focus on altering the intracellular signaling of neurotrophic factors like Smad3 or on the mechanisms of aggregation of α-syn. It should be noted that most models do not capture the main hallmarks of PD in the same animal and hence, most models are only suitable to address one particular issue. However, interesting approximations for LB-like formation have been obtained with rotenone treatment, α-syn transgenesis with either wild-type or A53T mutation and in Smad3 deficient mice. Thus, the current models do appear to be useful to asses not only motor symptoms but also aetiological and predictive validity of neuroprotective molecules that could halt the pathological progression of the disease.
Non-human primates
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a neurotoxin used in non-human primates as the most relevant animal model of PD, given its ability to induce persistent parkinsonism in humans and on the induction of selective destruction of midbrain dopaminergic neurons [94-96]. Cardinal motor symptoms of PD are reproduced in this model, as well as other non-motor symptoms such as constipation, salivation, sleep disturbance and cognitive deficits [97]. The MPTP model has limitations as the rapid toxicity results in the acute onset of neurodegeneration and neurological symptoms. Although increased α-syn immunoreactivity (−ir) within surviving neurons is detected, MPTP-affected dopaminergic neurons do not develop LBs or LNs [98].
Rodents
MPTP in mice
In mice, the MPTP model must be induced in specific strains for it to be a consistent model, such as C57 and Swiss Webster mice [99]. The loss of dopaminergic neurons depends on the administration regime (from acute to chronic), ranging from 60% to 90% [88]. Acute or sub-chronic administration does not lead to the formation of inclusions and while α-syn inclusions have been detected in the study of some chronic models, this is not always replicated. In this sense, α-syn-ir was detected in dopaminergic neurons when chronic MPTP treatment was coupled with probenecid administration to block the rapid clearance of MPTP and its derivates. On examination by electron microscopy, the neurons that accumulate α-syn appeared to contain lipid droplets or secondary lysosomes covered by proteins. Although this is quite a distinct morphology to the straight filaments detected in human LBs, it was suggested that lipofuscins may be important for the development of LBs [100,101]. When MPTP was administered continuously using an osmotic minipump, ubiquitin-ir and α-syn-ir nigral inclusions formed, although their co-localization in the same cell or aggregate was not studied. Ultrastructurally, these inclusions appeared as concentric membranes containing α-syn that was transformed into fibrillar morphology [102]. However, other studies failed to find such inclusions following acute, semi-chronic or chronic MPTP treatment with probenecid or using osmotic minipumps [103-105]. Thus, further work is required before this model can be used to study aggregate formation.
Rotenone in rats
Rotenone is an insecticide that accumulates in the mitochondria, inhibiting complex I and promoting oxidative stress [106], as well as inhibiting the ubiquitin-proteasome system in vitro [107]. While this model is one of the most promising models for PD studies, it has several drawbacks since rotenone is not a selective dopaminergic neurotoxin, it produces high rates of mortality (~30%) and there is also strong variability in neurodegeneration (only observed in 50% of rats). Interestingly, peripheral treatment with rotenone induces the formation of α-syn inclusions, with a dense core and fibrillar surrounding that resembles those observed in PD [108,109]. The aggregation of α-syn may be enhanced by neonatal lipopolysaccharide (LPS) treatment, suggesting some cooperation between perinatal inflammatory processes and exposure to this pesticide [110]. In vitro studies have shown that rotenone induces a conformational change in α-syn and that it accelerates the rate of fibril formation [111,112]. Indeed, like the human pathology α-syn deposits are observed in the myenteric plexus [113]. In order to avoid peripheral rotenone toxicity and hence, the high mortality rate in rats, intracerebral administration of this toxin has been used, showing significant nigro-striatal neurodegeneration but not the appearance of α-syn inclusions [114]. Indeed, variability in the results obtained following peripheral administration (subcutaneous versus intravenous infusion) has limited the use of this model as a reliable tool to study PD [115,116].
Combined administration of other herbicides and fungicides, such as paraquat and Maneb also produces a high mortality rate and modest nigro-striatal neurodegeneration without LB deposition [88].
Proteasome inhibitors
The discovery of mutations in parkin and ubiquitin carboxy terminal hydrolase-1 (UCH-L1) in familial PD focused attention on the ubiquitin-proteasome system, suggesting that the toxicity of α-syn was associated with misfolding of the protein, and impaired proteasomal and lysosomal degradation [117]. Indeed, the combined systemic administration of the proteasome inhibitors PSI/epoxomicin produces a loss of nigro-striatal neurons and progressive motor disabilities. Moreover, inclusion bodies were observed not only in the SN but also, in the locus coeruleus, raphe and substantia innominata [118]. However, this model has proved very difficult to reproduce and the data has not been replicated in mice, rats or primates, in which only a partial response to proteasomal inhibitors is obtained [88]. This variability frustrates the use of this model in PD research. More recently, conditional genetic deletion of a proteasomal subunit in mice that disrupts 26S proteasome degradation was shown to induce the loss of dopaminergic neurons and the appearance of inclusions in SN neurons that contain ubiquitin and α-syn. Although no biochemical studies of aggregation or double-immunolabeling have been performed to clarify the nature of these inclusions, the inclusions appear to contain mitochondria and double-membraned autophagolysomes at the ultrastructural level. However, there was no resemblance of the radially oriented, straight filaments observed in LBs [119]. Indeed, in this model the accumulation of aberrant mitochondria into inclusions seems to be independent of α-syn [120].
Mitochondrial dysfunction
A loss of mitochondrial complex I is observed in sporadic PD that may lead to selective dopaminergic neurodegeneration due to oxidative stress [121]. Midbrain dopaminergic neurons of PD patients and elderly humans carry high levels of somatic mtDNA mutations [122]. Indeed, neurotoxins like MPTP and rotenone inhibit this mitochondrial complex [123]. Transgenic mice overexpressing wild-type α-syn induces mitochondrial fragmentation, which may predispose to neural degeneration [124], and transgenic mice overexpressing human A53T α-syn mutation also display mitochondrial abnormalities that may explain some aspects of the aggregated α-syn toxicity [125]. Disruption of the respiratory chain has been modeled in mice by deleting the gene encoding the mitochondrial transcription factor A (TFAM) in dopaminergic neurons, thereby inhibiting the transcription of mtDNA genes. Although these mice have an interesting phenotype, with impaired motor function and neurodegeneration, α-syn is not needed to express this phenotype. Indeed, the inclusions found in dopaminergic neurons have no α-syn but rather, abnormal mitochondrial membranes [126].
α-syn transgenesis
Different transgenic models have been developed that overexpress wild type, A53T, A30P and truncated α-syn. However, despite initial results with mutated α-syn in rodents, they have failed to translate into truly effective transgenic models of PD [127]. Attempts to overexpress or to knock-out α-syn in rodents have produced a variety of pathological abnormalities, including aggregate formation. However, no clear loss of dopaminergic neurons is observed. On the other hand, viral transduction induces rapid neurodegeneration but it is limited to the region targeted [125]. The use of cell-type promoters, such as tyrosine hydroxylase, is a limitation to transgenic overexpression that prevents the induction of the broad brain pathology observed in humans. However, this localized overexpression of α-syn (or truncated forms) may be useful to address specific features of PD. A variety of transgenic models using different promoters that drive broad expression have induced the graded appearance of α-syn aggregations with no fibrillar morphology [125,128,129]. One particularly interesting model is the transgenic A53T α-syn under the control of the mouse prion promoter, which overexpresses the mutant protein in neurons. These mice develop progressive motor failure at 8 months of age leading to paralysis and death [62]. Inclusions have been detected in different brain areas like the SN, raphe, pons, pontine reticular nuclei, locus coeruleus and deep cerebellar nuclei. The biochemical characterization of these inclusions showed SDS-insoluble α-syn aggregation into dimers, trimers and multimers, and these LB-like inclusions have a filamentous structure like human LBs [62,130]. However, as indicated, a major drawback of this model is that no dopaminergic neurodegeneration is detected in the SN. Conversely, there is neurodegeneration in areas not affected in PD, such as among motor neurons [127,131,132].
