Significance
Cofilin is an essential actin regulatory protein that severs filaments, which accelerates network remodeling by increasing the concentration of filament ends available for elongation and subunit exchange. The molecular basis of how cofilin binding interactions fragment filaments, which have stiffness comparable to commercial laboratory plastics, remains a central and unresolved mystery of cellular actin cytoskeleton reorganization. In this study we demonstrate that actin filament severing by vertebrate cofilin is driven by the linked dissociation of a single, site-specific cation that controls filament structure and mechanical properties, and that filament severing is an essential function of cofilin in cells. This work establishes that discrete interactions with cations serve a central regulatory function in mediating actin filament fragmentation by certain classes of severing proteins.
Keywords: cytoskeleton, persistence length, mechanics, electron cryomicroscopy
Abstract
Actin polymerization powers the directed motility of eukaryotic cells. Sustained motility requires rapid filament turnover and subunit recycling. The essential regulatory protein cofilin accelerates network remodeling by severing actin filaments and increasing the concentration of ends available for elongation and subunit exchange. Although cofilin effects on actin filament assembly dynamics have been extensively studied, the molecular mechanism of cofilin-induced filament severing is not understood. Here we demonstrate that actin filament severing by vertebrate cofilin is driven by the linked dissociation of a single cation that controls filament structure and mechanical properties. Vertebrate cofilin only weakly severs Saccharomyces cerevisiae actin filaments lacking this “stiffness cation” unless a stiffness cation-binding site is engineered into the actin molecule. Moreover, vertebrate cofilin rescues the viability of a S. cerevisiae cofilin deletion mutant only when the stiffness cation site is simultaneously introduced into actin, demonstrating that filament severing is the essential function of cofilin in cells. This work reveals that site-specific interactions with cations serve a key regulatory function in actin filament fragmentation and dynamics.
Actin polymerization powers the directed motility of eukaryotic cells and some pathogenic bacteria (1–3). Actin assembly also plays critical roles in endocytosis, cytokinesis, and establishment of cell polarity. Sustained motility requires filament disassembly and subunit recycling. The essential regulatory protein cofilin severs actin filaments (4–6), which accelerates actin network reorganization by increasing the concentration of filament ends available for subunit exchange (7).
Cofilin binding alters the structure and mechanical properties of filaments, which effectively introduces local “defects” that compromise filament integrity and promote severing (5). Filaments with bound cofilin have altered twist (8, 9) and are more compliant in both bending and twisting than bare filaments (10–13). It has been suggested that deformations in filament shape promote fragmentation at or near regions of topological and mechanical discontinuities, such as boundaries between bare and cofilin-decorated segments along partially decorated filaments (5, 12, 14–18).
Cations modulate actin filament structure and mechanical properties (19) and cofilin dissociates filament-associated cations (20), leading us to hypothesize that cation-binding interactions regulate filament severing by cofilin. Cations bind filaments at two discrete and specific sites positioned between adjacent subunits along the long-pitch helix of the filament (19, 21). These cation binding sites are referred to as “polymerization” and “stiffness” sites based on their roles in filament assembly and mechanics, respectively. These discrete sites bind both monovalent and divalent cations with a range of affinities (low millimolar for divalent and tens of millimolar for monovalent cations) (19, 21) but are predominantly occupied by Mg2+ and K+ under physiological conditions. Here we demonstrate that cation release from the stiffness site plays a central role in filament severing by vertebrate cofilin, both in vitro and in cells.
Results and Discussion
We tested whether cation occupancy and linked release are required for vertebrate cofilin to alter the structural and mechanical properties of filaments. Saccharomyces cerevisiae (herein referred to as yeast) actin lacks an acidic residue (Glu167 in subdomain 3) required to form the stiffness site and filaments display mechanical properties that are not influenced by cations (19). In contrast, cations have a strong effect on the stiffness of yeast actin filaments engineered with Glu167 at the stiffness site (A167E) (19, 22).
