Abstract
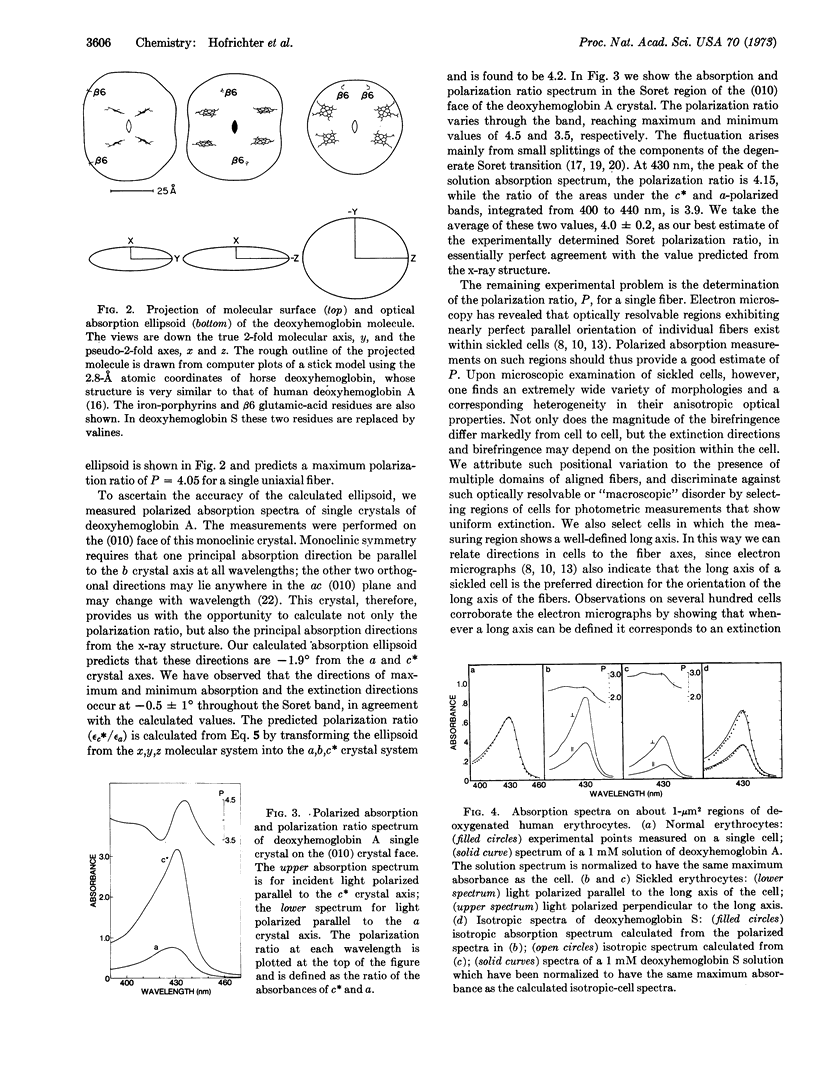
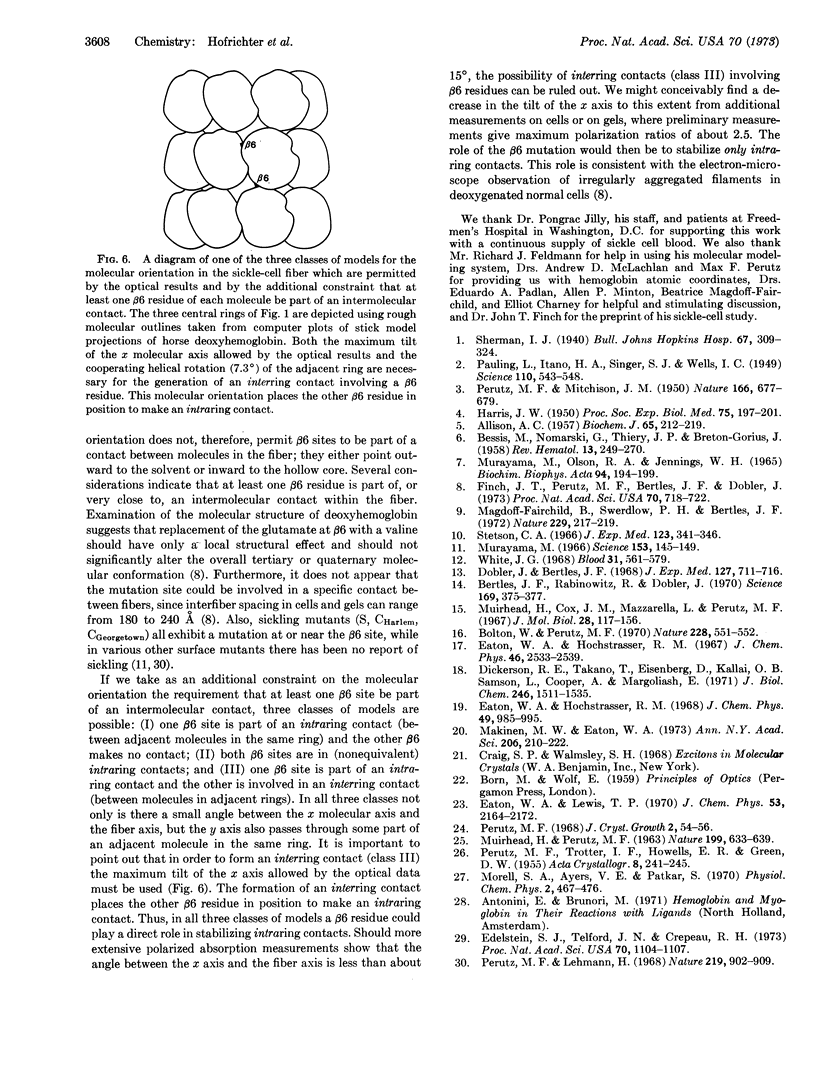
Possible orientations of deoxyhemoglobin S molecules within sickle-cell fibers are delimited by polarized absorption measurements on single sickled cells and single crystals of deoxyhemoglobin A. The polarization ratio of cells provides a lower limit for that of an individual fiber and, coupled with the absorption properties of the deoxyhemoglobin molecule, restricts the orientation of the long molecular (x) axis to within 22° of the fiber axis. Adopting the stacked ring model of Finch et al. for the molecular positions and the additional constraint that at least one mutated (β6) site is part of an intermolecular contact, our optical result requires that the true molecular dyad (y) axis pass through some part of an adjacent molecule in the same ring. This range of orientations for the y axis is approximately perpendicular to those described in existing models and places at least one β6 residue in position to be part of a contact between molecules in the same ring.
Keywords: anemia, linear dichroism, microspectrophotometry, deoxyhemoglobin A crystals
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Properties of sickle-cell haemoglobin. Biochem J. 1957 Feb;65(2):212–219. doi: 10.1042/bj0650212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESSIS M., NOMARSKI G., THIERY J. P., BRETON-GORIUS J. Etude sur la falciformation des globules rouges au microscope polarisant et au microscope électronique. II. L'intérieru du globule; comparaison avec les cristaux intra-globulaires. Rev Hematol. 1958 Apr-Jun;13(2):249–270. [PubMed] [Google Scholar]
- Bertles J. F., Rabinowitz R., Döbler J. Hemoglobin interaction: modification of solid phase composition in the sickling phenomenon. Science. 1970 Jul 24;169(3943):375–377. doi: 10.1126/science.169.3943.375. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Döbler J., Bertles J. F. The physical state of hemoglobin in sickle-cell anemia erythrocytes in vivo. J Exp Med. 1968 Apr 1;127(4):711–714. doi: 10.1084/jem.127.