LRRK2 transgenesis
LRRK2 null mice have no overt dopaminergic deficit or clear pathology in the brain, although age-dependent renal atrophy is observed that is associated with the aggregation of α-syn in renal tubules of the aged kidney [133,134]. The R1441C mutation in LRRK2 generated by a knock-in strategy in mice does not induce dopaminergic neurodegeneration but rather, DA neurotransmission and D2 receptor dysfunction. However, no obvious accumulation of α-syn is detected [135]. Mice overexpressing the G2019S mutation in LRRK2 do not reproduce any obvious gross neuropathological phenotypes nor is α-syn aggregation observed, although this LRRK2 mutation may accelerate the pathogenic phenotype of A53T α-syn mice [136-138].
Autophagy-lysosome system
Several studies have suggested a role for autophagy in PD. The autophagy-lysosome system is a catabolic pathway involved in protein and organelle degradation. Several types of autophagy have been described, including: microautophagy; macroautophagy, engulfing large structures; and chaperone-mediated autophagy that degrades only soluble proteins in a selective manner. Cellular homeostasis of autophagy is crucial to maintain the balance between healthy and unhealthy cells [139]. In PD and related LB diseases an accumulation of autophagosomes has been described, coupled with a reduction of lysosomal markers in nigral dopaminergic neurons, suggesting a defect in lysosome-mediated clearance of α-syn aggregates [140,141]. Indeed, α-syn is physiologically degraded by both the ubiquitin-proteasome and the autophagy-lysosome system (even by the chaperone-mediated mechanism), although the mutant forms of α-syn appear to inhibit their own degradation. Hence, these pathways would appear to participate in LB formation [142,143].
In mice, deletion of the gene essential for macroautophagy (Atg7) in dopaminergic neurons induces progressive moderate dopaminergic loss in the SN and striatal DA depletion at an age of 9 months, although most dopaminergic neurons are resistant to the long-term stress induced by impairing autophagy. These mice have ubiquitinated-SQSTM1 inclusions in dopaminergic neurons but no abnormal aggregation of α-syn [74]. In PD patients, the translocation to the nucleus of the TFEB transcription factor (a regulator of autophagy), is dampened in midbrain dopaminergic neurons and it co-localizes with α-syn in LBs. Using adeno-associated viral vectors to model α-syn transgenesis in rats, under the control of the synapsin-1 promoter and the WPRE enhancer, stimulation of TFEB and Beclin (an activator of autophagy) was shown to rescue nigral DA neurons from α-syn toxicity [144]. These results identify interesting new mechanisms, however no clear α-syn aggregates have yet been formed by manipulating the autophagy-lysosome system.
Neurotrophic factors: Smad3 deficiency
GDNF and its close relative Neurturin provide functional rescue of nigro-striatal dopaminergic neurons after 6-OHDA or MPTP treatment. These results led to clinical trials into the use of these molecules in patients with PD, although the use of exogenous GDNF in clinical trials produced inconclusive results. The neuroprotective effect of this neurotrophic factor may not be translated to the clinic as GDNF fails to protect against α-syn-induced toxicity, probably due to the blockade of GDNF signaling by α-syn [145]. Indeed, transgenesis of α-syn induced by viral overexpression in the nigro-striatum, induces dystrophic terminals in the striatum containing α-syn aggregates, which are not modified by exogenous administration of GDNF [146,147].
Recently, intracellular TGF-β1 signaling has been implicated in different pathological events related to PD in a mouse model, including LB-like formation. In humans, TGF-β1 is up-regulated in the striatum and in the ventricular cerebrospinal fluid of patients with PD [148,149]. Moreover, active TGF-β1 overexpression in the nigro-striatal system of MPTP-treated mice using adenoviral vectors produces poorer survival of dopaminergic neurons and higher levels of striatal DA depletion [150,151]. However, since the effects of TGF-β1 are dose- and context-dependent [152], its overexpression may introduce a bias in studies on animal models. Smad3 deficiency, a molecule involved in the intracellular TGF-β1 signaling cascade, provides us with a new and interesting model of PD, as it promotes selective postnatal neurodegeneration of dopaminergic midbrain neurons, strong MAO-mediated catabolism of DA in the striatum and oxidative stress, as well as dampening the trophic and astrocytic support to dopaminergic neurons. Interestingly, Smad3 deficiency induces the formation of α-syn inclusions in selected brain areas, which accumulate with age in a progressive and gene dosage dependent manner. These α-syn inclusions appear in both the perikaryon (SN and paralemniscal nucleus) and in neurites (in the motor and cingulate cortices, striatum, corpus callosum and spinal cord). Indeed, these α-syn deposits are phosphorylated at Ser129 and ubiquitinated, and they form a core/halo distribution that resembles the deposits observed in human LBs. In other brain areas α-syn expression is associated with an irregular morphology, increased α-syn-ir staining and neurite thickness (pontine nuclei, cerebellar white matter, cerebral peduncle, diencephalic nuclei and internal capsule). Moreover, α-syn inclusions are also detected in glial cells in the cerebellum and spinal cord. Biochemical analyses show the presence of detergent-insoluble dimers, trimers and oligomers of α-syn in the ventral midbrain, motor cortex and spinal cord [153].
There is currently increasing attention being paid to the non-motor symptoms of PD, such as cognitive impairment and behavioral disorders. The hippocampus is implicated in physiological learning and memory, as well as in the cognitive dysfunction seen in some PD patients where there is an interaction with the dopaminergic system [154]. The hippocampus of PD patients is also affected by the presence of LBs [31] and in mouse models, α-syn modulates adult neurogenesis in the dentate gyrus [155]. Indeed, the accumulation of extracellular α-syn oligomers impairs hippocampal LTP and it enhances basal synaptic transmission [156]. Both triplication and point mutations of SNCA are associated with cortical and subcortical LB accumulation, and such patients are clinically diagnosed as PD with dementia [47,64]. These data suggest a role for the α-syn aggregates in the cognitive impairment observed in demented PD patients. Similarly, Smad3 deficient mice have α-syn inclusions in the neuronal layers of the hippocampus. Smad3 is strongly expressed in hippocampal neurons and Smad3 deficiency induces a strong decrease in adult neurogenesis in the dentate gyrus, inducing the apoptosis of early stage and highly proliferative intermediate precursor cells (IPCs). Indeed, Smad3 deficiency abolishes the induction of LTP in the dentate gyrus but not in the CA1, highlighting the specificity of this effect. Both neurogenesis and LTP induction in the adult hippocampus are two aspects of hippocampal brain plasticity related to learning and memory that decline with age, and as a result of neurological disorders [157]. Smad3 deficiency sheds light on a new interesting pathological mechanism and provides a new model of PD to be explored in which dopaminergic dysfunction, widespread α-syn inclusions and cognitive impairment co-exist.