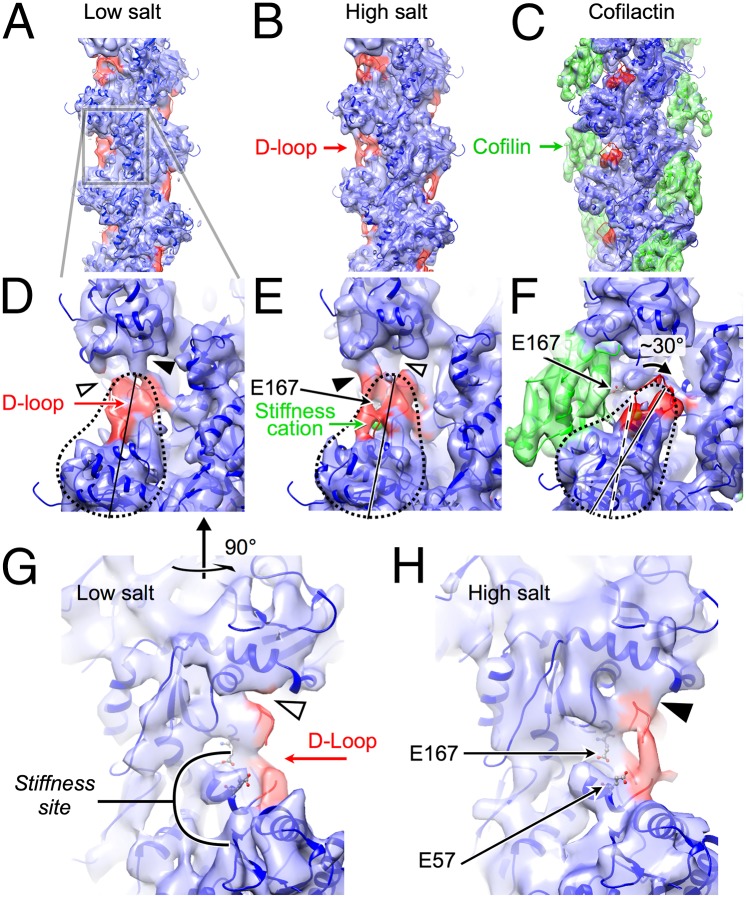
To investigate the structural basis of the filament stiffness change introduced by the A167E substitution, we solved structures of A167E yeast actin filaments in low and high [Mg2+] conditions by electron cryomicroscopy. Although the subunit conformational heterogeneity in filaments is evidently high (23), comparison of the density maps reveals cation-dependent structural differences that may reflect a shift toward a more rigid conformation at high Mg2+ concentrations (Fig. 1). In low [Mg2+], the predominant contact between the D-loop and the adjacent subunit is evidently proximal to the filament axis at a low filament radius (Fig. 1 D and G). In high [Mg2+], the D-loop strongly populates a conformational state displaying a high-radius contact with the adjacent subunit (Fig. 1 E and H). These two contact types correspond to D-loop conformational modes “2” and “3,” respectively, inferred from heterogeneity analysis of vertebrate actin filaments (23). These observations suggest that stiffness cation occupancy is coupled to the D-loop conformation and radial distribution of long-axis contacts between adjacent filament subunits (Fig. 1 D, E, G, and H), consistent with a role for the D-loop conformation in controlling filament mechanical properties (10, 24–26).
Fig. 1.
Electron cryomicroscopic reconstructions of A167E yeast actin reveal a salt-dependent change in the DNase I binding loop (D-loop) conformation. Overview of filaments (A, B, D, E, G, and H) without and (C and F) with bound cofilin. Ribbon diagram of the published atomic model of F-actin (34) (PDB ID code 3MFP) is rendered blue, except for the D-loop, which is colored red. The PDB model for cofilactin (8) (PDB ID code 3J0S) is colored similarly, except that the cofilin molecules are green. Experimental density maps (this work) are aligned with the atomic models and rendered as a semitransparent isosurface, colored to match the atomic models. (A, D, and G) Reconstruction under low-salt conditions (0.4 mM MgCl2). Converged estimate of the twist per subunit is 167.1°. Hollow and solid arrowheads in D, E, G, and H denote the apparent absence and presence (respectively) of long-axis contacts between the D-loop and the adjacent subunit. (B, E, and H) Reconstruction under high-salt conditions (5 mM MgCl2). The twist per subunit is indistinguishable from the low-salt dataset. The actin outer domain (subdomains 1 and 2) is indicated by the dashed pear shape. The two arrows in H point to E57 and the alpha helix harboring E167, which shows signs of increased order upon inclusion of high salt. (C and F) Reconstruction of A167E yeast actin filaments decorated with vertebrate cofilin under high-salt conditions (5 mM MgCl2). The filament twist per subunit refines to a value of 162.0°, as reported for cofilin bound to vertebrate actin (8). Cofilin rotates the outer domain clockwise (∼30°) and reorients the D-loop. The long-axis contact observed in bare actin under high-salt conditions is absent with bound cofilin. A lower-resolution (∼20 Å) reconstruction of cofilactin filaments under low-salt conditions did not reveal obvious differences in cofilin binding modes or conformations, and the filament pitch and rise were indistinguishable between low- and high-salt conditions.
