Significance
The assembly of normally soluble proteins into large fibrils, known as amyloid aggregation, is associated with a range of pathologies. Prefibrillar protein oligomers but not the grown fibers are believed to be the main toxic agents. It is unresolved if these oligomers are necessary for fibril assembly or just a dangerous byproduct. We show using computer simulations that, at physiological concentrations, amyloid formation must proceed through a two-step process including prefibrillar oligomers. We find that there is an optimal oligomeric size for amyloid nucleation and that classical nucleation theory cannot be applied to this process. Formation of oligomers and hence, fibrils, is controlled by the strength of nonspecific attractions, whose weakening may be crucial in preventing amyloid aggregation.
Keywords: amyloid, protein aggregation, protein oligomers, neurodegeneration, coarse-grained simulations
Abstract
Protein oligomers have been implicated as toxic agents in a wide range of amyloid-related diseases. However, it has remained unsolved whether the oligomers are a necessary step in the formation of amyloid fibrils or just a dangerous byproduct. Analogously, it has not been resolved if the amyloid nucleation process is a classical one-step nucleation process or a two-step process involving prenucleation clusters. We use coarse-grained computer simulations to study the effect of nonspecific attractions between peptides on the primary nucleation process underlying amyloid fibrillization. We find that, for peptides that do not attract, the classical one-step nucleation mechanism is possible but only at nonphysiologically high peptide concentrations. At low peptide concentrations, which mimic the physiologically relevant regime, attractive interpeptide interactions are essential for fibril formation. Nucleation then inevitably takes place through a two-step mechanism involving prefibrillar oligomers. We show that oligomers not only help peptides meet each other but also, create an environment that facilitates the conversion of monomers into the β-sheet–rich form characteristic of fibrils. Nucleation typically does not proceed through the most prevalent oligomers but through an oligomer size that is only observed in rare fluctuations, which is why such aggregates might be hard to capture experimentally. Finally, we find that the nucleation of amyloid fibrils cannot be described by classical nucleation theory: in the two-step mechanism, the critical nucleus size increases with increases in both concentration and interpeptide interactions, which is in direct contrast with predictions from classical nucleation theory.
During the process of amyloid formation, normally soluble proteins assemble into fibrils that are enriched in β-sheet content and have diameters of a few nanometers and lengths up to several micrometers. This phenomenon has been implicated in a variety of pathogenic processes, including Alzheimer’s and Parkinson’s diseases, type 2 diabetes, and systemic amyloidoses (1–3). The association with human diseases has largely motivated a long-standing effort to probe the assembly process, and numerous studies have aimed at elucidating the mechanism of amyloid aggregation (4). The basic nature of the aggregation reaction has emerged as a nucleation and growth process (5, 6), where the aggregates are created through a not well-understood primary nucleation event and can grow by recruiting additional peptides or proteins to their ends (7, 8). In this paper, we focus on the nature of this primary step in amyloid nucleation and the fundamental initial events that underlie amyloid formation.
Amyloidogenic peptides and proteins, when in their nonpathological cellular form, can range in the structures from mainly α-helical to β-sheet and even random coil, whereas the amyloid forms of proteins possess a generic cross–β-structure (9–14). The formation of amyloid is, hence, accompanied by marked changes in the conformations of the peptides and proteins that undergo this process. A pertinent question is whether this conformational change takes place simultaneously with the nucleation process or whether nucleation takes place first and is then followed by conformational change. These two possible scenarios of nucleation have been extensively discussed in the experimental and theoretical literature (5, 8, 15–19). We will refer in this work to the two scenarios simply as one-step nucleation (1SN), in which the β-sheet–enriched nucleus forms directly from the solution, and two-step nucleation (2SN), where soluble monomers first assemble into disordered oligomers, which subsequently convert into a β-sheet nucleus. Disordered oligomers, ranging in size between dimers and micrometer-sized particles, have been observed in some experiments (20–28). These findings highlight a central question regarding the role of disordered oligomers in fibril formation: are such clusters a necessary step in the process of fibril formation or just a byproduct?
From a biological and biomedical perspective, it is important to understand the conditions under which oligomeric clusters form, because such species exhibit high cytotoxicity (1, 29–31). Indeed, there is strong evidence that the disordered oligomers rather than fully grown fibrils are the main pathogenic species in protein aggregation diseases (31–33). As such, defining the role of the prefibrillar oligomers during amyloid formation will be crucial to develop intervention strategies that target these species (1, 30, 34, 35).
Mutations in the polypeptide sequence and extrinsic changes in the experimental conditions are known to alter the concentrations of aggregated species, their size, and their cytotoxicity (25, 36–39). For instance, mutations that increase hydrophobicity of the Alzheimer’s β-peptide (Aβ1–42) have a pronounced effect on its aggregation behavior and the size distribution of the resulting oligomers (23–26, 40), promoting toxicity and expediting the fibrillization process. In the same spirit, two extra hydrophobic residues in Aβ1–42 are believed to contribute to the more pronounced oligomerization and faster fibrillization compared with its alloform Aβ1–40 (24, 25, 40). Temperature, pH, and concentration of certain metals also affect oligomerization and pathways of fibrillization (41–44).
The common feature of the above experiments is that they modify the internal free energy difference between the soluble and the β-sheet–forming state, also called the β-sheet propensity, which has been extensively studied in the literature (45–48). However, they also modify interactions between peptides that aggregate, a crucial contribution that has not yet been systematically addressed.