Invertebrate models
Several invertebrate models have been developed that recapitulate key features of human PD. Drosophila models of α-syn overexpression show loss of dopaminergic neurons, locomotor dysfunction and formation of α-syn inclusions. Indeed, these inclusions are observed as a core with peripherally radiating filaments [158,159]. Despite the simplicity of the nematode model Caenorhabditis elegans, the presence of eight dopaminergic neurons has allowed their evaluation following parkinsonian toxic and genetic insult. Overexpression of human wild-type or mutant α-syn on C. elegans induces dopaminergic neurodegeneration and the formation of α-syn aggregates with fibrillar morphology [160-162]. It is interesting to note that both Drosophila and C. elegans do not express α-syn and thus, other genes like LRRK2 cannot be studied in the context of α-syn inclusion formation. These models also lack the complexity of vertebrates and hence, they are not perfect models of PD but rather, they may be useful for comprehensive genetic analysis and drug screening.
Dopamine metabolism and α-syn
Several studies associate α-syn with the DA metabolism in the presynaptic terminals. It is known that α-syn inhibits tyrosine hydroxylase, the rate limiting enzyme of DA biosynthesis [163,164], and aromatic amino acid decarboxylase, the enzyme that converts L-Dopa to DA [165]. Indeed, deregulation of DA biosynthesis in the SN induces increased toxicity to α-syn [166].
The Smad3 deficient mouse model described earlier also associates α-syn and DA metabolism, whereby Smad3 deficiency drives α-syn overexpression and aggregation, as well as the deregulation of DA turnover by inducing MAO-dependent DA catabolism, provoking a loss of dopaminergic neurons [153]. Other mouse models, such as α-syn, parkin, DJ-1 or PINK1 null mice, also display deregulated striatal DA metabolism, release or re-uptake, although without dopaminergic neuronal loss. Increased extracellular DA, decreased DAT, increased GSH levels and differences in the catabolism of DA have all been described in Parkin deficient mice [167-169]. However, neither the number of dopaminergic neurons nor the striatal DA levels and turnover are altered in DJ-1 or PINK knock-out mice. Nevertheless, the evoked DA overflow in these mice diminishes due to increased DA uptake [170] or to the decreased quantal release of DA [171], respectively. The α-syn knock-out mice have less striatal DA, enhanced activity-dependent DA release and smaller reserve pools of synaptic vesicles [172,173], which may be due to a uninhibited DA vesicular release [174]. All these mutant mice illustrate that impaired presynaptic DA metabolism, release or re-uptake may be common to PD, although the presence of α-syn aggregates and DA neuronal loss is only detected in Smad3 deficient mice. Indeed, 3,4-dihydroxyphenylacetaldehyde (DOPAL), a MAO intermediate metabolite of DA, can induce toxic aggregation of α-synuclein [175]. These data suggest that DA metabolism and α-syn interactions may underlie the susceptibility of SN neurodegeneration in PD [176].
It was recently suggested that the spread of the pathological elements in PD, as described in the Braak stages, can occur by neuron-to-neuron transmission of aggregates to healthy cells [177,178]. In this hypothesis, preformed fibrils of α-syn enter neurons, probably by endocytosis, to recruit soluble endogenous α-syn into insoluble LB- and LN-like aggregates [179,180]. Interestingly, peripheral inoculation of α-syn fibrils by intramuscular injections can propagate the pathogenic protein to brain nuclei [181]. However, another study showed that α-syn fibrils injected into transgenic mice overexpressing α-syn mutations promotes the widespread formation of α-syn inclusions in the brain of A53T but not E46K mutant mice, nor in non-transgenic mice. The authors suggest inespecificity with neurofilament of the antibodies used and that only A53T mutant mice have the capacity to induce α-syn aggregation upon exogenous administration of α-syn [182]. This aggregate transmission may be related to the existence of extracellular forms of α-syn that are released from neurons and glia by exocytosis [183]. Indeed, extracellular α-syn may induce DA release in the striatum, establishing a new link between α-syn and DA metabolism. Furthermore, α-syn function may be related to the reorganization of plasma membrane microdomains [184].
Conformational plasticity of α-syn
Advances in cell-free systems and model cell systems have shed light on the process of α-syn aggregation. The role proposed for α-syn is in the modulation of neurotransmitter release in the presynaptic nerve terminal, as well as influencing DA neurotransmitter biosynthesis, vesicle trafficking and exocytosis. In the cytoplasm and/or vesicle lumen, α-syn is present as an intrinsically disordered protein (IDP: i.e. a monomer that lacks a well-organized secondary structure), yet when bound to membranes it adopts several conformations, such as an extended α-helix or a broken-helix [185,186]. Indeed, despite the overwhelming evidence that α-syn is a disordered monomer in solution, two recent reports suggest that the native protein exists as a helical tetramer under physiological conditions, with reduced aggregation tendencies, and that the dissociation of the tetramer into monomeric subunits promotes toxic aggregation [187,188]. Indeed, when monomeric α-syn binds to membranes, it changes its conformation to a partially helical form [189].
Many IDPs are known to interact with a large number of proteins, serving as a nodes or hubs, in a way that IDPs undergo a disorder-to-order transition upon interaction with specific partners. More than 50 proteins have been reported to interact with α-syn, although it is unknown the proportion unfolded α-syn or that which has adopted a secondary structure as a consequence of binding within the cell [186].
The α-syn peptide has 140 amino acids, with 3 distinct regions: a N-terminal (1–60 residues) that contain four imperfect repeats of KTKEGV motifs; a NAC region (61–95 residues), with 3 additional KTKEGV repeats, and the hydrophobic and amyloidogenic NAC region; a C-terminus (96–140 residues) that is enriched in acidic and proline residues, and that facilitates interactions with different proteins [185].
The major constituent of LBs is a fibrillar form of α-syn that adopts a β-sheet structure and hence, the disordered monomer or helical tetramer is transformed into highly organized fibrils in the course of this pathology. In vitro studies suggest a nucleation-dependent process due to a conformational transition to anti-parallel β-sheet structures, including a committed step of α-syn dimer formation [190]. Nucleation follows a sigmoidal growth profile, with an initial lag phase where the protein changes to a partially folded intermediate to form nuclei with oligomers (Figure 1). This conformational change exposes the NAC domain that can participate in hydrophobic interactions that may initiate the aggregation process. During the growth phase, the nucleus adds monomers to form larger oligomers, polarized protofibrils and finally, fibrils. In the last steady-state phase both the fibrils and monomers appear to be in equilibrium, this model therefore reflecting the addition of monomers to existing aggregates [186,191-193]. Recently, a key conformational intermediate was characterized after oligomer formation, with the appearance of stable and compact oligomers that are more damaging to cells (Figure 1). Indeed, it seems that the assembly process can be reversed and that fibrils may disaggregate to form these stable, cytotoxic oligomers [194].
Figure 1.