We also solved a cryo-EM structure of A167E yeast actin filaments with bound vertebrate cofilin. The stiffness cation and cofilin binding sites overlap (Fig.1F and ref. 21), such that steric interactions preclude simultaneous occupancy by both ligands. Consistent with a prior cryo-EM study (8), cofilin induces a large (∼30°) rotation of the actin outer domain (subdomains 1 and 2; Fig. 1F), which repositions the D-loop away from the upper half of the stiffness site in the adjacent subunit. Thus, cofilin occupancy compromises the geometry and cation coordination of the predicted stiffness site. The overlapping binding sites and allosteric interactions give rise to strong thermodynamic coupling between cofilin binding and cation release (20).
The bending flexibilities of actin filaments with and without bound cofilin were quantitated from a correlation analysis of thermally driven shape fluctuations (27). Vertebrate cofilin binding increases the flexibility (i.e., lowers the bending persistence length, Lp) of vertebrate and A167E yeast actin filaments but has no detectable effect on the bending mechanics of WT yeast actin filaments (Fig. 2A and Fig. S1) despite binding with tight affinity (Kd <100 nM) (12), comparable to the affinity of vertebrate cofilin binding to A167E yeast actin filaments (Fig. S2). Thus, occupancy of the stiffness site is essential for vertebrate cofilin to alter the mechanical properties of actin filaments.
Fig. 2.
Stiffness-site cation release is required to alter actin filament mechanics and promote severing. (A) Vertebrate cofilin increases the bending flexibility (i.e., lowers bending Lp) of vertebrate and yeast A167E (stiffness-site mutant) actin filaments, but not WT yeast actin filaments. The cofilin binding densities in cofilactin samples are 0.9. Uncertainty bars represent the SD of the mean bending Lp (n = 200–500 filaments per each sample). (B) Time course of average Alexa-488–labeled WT and A167E yeast actin (600 nM) filament lengths upon addition of 300 nM vertebrate cofilin. The exponential fit (solid red line) yields the rate of 0.012 s−1. (C) Representative TIRF microscopic images of Alexa-488–labeled WT and A167E yeast actin filaments equilibrated with vertebrate cofilin at 0.5 binding density. (Scale bars, 5 µm.) (D) Cofilin binding density (ν)-dependence of average filament length (n = 200–500 filaments per each binding density). Uncertainty bars represent SEM (n = 4 per condition). Solid lines represent the best fits of the data to filament severing model presented in SI Text (Eq. S21) (12), yielding severing rate constants at boundaries relative to spontaneous fragmentation rate of bare actin filaments: k′sc = 0.9 (± 0.7) and 8.3 (± 3.2) for WT and A167E yeast actin filaments, respectively. Severing seems to be more efficient in real-time assays, presumably owing to inhibition of filament reannealing by flow.
Therefore, we evaluated whether cation interactions at the stiffness site and subsequent alterations in filament properties contribute to vertebrate cofilin severing activity, which we visualized in real time by total internal reflection fluorescence (TIRF) microscopy (Fig. S1). Vertebrate cofilin efficiently severs vertebrate actin filaments as well as A167E yeast actin filaments, as indicated by a dramatic reduction in average filament length (Fig. 2B), whereas we detected no severing of WT yeast actin filaments over the time range of observation (representative time course shown in Fig. 2B; severing rates shown in Fig. S1) (12). The average steady-state filament length (Fig. 2 C and D) confirms that WT yeast actin filaments are weakly severed by vertebrate cofilin, whereas A167E yeast actin filaments partially decorated with cofilin are severed more efficiently than bare or saturated filaments, similar to vertebrate actin filaments (12, 16).
Addition of phalloidin, which stiffens bare actin segments (28) and thus introduces a mechanical gradient at boundaries, promotes vertebrate cofilin-mediated severing of WT yeast actin filaments (Fig. S3). Furthermore, severing of A167E yeast actin filaments scales with the difference in stiffness between flexible cofilin-decorated segments and bare actin segments, whose rigidity and structure depends on stiffness site cation occupancy (Fig. S4), suggesting that the steepness of the mechanical gradient and structural change at boundaries determines filament severing efficiency. The gradient steepness is determined by the rigidity and conformational flexibility of bare segments with stiffness cation binding sites, because cofilactin segment rigidity is similar at all [Mg2+]. Both mechanical and topological discontinuities are likely to be required for efficient severing by cofilin, because regulatory proteins (e.g., tropomyosin and myosin) and ligands that stiffen filaments (29–31) without dramatically altering filament structure (32) do not sever filaments, even at partial occupancy (Fig. S3 and ref. 31).