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Electronic spectrum of single crystals of ferricytochrome-c. J Chem Phys. 1967 Apr 1;46(7):2533–2539. doi: 10.1063/1.1841081. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Single-crystal spectra of ferrimyoglobin complexes in polarized light. J Chem Phys. 1968 Aug 1;49(3):985–995. doi: 10.1063/1.1670263. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Lewis T. P. Polarized single-crystal absorption spectrum of 1-methyluracil. J Chem Phys. 1970 Sep 15;53(6):2164–2172. doi: 10.1063/1.1674310. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J., Telford J. N., Crepeau R. H. Structure of fibers of sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1104–1107. doi: 10.1073/pnas.70.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. W. Studies on the destruction of red blood cells. VIII. Molecular orientation in sickle cell hemoglobin solutions. Proc Soc Exp Biol Med. 1950 Oct;75(1):197–201. doi: 10.3181/00379727-75-18144. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- MURAYAMA M., OLSON R. A., JENNINGS W. H. MOLECULAR ORIENTATION IN HORSE HEMOGLOBIN CRYSTALS AND SICKLED ERYTHROCYTES. Biochim Biophys Acta. 1965 Jan 25;94:194–199. doi: 10.1016/0926-6585(65)90024-5. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Polarized single crystal absorption spectra of carboxy- and oxyhemoglobin. Ann N Y Acad Sci. 1973;206:210–222. doi: 10.1111/j.1749-6632.1973.tb43213.x. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Murayama M. Molecular mechanism of red cell "sickling". Science. 1966 Jul 8;153(3732):145–149. doi: 10.1126/science.153.3732.145. [DOI] [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- PERUTZ M. F., MITCHISON J. M. State of haemoglobin in sickle-cell anaemia. Nature. 1950 Oct 21;166(4225):677–679. doi: 10.1038/166677a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Stetson C. A., Jr The state of hemoglobin in sickled erythrocytes. J Exp Med. 1966 Feb 1;123(2):341–346. doi: 10.1084/jem.123.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. The fine structure of sickled hemoglobin in situ. Blood. 1968 May;31(5):561–579. [PubMed] [Google Scholar]