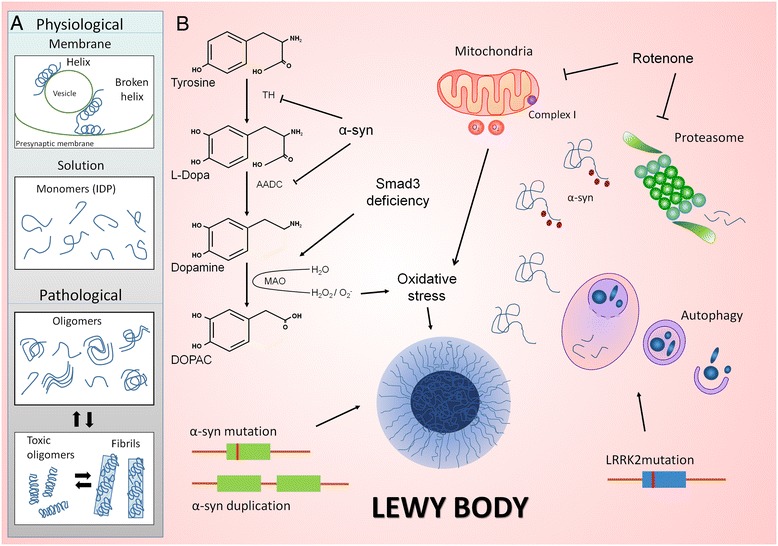
Models of α-syn aggregation and LBs formation. A α-Syn is present in the vesicle lumen and in the cytoplasma as an intrinsically disordered protein. α-Syn bound to membranes has distinct conformation such as an extended α-helix or a broken-helix. In the pathological context, disordered monomers may lead to oligomerization and fibril formation, following a nucleation-dependent process, in which monomers are added to existing aggregates. B Rotenone administration in rats, A53T α-syn transgenesis in mice and Smad3 deficient mice are interesting models to study LB formation. While A53T α-syn transgenesis and Smad3 deficiency can modulate DA metabolism, rotenone and Smad3 deficiency induce oxidative stress, mechanisms that may participate in LBs formation. Indeed, proteasome and autophagy inhibitors may impair degradation of α-syn. LRRK2 mutations may participate in LB formation by altering autophagy and α-syn solubility.
The pathogenic aggregation of α-syn can be modulated by endogenous and exogenous factor, such as metals and pesticides, genetic mutations in SNCA, post-translational modifications and protein-protein interactions [186]. It is not clear how α-syn bound to membranes, such as synaptic vesicles or presynaptic plasma membrane, can aggregate. One model proposes that the broken-helix membrane bound state of α-syn releases its C-terminal region, converting the protein into a partially helical membrane bound state, which may lead to oligomerization and fibril formation. Alternatively, α-helical forms of α-syn bound to membranes may inhibit fibril formation, and membrane-bound α-syn monomers may be protected from aggregation. Indeed, oligomers have a high propensity to bind to membranes, which may promote permeabilization and disrupt cellular homeostasis [195,196]. Furthermore, α-syn localizes to the nerve terminal, where modest increments in α-syn (such as those produced by gene duplication) inhibit neurotransmitter release by reducing synaptic vesicle density and by altering vesicle reclustering after endocytosis [197].
By contrast, oligomers might be kinetically detained by interactions with small molecules, inducing secondary structures such as annular pores or spheres [198-200]. The point mutations detected in the SNCA gene of PD patients are also endogenous factors that could accelerate α-syn aggregation in vitro, with A53T and G46L forming oligomers and fibrils, and A30P forming oligomers but not fibrils [201].
Post-translational modification of α-syn
Several post-translational modifications of α-syn may occur, such as phosphorylation, truncation, ubiquitination, nitration, sumoylation and enzymatic cross-linking. In LBs, phosphorylation at serine 129 is a common α-syn modification, although its role is unclear if we consider that overexpression of the phosphorylated serine 129 isoform in animal models does not produce toxicity [202,203]. The majority of α-syn is mono- to tri-ubiquinated [204], and while poly-ubiquitination serves as a signal for α-syn degradation by the proteasome, it does not seem to be required for α-syn fibrillation and LB formation. Nevertheless, an interplay between phosphorylation and ubiquitination may render the protein more susceptible to aggregation [205]. It is estimated that 85% of all human proteins undergo Nα-acetylation due to the activity of Nα-acetyltransferases, probably influencing the subcellular localization of proteins, their rate of synthesis and protein-protein interactions [206]. Tetramers of α-syn seem to be Nα-acetylated, as is probably are the aggregated α-syn isolated from PD deposits. However, Nα-acetylation does not seem to alter protein aggregation but more likely, lipid binding [185]. Small amounts of various C-terminal truncated forms of α-syn have been detected in LBs, which exhibit greater fibrillation capacity [204]. Furthermore, four alternatively spliced forms add another level of complexity. Compared to the canonical α-syn, the three alternative isoforms aggregate significantly less, forming shorter fibrils that are arranged in parallel or with anular structures [207].
Despite the huge amount of research into the properties of α-syn aggregation, there is still no coherent picture on the structure, dynamics, and the physiological and pathological roles of α-syn. New animal models for PD need to explore LB formation in the context of neurodegeneration and DA metabolism, such as the rotenone, α-syn transgenesis and Smad3 deficient mice, in order to clearly understand the pathological mechanism of LB formation as well as to attain effective therapies for this disease.
Acknowledgements
We thank the Spanish Institute of Health Carlos III (PI12/00258, CP04/00013) and CIBERNED (CB06/05/0033).
Abbreviations
- DA
Dopamine
- IDP
Intrinsically disordered protein
- LB
Lewy body
- LN
Lewy neurite
- PD
Parkinson’s disease
- SN
Substantia nigra
- α-syn
α-synuclein
Footnotes
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Rosa María Giráldez-Pérez, Email: rmgiraldez@us.es.
Mónica Antolín-Vallespín, Email: monica.antolin@hrc.es.
María Dolores Muñoz, Email: dolores.munoz@hrc.es.
Amelia Sánchez-Capelo, Email: amelia.capelo@hrc.es.