The two variants of yeast actin with different stiffness sites, WT and A167E mutant, provide an experimental tool to directly evaluate the role of cofilin severing activity in cells. Cofilin is essential for S. cerevisiae viability (33). Vertebrate cofilin does not sever yeast actin filaments (Fig. 2) and does not rescue the lethal phenotype associated with yeast cofilin deletion (Fig. 3A, sector 3) (22). With the yeast cofilin gene intact, the A167E yeast actin mutant is viable and displays little or no defect in growth (Fig. 3A, sectors 1 and 2) or actin patch and cable dynamics (Fig. 3 B and C, Table 1, and Movies S1 and S2) compared with WT cells. Strikingly, vertebrate cofilin in the A167E yeast actin background rescues the viability of yeast cofilin deletion (Fig. 3A, sector 4), indicating that the actin filament severing activity of cofilin is essential for its function in cells. Yeast cells with A167E actin and vertebrate cofilin assemble actin patches and actin cables (Fig. 3 B–G and Movies S3–S6) with patch internalization efficiencies comparable to those of WT and A167E cells (Table 1). Patches of yeast cells with A167E and vertebrate cofilin seem to be slightly more stable than those of WT cells, as indicated by the patch lifetimes (Table 1).
Fig. 3.

Actin filament-severing activity of cofilin is essential for viability and actin dynamics in yeast. (A) A single-point mutation in yeast actin allows human cofilin to function in yeast. Yeast strain MHY8403 (act1Δ cof1Δ/YCp50ACT1, pRS316COF1) was transformed with pairs of plasmids bearing the indicated alleles and spread on selection plates lacking leucine and histidine. Single colonies were then streaked onto FOA plates and incubated at 30 °C for 4 d. The act1-A167E hCOF strain (sector 4) grows slightly more slowly than the other two viable strains. (B–D) Representative images of actin cables in (B) ABP140-3GFP, (C) act1-A167E ABP140-3GFP, and (D) act1-A167E hCOF ABP140-3GFP yeast cells. (Scale bars, 5 μm in B–D). (E–G) Representative kymographs of actin patches in (E) Abp1-GFP, (F) act1-A167E ABP1-GFP, and (G) act1-A167E hCof1 ABP1-GFP yeast cells. Average patch lifetimes and percentages of internalization are summarized in Table 1 (n > 50 patches per strain).
Table 1.
Actin patch lifetimes and percentage of internalization in WT and mutant strains
| Strain | Lifetime, s | No. of patches | P value | Internalization percentage |
| WT | 12.7 ± 2.5 | 54 | 91 | |
| act1-A167E | 14.5 ± 3.1 | 68 | 0.0005 | 88 |
| act1-A167E hCof | 21.0 ± 6.3 | 63 | 0.0001 | 84 |
Uncertainties represent ± SD.
These results favor a model in which each bound cofilin dissociates a stiffness cation at an actin subunit interface, which compromises longitudinal and lateral contacts between adjacent filament subunits (Fig. 1). Loss of these contacts introduces a local change in compliance and mechanical phase boundary of differential stiffness within the filament that is susceptible to fragmentation by shape deformations (12). The absence of a stiffness site renders WT yeast actin filaments resistant to fragmentation by vertebrate cofilin because mechanical gradients and corresponding conformational changes and fluctuations at junctions between bare and cofilin-decorated segments are small or nonexistent without stiffness-site cations.
An alternative explanation is that the A167E substitution manifests its effect through a cation-independent mechanism. E167 forms a noncovalent ionic bridge with K61 of an adjacent subunit in high-resolution actin filament models derived from electron cryomicroscopy (34, 35), raising the possibility that the observed effects of the A-to-E substitution are simply a consequence of introducing a stabilizing ionic bridge. Several observations argue against this interpretation. Salts and electrolytes weaken ionic bridges between charged protein residues (36, 37), which would compromise intersubunit contacts and lower the filament stiffness (10, 38). In contrast, Mg2+ enhances intersubunit contacts (Fig. 1) and stiffens A167E yeast actin filaments (19). If A167E yeast actin filament severing were driven by cofilin-mediated rupture of intrinsic K61–E167 ionic bridges, filament severing would occur at any salt conditions, as long as cofilin was bound. In marked contrast, vertebrate cofilin severs weakly, if at all, in low [Mg2+] and displays a severing efficiency that scales with the Mg2+ concentration and the stiffness of bare filament segments (Fig. S4). These results indicate that both A167E mutation and stiffness cations are required for vertebrate cofilin to sever yeast actin filaments, thereby eliminating cation-independent severing pathways.