In this paper, we study the effect of nonspecific interactions between peptides on the amyloid nucleation process. Such nonspecific interactions do not depend on the atomistic details of the amino acids involved, allowing us to address question about amyloid aggregation and nucleation using a coarse-grained model. In particular, generic hydrophobic stretches in the sequence of Aβ have been shown to be sufficient to promote aggregation (49, 50). Mutations of nonpolar residues to other nonpolar residues had little or no effect on aggregation, whereas mutations that reduce charge and/or increase hydrophobicity enhanced it (50, 51). Furthermore, atomic force microscopy measurements have shown that the strength of overall interactions between amyloidogenic proteins correlates with their tendency to aggregate (52, 53).
We have performed extensive computer simulations that allowed us to observe both the 1SN and the 2SN mechanisms. These simulations reveal that 1SN and 2SN can be viewed as two limits of the same process, something that several previous studies have suspected (16, 18). Importantly, we observe that only 2SN is possible at low peptide concentrations, comparable with the levels that are found in vivo. Another key observation is that fibril nucleation typically does not proceed through the most prevalent oligomeric species but rather, through an oligomer with a size that is only observed as a result of rare fluctuations. As a consequence, such oligomers will be hard to capture experimentally, although their presence is required for nucleation to take place. Our simulations show that the free energy barrier for fibril nucleation through the two-step mechanism decreases with increasing strength of the interpeptide interactions. Furthermore, the critical nucleus size in the two-step mechanism is found to grow with the increase in the peptide concentrations as well as with stronger interpeptide interactions, which is in direct contrast with the classical nucleation. These results imply that weakening the nonspecific interactions between peptide monomers in solution and thereby, simultaneously increasing both the free energy barrier for oligomer formation and the free energy barrier for peptide conversion at a given oligomer size may be a crucial step in preventing amyloid aggregation.
Coarse-Grained Model of an Amyloidogenic Peptide Supported by Atomistic Simulations
In view of the experimental evidence that the formation of peptide oligomers is governed by generic features of the interactions between monomers (49, 50, 54), we can use a simple coarse-grained model of an amyloidogenic peptide. The great advantage of such a model is that it is sufficiently cheap to allow us to vary interpeptide interactions and explore a wide range of peptide concentrations. The model should be able to capture both the formation of amorphous oligomers and the nucleation of fibrils. Earlier studies (55) have shown that a minimal model that captures this phenomenology accounts for the fact that the peptides can be in two states: a soluble state that can form only disordered oligomers and a higher free energy state that can form β-sheet–like fibrils.
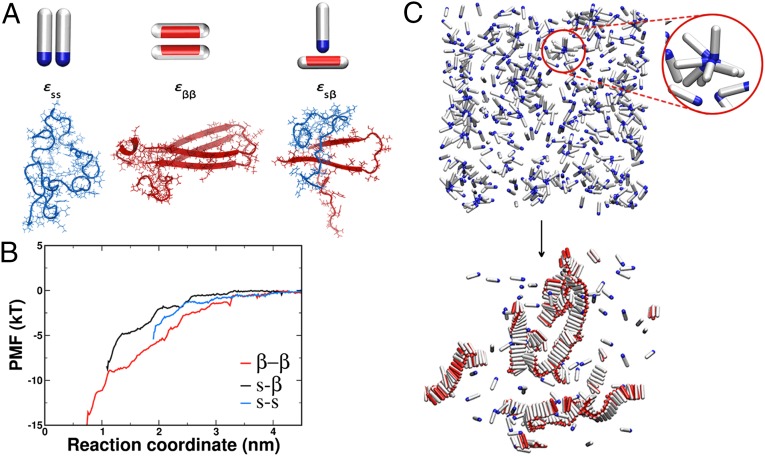
The soluble state of the amyloidogenic peptide is modeled as a hard spherocylinder with an attractive patch at the tip (Fig. 1A). The patch is the source of nonspecific attractions, with strength that is represented by the parameter εss. Such particles are able to make micellar-like oligomers, as represented in Fig. 1C, Upper, but not extended aggregates. The β-sheet–forming configuration is described as a hard spherocylinder with an attractive side patch (Fig. 1A). Peptides in the β-state interact with an interaction strength εββ if their patches point toward each other. In that case, they tend to pack parallel to one another, which leads to the fibril-like structure (Fig. 1C, Lower). The interaction strength between the soluble and the fibril-forming state is given by εsβ (Fig. 1A). In our Monte Carlo simulations, a soluble protein can convert into the β-prone state every simulation step with some small probability that mimics slow conversion of the soluble peptide into the β-prone configuration. The conversion, apart from being kinetically slow, is also thermodynamically unfavorable, which reflects the loss of the conformational entropy of the β-prone form compared with the soluble form (14). Hence, we penalize every conversion from the soluble to the β-form with a change in the excess chemical potential of , where k denotes Boltzmann’s constant. This value is chosen to reflect the fact that amyloidogenic proteins are typically not found in the β-sheet conformation in solution (54, 56). With respect to the current body of work on the importance of the β-sheet propensity, this places our model in the range of proteins with small to mid–β-propensity, such as Aβ. Additional details of the coarse-grained model can be found in SI Text, section I.
Fig. 1.
Possible interactions in the system. (A, Left) Two spherocylinders representing the soluble peptides interact through their attractive tips (blue). The interaction strength is given by . (A, Center) Spherocylinders in the β-form interact through their side patches (red). The strength of this interaction is . (A, Right) The interaction between the soluble peptide and the β-competent form is given by . Underneath each coarse-grained representation is its atomistic realization for the case of the Aβ1–42 peptide. Snapshots are taken at the shortest distance of the molecular dynamics umbrella sampling at 10 ns. (B) Potential of mean force (PMF) for the pair interactions in the Aβ1–42 system used to guide coarse-grained simulations. The red curve represents the interaction between two peptides in the β-form, the blue curve is the interaction between the random coil peptides, whereas the black line is the interaction between the random coil peptide and the peptide kept in the β-prone form. (C) Representative snapshots before the nucleation occurred and at the end of the simulation run. (Upper) Disordered oligomers formed by peptides in their soluble form. The red circle magnifies such an oligomer. (Lower) Amyloid-like fibrils formed by peptides in the β-sheet–competent form. For visual clarity, the red attractive patch in the β-form is depicted spanning the whole length of the spherocylinder body.