References
- 1.Goetz CG. The history of Parkinson’s disease: early clinical descriptions and neurological therapies. Cold Spring Harb Perspect Med. 2011;1:a008862. doi: 10.1101/cshperspect.a008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewy FH. Paralysis agitans. In: Lewandowsky M, editor. Pathologische anatomie. City: Springer; 1912. pp. 920–933. [Google Scholar]
- 3.Parent M, Parent A. Substantia nigra and Parkinson’s disease: a brief history of their long and intimate relationship. Can J Neurol Sci. 2010;37:313–319. doi: 10.1017/s0317167100010209. [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 7.Foltynie T, Brayne C, Barker RA. The heterogeneity of idiopathic Parkinson’s disease. J Neurol. 2002;249:138–145. doi: 10.1007/pl00007856. [DOI] [PubMed] [Google Scholar]
- 8.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, Jacobs AH, Herholz K, Heiss WD. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- 10.Rinne JO, Ma SY, Lee MS, Collan Y, Roytta M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson’s disease is related to disability of the patients. Parkinsonism Relat Disord. 2008;14:553–557. doi: 10.1016/j.parkreldis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shults CW. Lewy bodies. Proc Natl Acad Sci U S A. 2006;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Tortosa E, Newell K, Irizarry MC, Sanders JL, Hyman BT. alpha-Synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol. 2000;99:352–357. doi: 10.1007/s004010051135. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto M, Uchihara T, Hayashi M, Nakamura A, Kikuchi E, Mizutani T, Mizusawa H, Hirai S. Heterogeneity of nigral and cortical Lewy bodies differentiated by amplified triple-labeling for alpha-synuclein, ubiquitin, and thiazin red. Exp Neurol. 2002;177:88–94. doi: 10.1006/exnr.2002.7961. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa T, Adachi E, Orimo S, Nakamura A, Mizusawa H, Uchihara T. Pale neurites, premature alpha-synuclein aggregates with centripetal extension from axon collaterals. Brain Pathol. 2012;22:67–78. doi: 10.1111/j.1750-3639.2011.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris EH, Giasson BI. Role of oxidative damage in protein aggregation associated with Parkinson’s disease and related disorders. Antioxid Redox Signal. 2005;7:672–684. doi: 10.1089/ars.2005.7.672. [DOI] [PubMed] [Google Scholar]
- 19.Pollanen MS, Dickson DW, Bergeron C. Pathology and biology of the Lewy body. J Neuropathol Exp Neurol. 1993;52:183–191. doi: 10.1097/00005072-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Arima K, Ueda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M. Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res. 1998;808:93–100. doi: 10.1016/s0006-8993(98)00734-3. [DOI] [PubMed] [Google Scholar]
- 21.Galloway PG, Mulvihill P, Perry G. Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am J Pathol. 1992;140:809–822. [PMC free article] [PubMed] [Google Scholar]
- 22.Kanazawa T, Uchihara T, Takahashi A, Nakamura A, Orimo S, Mizusawa H. Three-layered structure shared between Lewy bodies and lewy neurites-three-dimensional reconstruction of triple-labeled sections. Brain Pathol. 2008;18:415–422. doi: 10.1111/j.1750-3639.2008.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 24.Beyer K, Ariza A. alpha-Synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol Neurobiol. 2013;47:509–524. doi: 10.1007/s12035-012-8330-5. [DOI] [PubMed] [Google Scholar]
- 25.Vitte J, Traver S, Maues De Paula A, Lesage S, Rovelli G, Corti O, Duyckaerts C, Brice A. Leucine-rich repeat kinase 2 is associated with the endoplasmic reticulum in dopaminergic neurons and accumulates in the core of Lewy bodies in Parkinson disease. J Neuropathol Exp Neurol. 2010;69:959–972. doi: 10.1097/NEN.0b013e3181efc01c. [DOI] [PubMed] [Google Scholar]
- 26.Miki Y, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. Accumulation of histone deacetylase 6, an aggresome-related protein, is specific to Lewy bodies and glial cytoplasmic inclusions. Neuropathology. 2011;31:561–568. doi: 10.1111/j.1440-1789.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanikawa S, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. Endosomal sorting related protein CHMP2B is localized in Lewy bodies and glial cytoplasmic inclusions in alpha-synucleinopathy. Neurosci Lett. 2012;527:16–21. doi: 10.1016/j.neulet.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Xia Q, Liao L, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J. Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci. 2008;13:3850–3856. doi: 10.2741/2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J. Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol. 2007;17:139–145. doi: 10.1111/j.1750-3639.2007.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging. 2004;25:19–23. doi: 10.1016/j.neurobiolaging.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. City: Springer; 2009. [PubMed] [Google Scholar]
- 32.van de Berg WD, Hepp DH, Dijkstra AA, Rozemuller JA, Berendse HW, Foncke E. Patterns of α-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonism Relat Disord Suppl. 2012;1:S28–S30. doi: 10.1016/S1353-8020(11)70011-6. [DOI] [PubMed] [Google Scholar]
- 33.Hishikawa N, Hashizume Y, Yoshida M, Sobue G. Widespread occurrence of argyrophilic glial inclusions in Parkinson’s disease. Neuropathol Appl Neurobiol. 2001;27:362–372. doi: 10.1046/j.1365-2990.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 34.Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 35.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132:2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 36.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115:409–415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 38.Rocca WA, McDonnell SK, Strain KJ, Bower JH, Ahlskog JE, Elbaz A, Schaid DJ, Maraganore DM. Familial aggregation of Parkinson’s disease: The Mayo Clinic family study. Ann Neurol. 2004;56:495–502. doi: 10.1002/ana.20228. [DOI] [PubMed] [Google Scholar]
- 39.Sveinbjornsdottir S, Hicks AA, Jonsson T, Petursson H, Gugmundsson G, Frigge ML, Kong A, Gulcher JR, Stefansson K. Familial aggregation of Parkinson’s disease in Iceland. N Engl J Med. 2000;343:1765–1770. doi: 10.1056/NEJM200012143432404. [DOI] [PubMed] [Google Scholar]
- 40.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 41.Schiesling C, Kieper N, Seidel K, Kruger R. Review: Familial Parkinson’s disease–genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol. 2008;34:255–271. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 42.Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev. 2011;91:1161–1218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 43.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 44.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 47.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 48.Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T. Parkinson’s disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, Nishioka K, Fuchs J, Gasser T, Maraganore DM, Adler CH, Larvor L, Chartier-Harlin MC, Nilsson C, Langston JW, Gwinn K, Hattori N, Farrer MJ. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63:743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, Cho J, Jeon BS. Alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 51.Kruger R, Kuhn W, Leenders KL, Sprengelmeyer R, Muller T, Woitalla D, Portman AT, Maguire RP, Veenma L, Schroder U, Schols L, Epplen JT, Riess O, Przuntek H. Familial parkinsonism with synuclein pathology: clinical and PET studies of A30P mutation carriers. Neurology. 2001;56:1355–1362. doi: 10.1212/wnl.56.10.1355. [DOI] [PubMed] [Google Scholar]
- 52.Markopoulou K, Dickson DW, McComb RD, Wszolek ZK, Katechalidou L, Avery L, Stansbury MS, Chase BA. Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson’s disease. Variability in familial Parkinson’s disease. Acta Neuropathol. 2008;116:25–35. doi: 10.1007/s00401-008-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 54.Mellick GD, Maraganore DM, Silburn PA. Australian data and meta-analysis lend support for alpha-synuclein (NACP-Rep1) as a risk factor for Parkinson’s disease. Neurosci Lett. 2005;375:112–116. doi: 10.1016/j.neulet.2004.10.078. [DOI] [PubMed] [Google Scholar]