We emphasize that E167 need not directly coordinate the stiffness cation as predicted from structural bioinformatics and mutagenesis (19), nor does the cation have to be positioned at regions of enhanced intersubunit contacts to adhere to a site-specific cation-dependent mechanism. Cation effects on actin structure and mechanics could arise from allosteric interactions originating at a distal site, rather than through direct coordination with E167 or the D-loop. A mechanism in which cation binding alters the filament subunit conformation and promotes formation of an ionic bridge between K61 and E167 or other stabilizing, longitudinal and/or lateral intersubunit interaction would also account for the observed salt requirements of filament stiffening and severing.
Vertebrate cofilin adopts two distinct conformational states, or binding modes, that exist in a reversible equilibrium on actin filaments (12, 13, 39). One of these binding modes may be associated with enhanced filament elasticity, severing, and/or acceleration of terminal filament subunit release (see ref. 40 for relevance of binding mode to Plasmodium cofilin). The observed cation effects on vertebrate cofilin severing activity could potentially originate from a salt-dependent shift in the equilibrium of these conformations. We do not favor this mechanism given existing data because A167E yeast actin filaments with bound cofilin are structurally indistinguishable in low- and high-Mg2+ conditions at the current resolution (Fig. 1), A167E cofilactin filaments (ν = 0.9) sever weakly in both low and high Mg2+ (Fig. S4), and vertebrate cofilin severs WT yeast actin filaments weakly at all salt concentrations tested.
Actin filament severing by vertebrate cofilin requires stiffness cations to create a mechanical and topological gradient, explaining why it weakly severs WT yeast actin filaments lacking a stiffness cation site (12). Yeast cofilin, however, alters the mechanics of both WT yeast and vertebrate actin filaments and efficiently severs them (12). The molecular mechanism and pathway of severing must therefore vary, in some contexts, between yeast and vertebrate cofilins. Alteration of WT yeast actin filament flexibility and severing by yeast cofilin must originate from E167- and stiffness cation-independent perturbations. However, the severing activities of both yeast and vertebrate cofilins correlate with the ability to alter filament mechanical properties (12), favoring a shared mechanical mechanism for severing (5, 11, 12, 15, 19, 41). These differences among cofilin orthologs are presumably explained by their relatively low sequence identity (∼40%), which may afford yeast cofilin with additional or distinct (yeast) actin interactions from vertebrate cofilin (42). Gelsolin severs actin filaments by a different mechanism than vertebrate cofilin, involving insertion of gelsolin subdomains between adjacent filament subunits (43, 44), and efficiently fragments both WT and A167E yeast actin filaments (Fig. S5). This indicates that a stiffness cation-linked mechanism is not a general feature of filament severing proteins, although other cofilin isoforms and orthologs may operate by a cation-dependent mechanism similar to vertebrate cofilin. Other filament binding proteins (e.g., tropomyosin and myosin) that bind at or near the stiffness site could potentially disrupt site geometry and cation occupancy (21). This may seem at variance with the observed stiffening effects of these proteins (29–31). However, this could be realized if cation-dependent interactions are replaced with more stabilizing protein–protein interactions (e.g., directly binding adjacent filament subunits).
The work presented here establishes that discrete and specific cation interactions play a fundamental role in regulating the structure and mechanical properties of actin filaments and the severing activity of vertebrate cofilin. Thus, filament-associated cations act as regulatory cofactors of actin cytoskeleton dynamics.
Materials and Methods
Protein and Sample Preparations.
Yeast actin (WT and A167E) was purified using DNase I affinity chromatography with the previously described modifications (45) and labeled with pyrene for equilibrium binding experiments or Alexa 488-succimidyl ester (12, 19) as described for TIRF microscopy imaging. Vertebrate actin was purified from rabbit skeletal muscle acetone powder, gel-filtered over Sephacryl S-300 equilibrated in buffer A (0.2 mM ATP, 0.2 mM CaCl2, 0.5 mM DTT, 1 mM NaN3, and 2 mM Tris⋅HCl, pH 8.0), and labeled with pyrene and Alexa-488- succimidyl ester as described above. Ca-ATP-G-actin was converted into Mg-ATP-G-actin with 200 µM EGTA and MgCl2 equal to the [G-actin] plus 10 µM. Mg-ATP-G-actin was polymerized by adding 0.1 volume of 10× polymerization KMI6.8 buffer (50 mM KCl, 2 mM MgCl2, 2 mM DTT, 0.2 mM ATP, and 10 mM imidazole, pH 6.8). Recombinant human cofilin-1 was purified (12, 14) and concentration determined as described (46). Purified rabbit skeletal muscle myosin S1 was provided as a generous gift from David D. Thomas, University of Minnesota, Minneapolis, and labeled with N-ethylmaleimide (NEM) as described (47). Human plasma gelsolin was purchased from Cytoskeleton, Inc.
Equilibrium Binding Assay.