To obtain estimates of the interaction parameters , , and , we carried out fully atomistic simulations in the system of the Aβ1–42 peptide in explicit water and used umbrella sampling to determine the three possible pair interactions. Details of the simulation procedure are explained in SI Text, section II and Fig. S1. As expected, the interaction between two peptides in the hairpin conformation packed in the β-sheet, reflecting the interaction, is the strongest , which is shown by the depth of the potential in Fig. 1B. To obtain an estimate of the interaction between the soluble and the β-prone peptide, we constrained one of the peptides to be in the U-turn shape of a β-hairpin configuration and sampled its interaction with the random coil peptide. Although the atomistic properties of the isolated β-prone peptide are still unknown, our purpose here is to use a conformation that can easily act as a platform for fibril assembly. We find that the interaction energy of this s–β complex at the shortest sampled distance is . The interaction between the peptides in random coil conformation is the smallest of the three () but still clearly attractive. In our model, the strength of this attractive interaction is represented by . It is important to note that the shortest sampled interpeptide separation in each of the three cases is limited by the sum of the peptides’ radii of gyration. Of course, it is an oversimplification to try to capture the interaction energy between two complex fluctuating peptides by a single number. However, we argue that the relative interaction strengths that we compute in our atomistic simulations are indicative of the relative strengths of the interactions in the first steps of assembly. We use the atomistic simulations mainly to justify why, in our coarse-grained model, we choose the following ordering of interaction strengths: . An additional set of umbrella simulations was performed on the A2V mutation of Aβ42, which is reported to be responsible for early-onset Alzheimer’s disease (57), with the purposes of evaluating the relative strengths of interactions for an additional system and further supporting the choice of energy scale in the coarse-grained model. The Aβ42–A2V simulations show the same trend for the interaction strengths as the wild type (WT) with (14.6, 5.9, and , respectively) (Fig. S2D). (We do not discuss implications of this mutation on the mechanism underlying the A2V substitution in Aβ-fibrillogenesis. To accurately compare it with the WT, we would also need to assess the free energy barrier for the β-conversion associated with both variants, which is not within the scope of this work.)
1SN Vs. 2SN
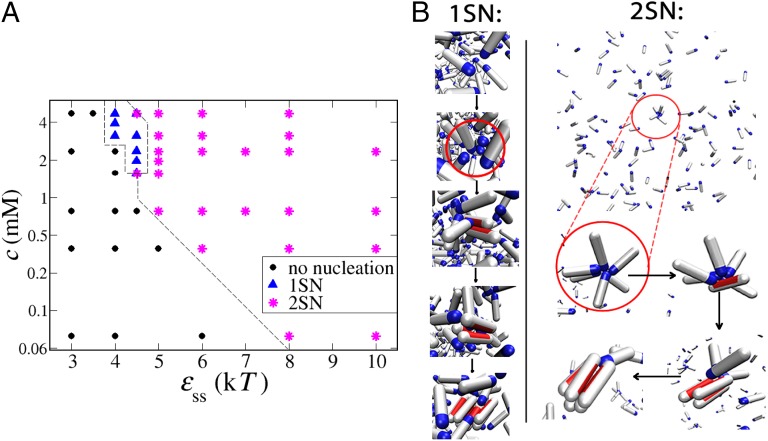
Our Monte Carlo simulations allow us to study amyloid nucleation pathways over a range of peptide concentrations and interpeptide interaction strengths. A tentative phase diagram is shown in Fig. 2A. When interactions between peptides in the solution are sufficiently strong (), disordered micellar-like oligomers are formed before the observation of elongated fibrils. These oligomers exist in the solution for a long time before transforming into a β-sheet nucleus through a series of single-peptide conversions, during which each converted peptide is in contact with other random coils within the aggregate. This two-step mechanism (2SN) is often referred to in the literature as the nucleated conformational conversion and was observed for both -peptides (18) and prion proteins (17) under certain experimental conditions. The β-sheet protofibril can further grow by either addition of monomers from the solution or merging with another oligomer. The preferred mode of growth will depend on the exact conditions: at lower concentrations and lower -values, the system is well below the critical micelle concentration, and there will be many more monomers than oligomers in solution (58). In that case, the growth will mainly be through monomer addition (17). Above the critical micelle concentration, most of peptides will be incorporated within oligomers, and few monomers will remain in solution. In that case, the addition of disordered oligomers at the end of the growing fiber becomes the more probable fibril growth mechanism (18).
Fig. 2.
Mechanism of amyloid nucleation. (A) Tentative phase diagram. At low concentrations and low interpeptide interactions, no nucleation was observed (black circles). The one-step mechanism is possible only at low interpeptide interactions and high concentrations (blue triangles). In any other case, the nucleation proceeded through the two-step mechanism (magenta asterisks). All points are collected for . The dashed line is a guide to the eye. (B) Pre- and postnucleation snapshots for two points in the phase diagram. (Left) The 1SN case: and . The red circle zooms into the area where nucleation happens. (Right) The 2SN case at low peptide concentration: and . The nucleating oligomer is circled in red in the first snapshot and magnified in the rest of the snapshots.
Our atomistic simulations show that the attractions between soluble peptides originate mainly from polar interactions, with some participation of hydrogen bonding (Fig. S2 B and C). Recent molecular simulations support this observation and indicate that the polar N-terminal region can act as a catalytic region that initiates and accelerates assembly of Aβ-peptides (59, 60).