- 55.Farrer M, Maraganore DM, Lockhart P, Singleton A, Lesnick TG, de Andrade M, West A, de Silva R, Hardy J, Hernandez D. alpha-Synuclein gene haplotypes are associated with Parkinson’s disease. Hum Mol Genet. 2001;10:1847–1851. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- 56.Tan EK, Chai A, Teo YY, Zhao Y, Tan C, Shen H, Chandran VR, Teoh ML, Yih Y, Pavanni R, Wong MC, Puvan K, Lo YL, Yap E. Alpha-synuclein haplotypes implicated in risk of Parkinson’s disease. Neurology. 2004;62:128–131. doi: 10.1212/01.wnl.0000101721.25345.dc. [DOI] [PubMed] [Google Scholar]
- 57.Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boassa D, Berlanga ML, Yang MA, Terada M, Hu J, Bushong EA, Hwang M, Masliah E, George JM, Ellisman MH. Mapping the subcellular distribution of alpha-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson’s disease pathogenesis. J Neurosci. 2013;33:2605–2615. doi: 10.1523/JNEUROSCI.2898-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ono K, Ikeda T, Takasaki J, Yamada M. Familial Parkinson disease mutations influence alpha-synuclein assembly. Neurobiol Dis. 2011;43:715–724. doi: 10.1016/j.nbd.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 60.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 61.Yamaguchi K, Cochran EJ, Murrell JR, Polymeropoulos MH, Shannon KM, Crowther RA, Goedert M, Ghetti B. Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha-synuclein gene. Acta Neuropathol. 2005;110:298–305. doi: 10.1007/s00401-005-1042-4. [DOI] [PubMed] [Google Scholar]
- 62.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 63.Gaugler MN, Genc O, Bobela W, Mohanna S, Ardah MT, El-Agnaf OM, Cantoni M, Bensadoun JC, Schneggenburger R, Knott GW, Aebischer P, Schneider BL. Nigrostriatal overabundance of alpha-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012;123:653–669. doi: 10.1007/s00401-012-0963-y. [DOI] [PubMed] [Google Scholar]
- 64.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 65.Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 68.Paisan-Ruiz C, Nath P, Washecka N, Gibbs JR, Singleton AB. Comprehensive analysis of LRRK2 in publicly available Parkinson’s disease cases and neurologically normal controls. Hum Mutat. 2008;29:485–490. doi: 10.1002/humu.20668. [DOI] [PubMed] [Google Scholar]
- 69.Wider C, Ross OA, Wszolek ZK. Genetics of Parkinson disease and essential tremor. Curr Opin Neurol. 2010;23:388–393. doi: 10.1097/WCO.0b013e32833b1f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paisan-Ruiz C. LRRK2 gene variation and its contribution to Parkinson disease. Hum Mutat. 2009;30:1153–1160. doi: 10.1002/humu.21038. [DOI] [PubMed] [Google Scholar]
- 71.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case–control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 73.Ross OA, Toft M, Whittle AJ, Johnson JL, Papapetropoulos S, Mash DC, Litvan I, Gordon MF, Wszolek ZK, Farrer MJ, Dickson DW. Lrrk2 and Lewy body disease. Ann Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- 74.Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M, Holstein GR, Yue Z. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci. 2012;32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mamais A, Raja M, Manzoni C, Dihanich S, Lees A, Moore D, Lewis PA, Bandopadhyay R. Divergent alpha-synuclein solubility and aggregation properties in G2019S LRRK2 Parkinson’s disease brains with Lewy Body pathology compared to idiopathic cases. Neurobiol Dis. 2013;58:183–190. doi: 10.1016/j.nbd.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pramstaller PP, Schlossmacher MG, Jacques TS, Scaravilli F, Eskelson C, Pepivani I, Hedrich K, Adel S, Gonzales-McNeal M, Hilker R, Kramer PL, Klein C. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005;58:411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 77.Spratt DE, Martinez-Torres RJ, Noh YJ, Mercier P, Manczyk N, Barber KR, Aguirre JD, Burchell L, Purkiss A, Walden H, Shaw GS. A molecular explanation for the recessive nature of parkin-linked Parkinson’s disease. Nat Commun. 2013;4:1983. doi: 10.1038/ncomms2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lonskaya I, Desforges NM, Hebron ML, Moussa CE. Ubiquitination increases parkin activity to promote autophagic alpha-synuclein clearance. PLoS One. 2013;8:e83914. doi: 10.1371/journal.pone.0083914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawahara K, Hashimoto M, Bar-On P, Ho GJ, Crews L, Mizuno H, Rockenstein E, Imam SZ, Masliah E. alpha-Synuclein aggregates interfere with Parkin solubility and distribution: role in the pathogenesis of Parkinson disease. J Biol Chem. 2008;283:6979–6987. doi: 10.1074/jbc.M710418200. [DOI] [PubMed] [Google Scholar]
- 80.Rogaeva E, Johnson J, Lang AE, Gulick C, Gwinn-Hardy K, Kawarai T, Sato C, Morgan A, Werner J, Nussbaum R, Petit A, Okun MS, McInerney A, Mandel R, Groen JL, Fernandez HH, Postuma R, Foote KD, Salehi-Rad S, Liang Y, Reimsnider S, Tandon A, Hardy J, St George-Hyslop P, Singleton AB. Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease. Arch Neurol. 2004;61:1898–1904. doi: 10.1001/archneur.61.12.1898. [DOI] [PubMed] [Google Scholar]
- 81.Annesi G, Savettieri G, Pugliese P, D'Amelio M, Tarantino P, Ragonese P, La Bella V, Piccoli T, Civitelli D, Annesi F, Fierro B, Piccoli F, Arabia G, Caracciolo M, Ciro Candiano IC, Quattrone A. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann Neurol. 2005;58:803–807. doi: 10.1002/ana.20666. [DOI] [PubMed] [Google Scholar]
- 82.Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 2011;17:485–493. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schellinck HM, Cyr DP, Brown RE. How many ways an mouse behavioral experiments go wrong? Confounding variables in mouse models of neurodegenerative diseases and how to control them. In: Brockmann HJ, editor. Advances in the Study of Behavior. Burlington: Academic Press; 2010. pp. 255–366. [Google Scholar]
- 86.van der Staay FJ, Arndt SS, Nordquist RE. Evaluation of animal models of neurobehavioral disorders. Behavioral and Brain Functions. 2009;5:11. doi: 10.1186/1744-9081-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 88.Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lane E, Dunnett S. Animal models of Parkinson’s disease and L-dopa induced dyskinesia: how close are we to the clinic? Psychopharmacology (Berl) 2008;199:303–312. doi: 10.1007/s00213-007-0931-8. [DOI] [PubMed] [Google Scholar]
- 90.Kieburtz K, Ravina B. Why hasn't neuroprotection worked in Parkinson’s disease? Nat Clin Pract Neurol. 2007;3:240–241. doi: 10.1038/ncpneuro0491. [DOI] [PubMed] [Google Scholar]
- 91.Fishbein I, Kuo YM, Giasson BI, Nussbaum RL. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain. 2014;137:3235–3247. doi: 10.1093/brain/awu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ginns EI, Mak SK, Ko N, Karlgren J, Akbarian S, Chou VP, Guo Y, Lim A, Samuelsson S, LaMarca ML, Vazquez-DeRose J, Manning-Bog AB. Neuroinflammation and alpha-synuclein accumulation in response to glucocerebrosidase deficiency are accompanied by synaptic dysfunction. Mol Genet Metab. 2014;111:152–162. doi: 10.1016/j.ymgme.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Rothaug M, Zunke F, Mazzulli JR, Schweizer M, Altmeppen H, Lullmann-Rauch R, Kallemeijn WW, Gaspar P, Aerts JM, Glatzel M, Saftig P, Krainc D, Schwake M, Blanz J. LIMP-2 expression is critical for beta-glucocerebrosidase activity and alpha-synuclein clearance. Proc Natl Acad Sci U S A. 2014;111:15573–15578. doi: 10.1073/pnas.1405700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jenner P. The contribution of the MPTP-treated primate model to the development of new treatment strategies for Parkinson’s disease. Parkinsonism Relat Disord. 2003;9:131–137. doi: 10.1016/s1353-8020(02)00115-3. [DOI] [PubMed] [Google Scholar]