Equilibrum binding of human cofilin to yeast actin filaments was monitored by pyrene fluorescence (λex 366 nm; the fluorescence emission at 90° was scanned from 390 nm to 420 nm) with a Photon Technologies International QuantaMaster40 fluorimeter at 22 °C in MI6.8 buffer. The binding affinity of human cofilin to vertebrate and A167E mutant yeast actin filaments was determined from the best fits of the data to a quadratic form of a two-state equilibrium binding equation (16) or a cooperative binding model with nearest-neighbor interactions (14). Cofilin binding densities were calculated from the measured binding constants (14, 20).
Actin Filament Bending Persistence Length (Lp) Analysis.
Images of thermally fluctuating or surface-adsorbed Alexa-labeled yeast actin filaments were acquired at room temperature (∼22 °C) using an iMic digital objective-based TIRF microscope (Till Photonics) equipped with a 100× oil objective (Olympus), an iXon 897 EMCCD camera (Andor Technology), and LiveAcquisition image software (Till Photonics). Microscope slides were cleaned with absolute ethanol then 0.1 M KOH, followed by extensive rinsing with MilliQ water. The actin filament Lp values were determined from an angular correlation analysis of digitized filament images (>20 images, n = 200–500 individual filaments) using a custom MATLAB script as described in detail (11, 12, 19, 27).
Filament Severing Assays.
Filament severing activity was assayed in real time and under steady-state conditions. For the real-time severing assay, direct visualization of Alexa-488–labeled actin filament severing was performed using TIRF microscopy (12, 16). Filaments were immobilized on the NEM-inactiated muscle myosin-coated (with 150–200 nM for 5 min; ref. 16) coverslip and human cofilin was added to the flow cell at the indicated concentration in KMI6.8 buffer supplemented with 0.1 mg/mL glucose oxidase, 15 mM glucose, and 20 µg/mL catalase. The severing activities were quantified by counting cumulative severing events per each frame, dividing those by total length of actin filaments and elapsed time for each frame.
In the equilibrium length severing assay, samples of 3 µM Alexa-488–labeled actin filaments and human cofilin-1 concentrations yielding a range of binding densities were equilibrated for 60 min at room temperature then serially diluted in KMI6.8 buffer containing cofilin at concentrations to not alter the binding density (12). In the phalloidin competition experiment, 2 µM Alexa-488–labeled actin filaments equilibrated with cofilin and phalloidin were diluted in KMI6.8 buffer containing cofilin, yielding a range of binding densities and phalloidin (31). In the gelsolin severing experiment, preformed Alexa-488–labeled yeast actin filaments were equilibrated with gelsolin (at a molar ratio of 1:370) in buffer B [10 mM Tris⋅Cl (pH 7.4), 50 mM KCl, 0.2 mM MgCl2, 0.1 mM ATP, 0.5 mM DTT, and 0.2 mM CaCl2] at room temperature for 5–10 min, followed by dilution in buffer B for imaging (45). Filaments were immobilized to poly-l-lysine treated slides and imaged as done for the determination of filament flexural rigidity. The average filament length (Lavg) was determined from the population mean because the length distributions deviate from an exponential function, presumably because of contributions from filament annealing, severing, and elongation from both filament ends (48). The Lavg value at steady state is inversely proportional to the severing rate according to ,where n is the total number of actin filament subunits and kanneal and ksev are the annealing (unaffected by cofilin occupancy; Fig. S6) and severing rates, respectively. The full derivation of this relationship is included in SI Text.
Sample Preparation for Electron Cryomicroscopy.
Unlabeled A167E mutant yeast actin was polymerized in MI6.8 buffer (0.4 mM for low-salt condition or 5 mM MgCl2 for high-salt condition, 2 mM DTT, 0.2 mM ATP, and 10 mM imidazole, pH 6.8), and preformed A167E actin filaments were equilibrated with or without human cofilin-1 (ν = 0.9) with a final actin concentration of ∼5–10 µM. Samples (4–5 µL) were applied onto Quantifoil holey carbon grids (R1.2/1.3; no glow discharge was applied), incubated for 1–2 min in a humidified chamber, and plunge-frozen into liquid ethane using an FEI Vitrobot Mark II automated system. Samples were imaged at 27,000× magnification in an FEI-F20 electron microscope equipped with a side-entry cryo-holder, using a Gatan K2 Summit direct electron-counting camera operating in single-counting movie mode (image size was 3,710 × 3,838 pixels, pixel size was 1.867Å). A total of 60–69 frames were obtained for each sample area, with a frame collection rate of three per second and a net dose over all frames of ∼45 electrons per square angstrom of sample area. Movie frames were aligned and summed using the dosef_driftcorr motion correction software package (49), with minor modifications to permit central processing unit-only computations in the absence of a suitable graphics card. Defocuses and astigmatism parameters for the micrographs were estimated using the CTFFIND3 program (50) and were subsequently used to phase-flip the micrographs before initial extraction of filament images. Short overlapping box segments of selected filaments were then extracted from the resulting micrographs and rotated to an approximately vertical orientation using the user-specified path of the filaments. The rotation was accomplished with a fast sinc-interpolation algorithm based on the discrete cosine transform, implemented as a set of MATLAB codes (http://www.elisanet.fi/antti.happonen/fastint/) and executed in the Octave open-source numerical analysis package (www.gnu.org/software/octave/doc/interpreter). Segment dimension was 400 pixels, and box centers were spaced ∼13 pixels apart corresponding to one actin repeat per segment.