If the interactions between peptides are weak (), we observe that fibril nucleation can happen only when the peptide concentration is high—above 2 mM in our case. At high concentrations, random fluctuations can push monomers close to each other, and a nucleus can be born from the solution in a single step without any noticeable preceding aggregate (1SN). This event is followed by the rapid growth by monomer addition and sometimes referred to as the nucleated polymerization (5, 8). It is important to point out that the lifetime of the disordered oligomers is the key feature that distinguishes this mechanism from the 2SN route discussed above. Fig. 2B, Left shows the representative snapshots during the 1SN nucleation process. Closer inspection reveals that, because of random fluctuations, several soluble peptides interact with each other and other peptides that can convert to the β-prone state because of the stabilizing interaction with the soluble peptides. Interpeptide interactions are crucial even in this case, but the event is transient: it does not require the presence of long-lived oligomers. (Long lived in our simulations means that, after oligomers form, they tend to stay stable for most of the simulation time.) We should emphasize that the cross-over from 1SN to 2SN is not abrupt, and in the regime of high peptide concentrations and intermediate interactions, one can observe nucleation from short-living oligomers, which exhibit features of both limits.
It is worth adding that, at much higher peptide concentrations and interpeptide interactions than the ones reported here, kinetically arrested amorphous aggregates can be observed (61, 62). We did not study this region, because we focus on more physiologically relevant concentrations and interaction values.
Nucleation at Low Peptide Concentrations
The most relevant regime for amyloidogenic peptides under physiological conditions is the regime of low peptide concentrations (micro- and nanomolar). Our simulations clearly show that, in this regime, 1SN is extremely unlikely—so much so that we did not observe a single 1SN event in our longest ( Monte Carlo steps) simulation runs. The explanation is simple: in this regime, the probability of a random encounter of enough monomers to initiate fibril nucleation is completely negligible. At low peptide concentrations, nucleation can only be achieved if there are appreciable attractive interactions between soluble monomers. Such nonspecific attractions favor the formation of long-lived peptide clusters, in which subsequent fibril nucleation can take place. For instance, at a concentration of , we observe fibrils above the threshold of . In this case, nucleation originates inside oligomers that form only rarely but are relatively long-lived. Fig. 2B, Right shows an example of the 2SN mechanism that we observe. Direct experimental observation of the first stages of 2SN may be challenging, because the concentration of the oligomers is very small. However, our simulations suggest that it should be straightforward to obtain indirect evidence of the 2SN mechanism: proteins that do not attract in solution and cannot form prefibrillar clusters are very unlikely to form fibrils at low concentrations.
Role of Oligomers in the Nucleation Process: Free Energy Analysis
Nonspecific attractions between soluble peptides favor the formation of relatively long-lived oligomers. The existence of oligomers lowers the nucleation barrier for the subsequent fibril formation, because as our atomistic simulations show, the -interaction is more favorable than the ss-interaction, and the -interaction is even more favorable. Hence, the energetic price to pay for the conversion from the s-state to the β-state inside an oligomer is compensated for by the gain in interaction energy between the peptides. Increased number of hydrophobic contacts between the β-form and the soluble peptide is the reason why the -interaction is favorable (Fig. S2A). This enhanced hydrophobic interaction stabilizes the amyloidogenic β-form when in contact with other random coils within a disordered aggregate. To quantify this effect, we performed Monte Carlo umbrella sampling simulations to compute the free energy barrier for fibril formation inside an oligomer in our coarse-grained system. Details of the simulation method are given in SI Text, section II and Fig. S1.
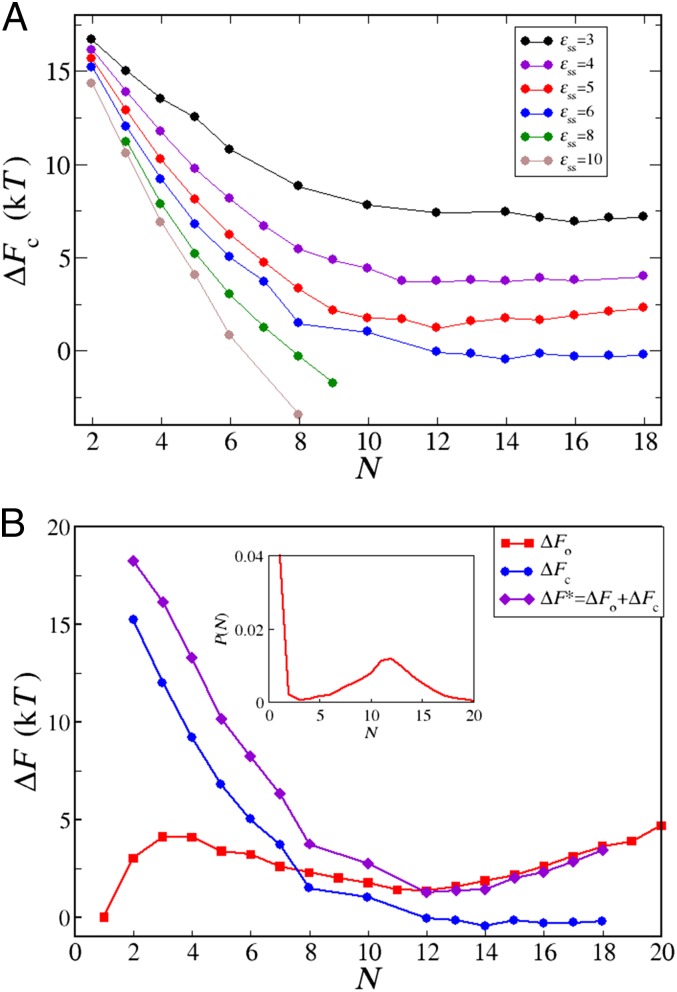
We find that the first conversion step (i.e., the one involving the first soluble monomer converting to a β-form within the oligomer) determines the height of the free energy barrier. The finding that the first conversion has the highest free energy cost is not too surprising: in this process, the conformational entropy of one random coil is lost, and there is only a small gain caused by interactions of the β-form with the remaining random coils. As soon as the second monomer converts to the β-form, a β-sheet interaction is established, which is enthalpically very favorable because of the increase in hydrophobic contacts and hydrogen bonds (Fig. S2 A and C). Every subsequent contact between the β-sheets lowers the free energy of the oligomer even more; thus, the rate-limiting step is the formation of the first β-form peptide. Hence, in our simulations, the critical nucleus for fibrillization is an oligomer (of undetermined size) with exactly one converted peptide. The smallest possible fibril is then made of two β-peptides, whereas in practice, such small fibrils quickly continue growing.