- 95.Jenner P. Functional models of Parkinson’s disease: a valuable tool in the development of novel therapies. Ann Neurol. 2008;64(Suppl 2):S16–S29. doi: 10.1002/ana.21489. [DOI] [PubMed] [Google Scholar]
- 96.Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A, Marsden CD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the common marmoset. Neurosci Lett. 1984;50:85–90. doi: 10.1016/0304-3940(84)90467-1. [DOI] [PubMed] [Google Scholar]
- 97.McDowell K, Chesselet MF. Animal models of the non-motor features of Parkinson’s disease. Neurobiol Dis. 2012;46:597–606. doi: 10.1016/j.nbd.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halliday G, Herrero MT, Murphy K, McCann H, Ros-Bernal F, Barcia C, Mori H, Blesa FJ, Obeso JA. No Lewy pathology in monkeys with over 10 years of severe MPTP Parkinsonism. Mov Disord. 2009;24:1519–1523. doi: 10.1002/mds.22481. [DOI] [PubMed] [Google Scholar]
- 99.Sonsalla PK, Heikkila RE. Neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and methamphetamine in several strains of mice. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:345–354. doi: 10.1016/0278-5846(88)90054-1. [DOI] [PubMed] [Google Scholar]
- 100.Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- 101.Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord. 2008;14(Suppl 2):S112–S115. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alvarez-Fischer D, Guerreiro S, Hunot S, Saurini F, Marien M, Sokoloff P, Hirsch EC, Hartmann A, Michel PP. Modelling Parkinson-like neurodegeneration via osmotic minipump delivery of MPTP and probenecid. J Neurochem. 2008;107:701–711. doi: 10.1111/j.1471-4159.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- 104.Gibrat C, Saint-Pierre M, Bousquet M, Levesque D, Rouillard C, Cicchetti F. Differences between subacute and chronic MPTP mice models: investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J Neurochem. 2009;109:1469–1482. doi: 10.1111/j.1471-4159.2009.06072.x. [DOI] [PubMed] [Google Scholar]
- 105.Shimoji M, Zhang L, Mandir AS, Dawson VL, Dawson TM. Absence of inclusion body formation in the MPTP mouse model of Parkinson’s disease. Brain Res Mol Brain Res. 2005;134:103–108. doi: 10.1016/j.molbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 106.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang XF, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis. 2006;23:198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 108.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 109.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 110.Tien LT, Kaizaki A, Pang Y, Cai Z, Bhatt AJ, Fan LW. Neonatal exposure to lipopolysaccharide enhances accumulation of alpha-synuclein aggregation and dopamine transporter protein expression in the substantia nigra in responses to rotenone challenge in later life. Toxicology. 2013;308:96–103. doi: 10.1016/j.tox.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silva BA, Einarsdottir O, Fink AL, Uversky VN. Biophysical Characterization of alpha-Synuclein and Rotenone Interaction. Biomolecules. 2013;3:703–732. doi: 10.3390/biom3030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 113.Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol Dis. 2009;36:96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 114.Mulcahy P, Walsh S, Paucard A, Rea K, Dowd E. Characterisation of a novel model of Parkinson’s disease by intra-striatal infusion of the pesticide rotenone. Neuroscience. 2011;181:234–242. doi: 10.1016/j.neuroscience.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 115.Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci. 2009;30:475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Lapointe N, St-Hilaire M, Martinoli MG, Blanchet J, Gould P, Rouillard C, Cicchetti F. Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J. 2004;18:717–719. doi: 10.1096/fj.03-0677fje. [DOI] [PubMed] [Google Scholar]
- 117.Cookson MR, van der Brug M. Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol. 2008;209:5–11. doi: 10.1016/j.expneurol.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol. 2004;56:149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- 119.Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, Gray T, Topham I, Fone K, Rezvani N, Mee M, Soane T, Layfield R, Sheppard PW, Ebendal T, Usoskin D, Lowe J, Mayer RJ. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci. 2008;28:8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paine SM, Anderson G, Bedford K, Lawler K, Mayer RJ, Lowe J, Bedford L. Pale body-like inclusion formation and neurodegeneration following depletion of 26S proteasomes in mouse brain neurones are independent of alpha-synuclein. PLoS One. 2013;8:e54711. doi: 10.1371/journal.pone.0054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 123.Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- 124.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin X, Parisiadou L, Sgobio C, Liu G, Yu J, Sun L, Shim H, Gu XL, Luo J, Long CX, Ding J, Mateo Y, Sullivan PH, Wu LG, Goldstein DS, Lovinger D, Cai H. Conditional expression of Parkinson’s disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chesselet MF, Richter F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011;10:1108–1118. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- 130.Tsika E, Moysidou M, Guo J, Cushman M, Gannon P, Sandaltzopoulos R, Giasson BI, Krainc D, Ischiropoulos H, Mazzulli JR. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, Sondel J, Kotilinek L, Day J, Schwarzschild MA, Cha JH, Newell K, Miller DW, Ueda K, Young AB, Hyman BT, Ashe KH. Motor dysfunction and gliosis with preserved dopaminergic markers in human alpha-synuclein A30P transgenic mice. Neurobiol Aging. 2003;24:245–258. doi: 10.1016/s0197-4580(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 132.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andres-Mateos E, Mejias R, Sasaki M, Li X, Lin BM, Biskup S, Zhang L, Banerjee R, Thomas B, Yang L, Liu G, Beal MF, Huso DL, Dawson TM, Dawson VL. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29:15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, 3rd, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herzig MC, Bidinosti M, Schweizer T, Hafner T, Stemmelen C, Weiss A, Danner S, Vidotto N, Stauffer D, Barske C, Mayer F, Schmid P, Rovelli G, van der Putten PH, Shimshek DR. High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS One. 2012;7:e36581. doi: 10.1371/journal.pone.0036581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, Liu Y, Swing DA, Beal MF, Troncoso JC, McCaffery JM, Jenkins NA, Copeland NG, Galter D, Thomas B, Lee MK, Dawson TM, Dawson VL, Moore DJ. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 141.Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 143.Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A. 2013;110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A. alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med. 2012;4:163ra156. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- 146.Decressac M, Ulusoy A, Mattsson B, Georgievska B, Romero-Ramos M, Kirik D, Bjorklund A. GDNF fails to exert neuroprotection in a rat alpha-synuclein model of Parkinson’s disease. Brain. 2011;134:2302–2311. doi: 10.1093/brain/awr149. [DOI] [PubMed] [Google Scholar]
- 147.Lo Bianco C, Deglon N, Pralong W, Aebischer P. Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson’s disease. Neurobiol Dis. 2004;17:283–289. doi: 10.1016/j.nbd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 148.Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P, Nagatsu T. Transforming growth factor-beta-1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci Lett. 1995;193:129–132. doi: 10.1016/0304-3940(95)11686-q. [DOI] [PubMed] [Google Scholar]
- 149.Vawter MP, Dillon-Carter O, Tourtellotte WW, Carvey P, Freed WJ. TGF beta 1 and TGF beta 2 concentrations are elevated in Parkinson’s disease in ventricular cerebrospinal fluid. Exp Neurol. 1996;142:313–322. doi: 10.1006/exnr.1996.0200. [DOI] [PubMed] [Google Scholar]