SPARX Alignment Step.
Initial alignment parameters and a corresponding 3D reconstruction were obtained with the SPARX implementation (51) of the IHRSR method (52), using an atomic model of the actin filament (34) as an initial reference volume after low-pass filtering to 50 Å. Box segments were binned four times for the SPARX refinement, which was done in three passes with successively finer sampling for Euler angles and shift parameters (sampling of Euler angles was 10, 4, and 1.5° for the first, second, and third pass, respectively; corresponding sampling spacing in the shift parameters was 2, 1, and 0.5 pixels, respectively). The resulting alignment parameters were assessed to check for consistency in the relative axial rotation from one subunit to the next in contiguous regions of the same filament (axial rotation for consecutive subunits is expected to change by 167.1° and 162° in the absence and presence of cofilin, respectively). Filaments with more than 10 discontinuities in the axial rotation angle were discarded from consideration in subsequent phases of the analysis.
FREALIGN Refinement Step.
The SPARX alignment parameters were mapped back onto the original micrographs and used to reextract and rotate new instances of unbinned and non-phase-flipped box segments, resulting in a raw image stack for which the expected image shifts and in-plane rotation Euler angles were approximately zero. This second image stack, along with associated alignment parameters derived from SPARX, was then input to the FREALIGN package (53) for further cycles of refinement and reconstruction. To minimize the possibility of overrefinement, a resolution cutoff was applied such that information beyond a conservative threshold was excluded from the refinement target. This threshold was gradually lowered from 30 Å to 15 Å over five or six successive rounds of refinement. Subsequent to the first round of FREALIGN refinement, the resulting 3D reconstruction was low-pass-filtered to 40 Å and used to generate a cosine-edged soft mask (fall-off width of 20 Å) that encompassed ∼200% of the expected molecular volume of the filaments. This mask was then applied to the reference volume before each subsequent round of refinement, to minimize alignment errors due to noise in the solvent region. The resolution of the reconstructions was estimated by a gold-standard procedure (54) in which the filaments were separated into two groups, and all SPARX and FREALIGN refinement steps were performed separately on both groups. The resulting pair of fully independent reconstructions was aligned by rotation and translation along the z axis to maximize the correlation coefficient, before computation of the Fourier shell correlation within the masked region. The resolution of all three final reconstructions was 10–11 Å as indicated by the 0.143 criterion. A B-factor of −600 was applied to the reconstructions before rendering for display. Atomic coordinates of actin or cofilactin were fit into density maps using the Fit in Map function of UCSF Chimera package from the Computer Graphics Laboratory, University of California, San Francisco. All molecular graphics images were rendered by UCSF Chimera.
Yeast Cell Analyses.
All S. cerevisiae strains were congenic with the BY4741 (a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) strain used to make the yeast gene deletion collection (Open Biosystems). Standard methods were used to manipulate yeast cells (55). MHY8282 (a cof1Δ::kanMX/pRS316COF1) was used to test complementation by different cofilin genes by a plasmid-shuffle assay, and MHY8403 (α act1Δ::natMX cof1Δ::kanMX/pRS316COF1, YCp50ACT1) was used for testing various pairs of actin and cofilin genes. To make MHY8282, the diploid cof1Δ::kanMX/ COF1 heterozygous strain 202C8 (Open Biosystems) was transformed with pRS316COF1 and sporulated, and viable haploid cof1Δ [pRS316COF1] progeny were isolated by tetrad dissection. To make MHY8403, MHY8282 was first crossed to SVY12 (α act1Δ::natMX MET15/YCp50ACT1) (56), a gift from David Amberg, State University of New York Upstate Medical University, Syracuse, NY. The resulting diploid strain was sporulated, and tetrads were dissected. MHY8403 was a Met+ nonparental ditype segregant.