Our umbrella sampling simulations allow us to calculate the free energy change for converting one peptide into the β-state within an oligomer of the size N when varying the peptide interactions (Fig. 3A). We find that the barrier for the conversion systematically decreases with increasing size of the oligomer for a fixed interaction. Such behavior is to be expected as the number of possible -interactions increases with the oligomer size. Of course, the effect of the oligomer size eventually saturates when the maximum number of geometrically allowed interactions is reached.
Fig. 3.
(A) The free energy barrier for conversion of one random coil peptide into the β-form within a disordered oligomer of the size N for various values of interpeptide interaction. From top to bottom, . In all cases, . (B) Partition of the free energy barrier for nucleation from solution. The red line and red squares show free energy for oligomerization, , obtained from the oligomer size distribution (shown in Inset) at mM and . The blue line and blue circles show free energy for conversion of one peptide into the β-prone state within an oligomer of size N, , for and . Note that the blue line in B corresponds to the blue line in A. The violet line and violet diamonds show the free energy barrier for formation of a critical nucleus from solution, , at mM and obtained as .
Not surprisingly, the nucleation barrier also systematically decreases with stronger interpeptide interactions. This finding can be seen to emerge from the assumption that shifts together with : strong is compensated for by strong(er) . To inspect the origin of this correlation, we measured for different values of s − β stabilizations (Fig. S3) and found that the nucleation barrier drops down dramatically with stronger interaction. This effect is likely to contribute to the higher rate of nucleation of 1–42 compared with 1–40 (63) and peptides with hydrophobic mutations compared with their respective WTs. However, at very high values of (over 10), the oligomer reorganization might become very slow, and the process can be kinetically arrested. Despite the fact that the exact number of converted peptides that give rise to nucleation can be dependent on the simulation model or the protein under study and is governed by the ratio, the trends described here should be general.
Two Competing Free Energy Contributions Cause Nonclassical Scaling of the Critical Nucleus Size
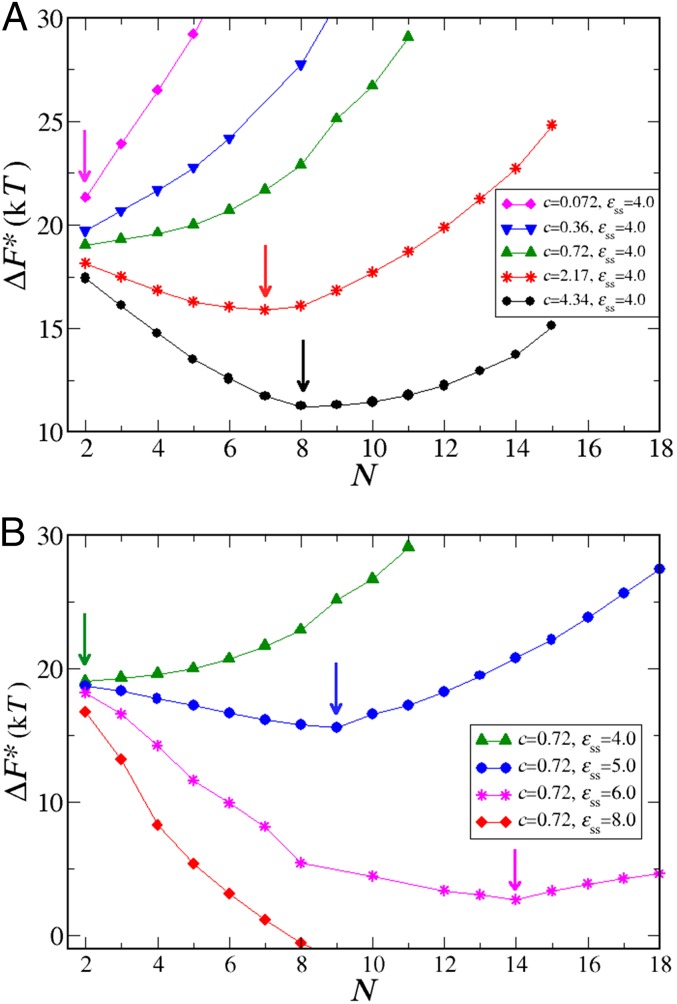
The overall nucleation barrier for fibril nucleation is the sum of two contributions. The first contribution is the free energy cost of forming an oligomer of size N. The second contribution is the free energy cost of nucleating a fibril in such an oligomer. Both free energy barriers depend on N. We can compute , the free energy barrier for the formation of an oligomer of size N, from measurements of the oligomer size distribution, , using . Examples of measurements for several values of c and are shown in Fig. S4. For dilute solutions of clusters, is of the form , where is a concentration-independent free energy, and c is the monomer concentration. Clearly, for low values of c, will grow rapidly with cluster size, which has important consequences for the overall behavior of the free energy barrier for fibril nucleation. For low monomer concentrations, the lowest overall nucleation barrier will correspond to nucleation of a fibril in a relatively small oligomer. For higher concentrations, fibril nucleation in larger oligomers will dominate. Fig. 3B shows an example of the various contributions to the fibril nucleation barrier for one specific choice of the monomer concentration and the value of . The shift of the nucleation barrier with monomer concentration is shown in Fig. 4A, while the arrows indicate the respective dominant nucleus sizes. Fig. 4B shows the variation of the nucleation barriers with the nonspecific monomer–monomer interaction (). Fig. 4B clearly shows that, all other things being equal, stronger nonspecific interactions between soluble peptides tend to decrease the fibril nucleation barrier very substantially. The underlying reason is that decreases strongly with increasing . We stress that the nucleation of fibrils cannot be described by classical nucleation theory: that theory would predict that the size of the critical nucleus would grow both with a decrease in concentration and in the interpeptide interactions. The explanation is that, unlike the classical nucleation case, we are always working in the regime where the condensed phase of the soluble peptides is thermodynamically unstable. Hence, large clusters are disfavored, particularly at low concentrations. However, fibril nucleation must proceed through a cluster, and that process is easier in large clusters (if they can form). It is the compromise between these two trends that leads to the observed variation of the critical nucleus size with monomer concentration and .