- 150.Sánchez-Capelo A, Colin P, Guibert B, Biguet NF, Mallet J. Transforming growth factor beta 1 overexpression in the nigrostriatal system increases the dopaminergic deficit of MPTP mice. Mol Cell Neurosci. 2003;23:614–625. doi: 10.1016/s1044-7431(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 151.Sánchez-Capelo A, Corti O, Mallet J. Adenovirus-mediated over-expression of TGF beta 1 in the striatum decreases dopaminergic cell survival in embryonic nigral grafts. Neuroreport. 1999;10:2169–2173. doi: 10.1097/00001756-199907130-00031. [DOI] [PubMed] [Google Scholar]
- 152.Sánchez-Capelo A. Dual role for TGF-beta 1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 153.Tapia-González S, Giraldez-Pérez RM, Cuartero MI, Casarejos MJ, Mena MA, Wang XF, Sánchez-Capelo A. Dopamine and alpha-synuclein dysfunction in Smad3 null mice. Mol Neurodegener. 2011;6:72. doi: 10.1186/1750-1326-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Calabresi P, Castrioto A, Di Filippo M, Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson’s disease. Lancet Neurol. 2013;12:811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- 155.Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, Mante M, Zhao C, Winkler J, Masliah E, Gage FH. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci. 2012;32:16906–16916. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Diogenes MJ, Dias RB, Rombo DM, Vicente Miranda H, Maiolino F, Guerreiro P, Nasstrom T, Franquelim HG, Oliveira LM, Castanho MA, Lannfelt L, Bergstrom J, Ingelsson M, Quintas A, Sebastiao AM, Lopes LV, Outeiro TF. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012;32:11750–11762. doi: 10.1523/JNEUROSCI.0234-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tapia-González S, Muñoz MD, Cuartero MI, Sánchez-Capelo A. Smad3 is required for the survival of proliferative intermediate progenitor cells in the dentate gyrus of adult mice. Cell Communication and Signaling. 2013;11:93. doi: 10.1186/1478-811X-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 159.Mizuno H, Fujikake N, Wada K, Nagai Y. alpha-Synuclein Transgenic Drosophila As a Model of Parkinson’s Disease and Related Synucleinopathies. Parkinsons Dis. 2010;2011:212706. doi: 10.4061/2011/212706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M, Mitani S, Iwatsubo T. Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem. 2006;281:334–340. doi: 10.1074/jbc.M504860200. [DOI] [PubMed] [Google Scholar]
- 161.Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- 162.van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu D, Jin L, Wang H, Zhao H, Zhao C, Duan C, Lu L, Wu B, Yu S, Chan P, Li Y, Yang H. Silencing alpha-synuclein gene expression enhances tyrosine hydroxylase activity in MN9D cells. Neurochem Res. 2008;33:1401–1409. doi: 10.1007/s11064-008-9599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- 165.Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem. 2006;99:1188–1196. doi: 10.1111/j.1471-4159.2006.04146.x. [DOI] [PubMed] [Google Scholar]
- 166.Ulusoy A, Bjorklund T, Buck K, Kirik D. Dysregulated dopamine storage increases the vulnerability to alpha-synuclein in nigral neurons. Neurobiol Dis. 2012;47:367–377. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 167.Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 168.Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de Yebenes J. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- 169.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 171.Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 173.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gitler AD, Shorter J. Prime time for alpha-synuclein. J Neurosci. 2007;27:2433–2434. doi: 10.1523/JNEUROSCI.0094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 176.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 179.Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sacino AN, Brooks M, Thomas MA, McKinney AB, McGarvey NH, Rutherford NJ, Ceballos-Diaz C, Robertson J, Golde TE, Giasson BI. Amyloidogenic alpha-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol. 2014;127:645–665. doi: 10.1007/s00401-014-1268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee HJ, Bae EJ, Lee SJ. Extracellular alpha–synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 184.Ronzitti G, Bucci G, Emanuele M, Leo D, Sotnikova TD, Mus LV, Soubrane CH, Dallas ML, Thalhammer A, Cingolani LA, Mochida S, Gainetdinov RR, Stephens GJ, Chieregatti E. Exogenous alpha-Synuclein Decreases Raft Partitioning of Cav2.2 Channels Inducing Dopamine Release. J Neurosci. 2014;34:10603–10615. doi: 10.1523/JNEUROSCI.0608-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alderson TR, Markley JL. Biophysical characterization of alpha-synuclein and its controversial structure. Intrinsically Disord Proteins. 2013;1:18–39. doi: 10.4161/idp.26255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deleersnijder A, Gerard M, Debyser Z, Baekelandt V. The remarkable conformational plasticity of alpha-synuclein: blessing or curse? Trends Mol Med. 2013;19:368–377. doi: 10.1016/j.molmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 187.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beyer K. Mechanistic aspects of Parkinson’s disease: alpha-synuclein and the biomembrane. Cell Biochem Biophys. 2007;47:285–299. doi: 10.1007/s12013-007-0014-9. [DOI] [PubMed] [Google Scholar]
- 190.Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 191.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci U S A. 2000;97:4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 194.Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM, Klenerman D. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hogen T, Levin J, Schmidt F, Caruana M, Vassallo N, Kretzschmar H, Botzel K, Kamp F, Giese A. Two different binding modes of alpha-synuclein to lipid vesicles depending on its aggregation state. Biophys J. 2012;102:1646–1655. doi: 10.1016/j.bpj.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stockl MT, Zijlstra N, Subramaniam V. alpha-Synuclein oligomers: an amyloid pore? Insights into mechanisms of alpha-synuclein oligomer-lipid interactions. Mol Neurobiol. 2013;47:613–621. doi: 10.1007/s12035-012-8331-4. [DOI] [PubMed] [Google Scholar]
- 197.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 199.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer's disease. FASEB J. 2004;18:962–964. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 200.Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- 201.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 202.Azeredo da Silveira S, Schneider BL, Cifuentes-Diaz C, Sage D, Abbas-Terki T, Iwatsubo T, Unser M, Aebischer P. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson’s disease. Hum Mol Genet. 2009;18:872–887. doi: 10.1093/hmg/ddn417. [DOI] [PubMed] [Google Scholar]
- 203.Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Oueslati A, Fournier M, Lashuel HA. Role of phost-translational modifications in modulating the structure, function and toxicity of a-synuclein: implications for Parkinson’s disease pathogenesis and therapies. In: Björklund A, Cenci MA, editors. Progress in Brain Research. City: Academic Press; 2010. pp. 115–145. [DOI] [PubMed] [Google Scholar]
- 205.Haj-Yahya M, Fauvet B, Herman-Bachinsky Y, Hejjaoui M, Bavikar SN, Karthikeyan SV, Ciechanover A, Lashuel HA, Brik A. Synthetic polyubiquitinated alpha-Synuclein reveals important insights into the roles of the ubiquitin chain in regulating its pathophysiology. Proc Natl Acad Sci U S A. 2013;110:17726–17731. doi: 10.1073/pnas.1315654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci U S A. 2009;106:8157–8162. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bungeroth M, Appenzeller S, Regulin A, Volker W, Lorenzen I, Grotzinger J, Pendziwiat M, Kuhlenbaumer G. Differential aggregation properties of alpha-synuclein isoforms. Neurobiol Aging. 2014;35:1913–1919. doi: 10.1016/j.neurobiolaging.2014.02.009. [DOI] [PubMed] [Google Scholar]