Fig. 4.
Free energy barrier for nucleation from solution. (A) For constant interpeptide interaction and various peptide concentrations, , and all peptide concentrations are expressed in mM. (B) For various interpeptide interactions (expressed in kT) and constant peptide concentrations, . In all cases, . Arrows indicate critical nucleus sizes for several chosen parameters.
Discussion and Conclusions
Our numerical study reveals the crucial effect of nonspecific interpeptide attractions on the pathways of amyloid aggregation. We find that, for peptides that do not attract, the classical 1SN mechanism is possible but only at very high peptide concentrations. At low peptide concentrations, attractive interpeptide interactions are essential for fibril formation. Nucleation then inevitably takes place through a two-step mechanism, where the formation of disordered oligomers preceded fibril nucleation. Although direct measurements of such nonspecific interactions have been challenging to achieve, the determination of second virial coefficients with advanced light scattering techniques (64) is a promising avenue to access these values for amyloidogenic peptides and their mutants in solution.
We recall that this study focused on peptides with low to mid–β-propensity (). If the β-state was stable and could exist in solution, oligomers would not be needed to aid nucleation, and the 1SN scenario would become more probable. However, the available experimental data suggest that the β-state is usually not present in solution, at least not for the Aβ-peptide. It has been observed that mutants that further decrease β-sheet propensity (such as the E22GAβ) exhibit enhanced oligomer formation over the WT (51, 65, 66), which is in agreement with our findings. Our coarse-grained model, although supported by atomistic simulations, is, of course, highly simplified, because we consider only two peptide states. In reality, we expect there to be several intermediate conformations on the pathway between the soluble and the β-form. Qualitatively, the key physical behavior originates from the fact that the mixed interaction energy is intermediate between that of the pure soluble and the β-form. As such, these results are expected to generalize to other coarse-graining schemes that exhibit this property, including ones with more than one intermediate state.
In this framework, oligomers play a dual role. Disordered oligomers not only help peptides meet each other but also, create an environment that facilitates the conversion of monomers into the β-form. We stress that, although the oligomers that form because of attractive nonspecific interactions are rare (and therefore, hard to observe directly), their formation is a crucial on-pathway step in the amyloid formation process for peptides with a β-competent state that is not particularly stable. Similar 2SN processes involving amorphous precursors have been identified in very different physical phenomena, such as protein crystal nucleation (67), nucleation of sickle cell hemoglobin polymers (68), and biomineralization (69). The surprising finding of this work is that, at realistic (i.e., low) peptide concentrations, fibril formation must proceed through an intermediate amorphous oligomer and that this pathway leads to highly nontrivial predictions for the dependence of the critical nucleus size and nucleation barrier on monomer concentration and interpeptide interaction. Because oligomeric species formed under such conditions consist predominantly of non–β-structures, it is interesting to speculate that nature might have evolved a portfolio of chaperones to target specifically both the non–β- as well as the conventional β-aggregated forms to curtail pathological aggregation. Recent evidence suggests that such a situation might, indeed, occur for extracellular chaperones (70, 71). Although these simulations focused on the Aβ1–42 peptide, we expect aspects of the mechanism proposed here to be applicable to other amyloid proteins, such as the prion protein or α-synuclein. The conformational change from the soluble into the β-prone state is a ubiquitous feature underlying the amyloid aggregation, and the importance of disordered oligomers could be of a more general nature.
Supplementary Material
Acknowledgments
We thank Michele Vendruscolo, Iskra Staneva, and William M. Jacobs, for helpful discussions. A.Š. acknowledges support from the Human Frontier Science Program and Emmanuel College. Y.C.C. and D.F. are supported by Engineering and Physical Sciences Research Council Programme Grant EP/I001352/1. T.P.J.K. acknowledges the Frances and Augustus Newman Foundation, the European Research Council, and the Biotechnology and Biological Sciences Research Council. D.F. acknowledges European Research Council Advanced Grant 227758.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410159111/-/DCSupplemental.
References
- 1.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426(6968):900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 2.Dobson CM. Protein folding and misfolding. Nature. 2003;426(6968):884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 4.Eichner T, Radford SE. A diversity of assembly mechanisms of a generic amyloid fold. Mol Cell. 2011;43(1):8–18. doi: 10.1016/j.molcel.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 6.Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15(6):384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 7.Oosawa F, Kasai M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- 8.Hofrichter J, Ross PD, Eaton WA. Kinetics and mechanism of deoxyhemoglobin S gelation: A new approach to understanding sickle cell disease. Proc Natl Acad Sci USA. 1974;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick AW, et al. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc Natl Acad Sci USA. 2013;110(14):5468–5473. doi: 10.1073/pnas.1219476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasmer C, et al. Amyloid fibrils of the HET-s(218-289) prion form a beta solenoid with a triangular hydrophobic core. Science. 2008;319(5869):1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 11.Morris KL, Serpell LC. From molecular to supramolecular amyloid structures: Contributions from fiber diffraction and electron microscopy. In: Otzen DE, editor. Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties. Weinheim, Germany: Wiley-VCH; 2013. pp. 63–84. [Google Scholar]
- 12.Tycko R, Wickner RB. Molecular structures of amyloid and prion fibrils: Consensus versus controversy. Acc Chem Res. 2013;46(7):1487–1496. doi: 10.1021/ar300282r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fändrich M, Schmidt M, Grigorieff N. Recent progress in understanding Alzheimer’s β-amyloid structures. Trends Biochem Sci. 2011;36(6):338–345. doi: 10.1016/j.tibs.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson R, et al. Structure of the cross-β spine of amyloid-like fibrils. Nature. 2005;435(7043):773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lomakin A, Teplow DB, Kirschner DA, Benedek GB. Kinetic theory of fibrillogenesis of amyloid beta-protein. Proc Natl Acad Sci USA. 1997;94(15):7942–7947. doi: 10.1073/pnas.94.15.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auer S, Ricchiuto P, Kashchiev D. Two-step nucleation of amyloid fibrils: Omnipresent or not? J Mol Biol. 2012;422(5):723–730. doi: 10.1016/j.jmb.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Serio TR, et al. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science. 2000;289(5483):1317–1321. doi: 10.1126/science.289.5483.1317. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Culyba EK, Powers ET, Kelly JW. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat Chem Biol. 2011;7(9):602–609. doi: 10.1038/nchembio.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellstrand E, Boland B, Walsh DM, Linse S. Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem Neurosci. 2010;1(1):13–18. doi: 10.1021/cn900015v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garzon-Rodriguez W, Sepulveda-Becerra M, Milton S, Glabe CG. Soluble amyloid Abeta-(1-40) exists as a stable dimer at low concentrations. J Biol Chem. 1997;272(34):21037–21044. doi: 10.1074/jbc.272.34.21037. [DOI] [PubMed] [Google Scholar]
- 21.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Y, Lynn DG, Berland KM. Direct observation of nucleation and growth in amyloid self-assembly. J Am Chem Soc. 2010;132(18):6306–6308. doi: 10.1021/ja910964c. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein SL, et al. Amyloid beta-protein: Monomer structure and early aggregation states of Abeta42 and its Pro19 alloform. J Am Chem Soc. 2005;127(7):2075–2084. doi: 10.1021/ja044531p. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein SL, et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1(4):326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitan G, et al. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci USA. 2003;100(1):330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleiholder C, Dupuis NF, Wyttenbach T, Bowers MT. Ion mobility-mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation. Nat Chem. 2011;3(2):172–177. doi: 10.1038/nchem.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabaté R, Estelrich J. Evidence of the existence of micelles in the fibrillogenesis of beta-amyloid peptide. J Phys Chem B. 2005;109(21):11027–11032. doi: 10.1021/jp050716m. [DOI] [PubMed] [Google Scholar]
- 28.Yong W, et al. Structure determination of micelle-like intermediates in amyloid beta -protein fibril assembly by using small angle neutron scattering. Proc Natl Acad Sci USA. 2002;99(1):150–154. doi: 10.1073/pnas.012584899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci USA. 2009;106(35):14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 31.Bucciantini M, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416(6880):507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 32.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 33.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 34.Hefti F, et al. The case for soluble Aβ oligomers as a drug target in Alzheimer’s disease. Trends Pharmacol Sci. 2013;34(5):261–266. doi: 10.1016/j.tips.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443(7113):774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 36.Carulla N, et al. Experimental characterization of disordered and ordered aggregates populated during the process of amyloid fibril formation. Proc Natl Acad Sci USA. 2009;106(19):7828–7833. doi: 10.1073/pnas.0812227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crescenzi O, et al. Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur J Biochem. 2002;269(22):5642–5648. doi: 10.1046/j.1432-1033.2002.03271.x. [DOI] [PubMed] [Google Scholar]
- 38.Melchor JP, McVoy L, Van Nostrand WE. Charge alterations of E22 enhance the pathogenic properties of the amyloid beta-protein. J Neurochem. 2000;74(5):2209–2212. doi: 10.1046/j.1471-4159.2000.0742209.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Nostrand WE, Melchor JP, Cho HS, Greenberg SM, Rebeck GW. Pathogenic effects of D23N Iowa mutant amyloid beta -protein. J Biol Chem. 2001;276(35):32860–32866. doi: 10.1074/jbc.M104135200. [DOI] [PubMed] [Google Scholar]
- 40.Urbanc B, Betnel M, Cruz L, Bitan G, Teplow DB. Elucidation of amyloid beta-protein oligomerization mechanisms: Discrete molecular dynamics study. J Am Chem Soc. 2010;132(12):4266–4280. doi: 10.1021/ja9096303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang D, Rauda I, Han S, Chen S, Zhou F. Aggregation pathways of the amyloid β(1-42) peptide depend on its colloidal stability and ordered β-sheet stacking. Langmuir. 2012;28(35):12711–12721. doi: 10.1021/la3021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhowmik D, et al. pH changes the aggregation propensity of amyloid-β without altering the monomer conformation. Phys Chem Phys. 2014;16(3):885–889. doi: 10.1039/c3cp54151g. [DOI] [PubMed] [Google Scholar]
- 43.Gorman PM, Yip CM, Fraser PE, Chakrabartty A. Alternate aggregation pathways of the Alzheimer beta-amyloid peptide: Abeta association kinetics at endosomal pH. J Mol Biol. 2003;325(4):743–757. doi: 10.1016/s0022-2836(02)01279-2. [DOI] [PubMed] [Google Scholar]
- 44.Srinivasan R, et al. pH-dependent amyloid and protofibril formation by the ABri peptide of familial British dementia. J Mol Biol. 2003;333(5):1003–1023. doi: 10.1016/j.jmb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Pellarin R, Guarnera E, Caflisch A. Pathways and intermediates of amyloid fibril formation. J Mol Biol. 2007;374(4):917–924. doi: 10.1016/j.jmb.2007.09.090. [DOI] [PubMed] [Google Scholar]
- 46.Bellesia G, Shea JE. Effect of beta-sheet propensity on peptide aggregation. J Chem Phys. 2009;130(14):145103. doi: 10.1063/1.3108461. [DOI] [PubMed] [Google Scholar]
- 47.Li MS, et al. Factors governing fibrillogenesis of polypeptide chains revealed by lattice models. Phys Rev Lett. 2010;105(21):218101. doi: 10.1103/PhysRevLett.105.218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C, Shea JE. Coarse-grained models for protein aggregation. Curr Opin Struct Biol. 2011;21(2):209–220. doi: 10.1016/j.sbi.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Kim W, Hecht MH. Generic hydrophobic residues are sufficient to promote aggregation of the Alzheimer’s Abeta42 peptide. Proc Natl Acad Sci USA. 2006;103(43):15824–15829. doi: 10.1073/pnas.0605629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim W, Hecht MH. Mutations enhance the aggregation propensity of the Alzheimer’s A beta peptide. J Mol Biol. 2008;377(2):565–574. doi: 10.1016/j.jmb.2007.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klimov DK, Thirumalai D. Dissecting the assembly of Abeta16-22 amyloid peptides into antiparallel beta sheets. Structure. 2003;11(3):295–307. doi: 10.1016/s0969-2126(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 52.Lyubchenko YL, Kim BH, Krasnoslobodtsev AV, Yu J. Nanoimaging for protein misfolding diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(5):526–543. doi: 10.1002/wnan.102. [DOI] [PubMed] [Google Scholar]
- 53.Lyubchenko YL, Sherman S, Shlyakhtenko LS, Uversky VN. Nanoimaging for protein misfolding and related diseases. J Cell Biochem. 2006;99(1):52–70. doi: 10.1002/jcb.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fändrich M, Fletcher MA, Dobson CM. Amyloid fibrils from muscle myoglobin. Nature. 2001;410(6825):165–166. doi: 10.1038/35065514. [DOI] [PubMed] [Google Scholar]
- 55.Bieler NS, Knowles TPJ, Frenkel D, Vácha R. Connecting macroscopic observables and microscopic assembly events in amyloid formation using coarse grained simulations. PLOS Comput Biol. 2012;8(10):e1002692. doi: 10.1371/journal.pcbi.1002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allison JR, Varnai P, Dobson CM, Vendruscolo M. Determination of the free energy landscape of alpha-synuclein using spin label nuclear magnetic resonance measurements. J Am Chem Soc. 2009;131(51):18314–18326. doi: 10.1021/ja904716h. [DOI] [PubMed] [Google Scholar]
- 57.Di Fede G, et al. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009;323(5920):1473–1477. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Israelachvili JN. Intermolecular and Surface Forces. Revised 3rd Ed Academic; London: 2011. [Google Scholar]
- 59.Chong SH, Park M, Ham S. Structural and thermodynamic characteristics that seed aggregation of amyloid-beta protein in water. J Chem Theory Comput. 2012;8(2):724–734. doi: 10.1021/ct200757a. [DOI] [PubMed] [Google Scholar]
- 60.Viet MH, Nguyen PH, Derreumaux P, Li MS. Effect of the English familial disease mutation (H6R) on the monomers and dimers of Aβ40 and Aβ42. ACS Chem Neurosci. 2014;5(8):646–657. doi: 10.1021/cn500007j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dima RI, Thirumalai D. Exploring protein aggregation and self-propagation using lattice models: Phase diagram and kinetics. Protein Sci. 2002;11(5):1036–1049. doi: 10.1110/ps.4220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aggeli A, et al. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide beta -sheet tapes, ribbons, fibrils, and fibers. Proc Natl Acad Sci USA. 2001;98(21):11857–11862. doi: 10.1073/pnas.191250198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meisl G, et al. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc Natl Acad Sci USA. 2014;111(26):9384–9389. doi: 10.1073/pnas.1401564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chi EY, et al. Roles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony-stimulating factor. Protein Sci. 2003;12(5):903–913. doi: 10.1110/ps.0235703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nilsberth C, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4(9):887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 66.Lashuel HA, et al. Mixtures of wild-type and a pathogenic (E22G) form of Abeta40 in vitro accumulate protofibrils, including amyloid pores. J Mol Biol. 2003;332(4):795–808. doi: 10.1016/s0022-2836(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 67.ten Wolde PR, Frenkel D. Enhancement of protein crystal nucleation by critical density fluctuations. Science. 1997;277(5334):1975–1978. doi: 10.1126/science.277.5334.1975. [DOI] [PubMed] [Google Scholar]
- 68.Galkin O, et al. Two-step mechanism of homogeneous nucleation of sickle cell hemoglobin polymers. Biophys J. 2007;93(3):902–913. doi: 10.1529/biophysj.106.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gebauer D, Coelfen H. Prenucleation clusters and non-classical nucleation. Nano Today. 2011;6(6):564–584. [Google Scholar]
- 70.Narayan P, et al. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β(1-40) peptide. Nat Struct Mol Biol. 2012;19(1):79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narayan P, et al. Amyloid-β oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry. 2012;51(46):9270–9276. doi: 10.1021/bi301277k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.