Plasmids were constructed by standard methods. A 2.0 kb EcoRI fragment containing yeast COF1 was isolated from pDD59, a gift from David Drubin, University of California, Berkeley, and subcloned into pRS316 (URA3, CEN) and YCplac111 (LEU2, CEN) (57, 58). A 3.0-kb BamHI–EcoRI ACT1 fragment was excised from a pRS314ACT1 plasmid kindly provided by Peter Rubenstein, University of Iowa, Iowa City, and ligated into pRS313 (HIS3, CEN). The act1-A167E mutant was identical to the above ACT1 gene except for the desired codon change (22). Peter Rubenstein provided a yeast strain carrying pRS314act1-A167E; this plasmid was recovered, and the BamHI–EcoRI insert was subcloned into pRS313. The presence of the mutation was confirmed by DNA sequencing. The ACT1 and act1-A167E inserts were subsequently excised from the pRS313 backbone by BamHI and SalI digestion and subcloned into pRS41H (hphNT1) (59).
For expression of human cofilin (hCOF) in yeast, the 501-bp hCOF open-reading frame was inserted between the yeast COF1 promoter (corresponding to the 251-bp upstream of the translational start site) and terminator (corresponding to the 200-bp downstream of the stop codon) sequences in the vector pBMH. BamHI and NotI restriction sites at the respective ends of the 952-bp chimeric DNA insert were cleaved, and the fragment was ligated into pRS315 (LEU2, CEN). The hCOF sequence in the resulting pRS315hCOF plasmid was verified by DNA sequencing.
To create yeast strains for imaging of actin patches and cables, the ABP1-GFP::HIS3 and ABP140-3GFP::HIS3 alleles were amplified by high-fidelity PCR from genomic DNA of yeast strains MAY013 and MAY045, respectively. The amplified DNA fragments were integrated into MHY8403 cells, and His+ colonies were screened by fluorescence microscopy for the expected staining patterns. The resulting strains, MHY8728 and MHY8731, respectively, were then transformed with pRS41H-ACT1 or pRS41H-act1-A167E together with either YCplac111COF1 or pRS315hCOF. Leu+ hygromycin B-resistant colonies were then streaked onto 5-FOA medium to identify cells that had lost the original URA3 plasmids carrying WT ACT1 and COF1.
Cell Imaging.
Yeast strains were cultured overnight in imaging media [1.7 g/L yeast nitrogen base, 0.1 g/L ammonium sulfate, 5 g/L casaminoic acids, 0.1 g/L uracil, 0.1 g/L adenin, and 2% (wt/vol) dextrose] and resuspended in the morning at an OD600 of 0.1 2–3 h before imaging. Cells were immobilized on coverslips coated with 0.1 g/L Con A (Sigma-Aldrich) and imaged in their medial focal plane on a Zeiss Axioplan microscope equipped with a 100×, 1.4 numerical aperture Plan-Apochromat objective lens. Images corresponding to the fluorescence signal of Abp1-GFP or Abp140-3GFP were collected with a Hamamatsu ORCA CCD camera and analyzed with Metavue version 6.2r6 (Universal Imaging).
Actin patch dynamics were analyzed as described (60, 61). The patch start and end times were determined as the first and last frames in which the patch could be distinguished from the background. The total patch lifetime was determined as the time difference between the end frame and the first frame plus the duration of a single frame. Kymographs were plotted along a line perpendicular to the membrane of the cell and passing through the center of the patch when it reached its maximum intensity. We defined the criteria for patch internalization when the maximum intensity of the patch was displaced of at least two pixels (corresponding to a distance of 129 nm) from its original point at the end of its lifetime.
Supplementary Material
Acknowledgments
We thank Drs. W. Austin Elam and Thomas D. Pollard for critically reading the manuscript and anonymous peer reviewers for their critical reviews and valuable suggestions. We gratefully acknowledge the Yale Center for Cellular and Molecular Imaging cryo-EM facility for support and maintenance of cryo-EM equipment including the F20 and Vitrobot. This work was supported by National Institutes of Health (NIH) Grants R01-GM097348 (to E.M.D.L.C.), GM053756 (to M.H.), and GM11053001 (to C.V.S.) and by American Cancer Society Grant IRG 5801255 (to C.V.S.). E.E.G. was supported by NIH Grant NIGMS 077190 (awarded to E. Reisler).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413397111/-/DCSupplemental.
References
- 1.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 2.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamburg JR. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15(1):185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 5.De La Cruz EM. How cofilin severs an actin filament. Biophys Rev. 2009;1(2):51–59. doi: 10.1007/s12551-009-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelot A, et al. Actin-filament stochastic dynamics mediated by ADF/cofilin. Curr Biol. 2007;17(10):825–833. doi: 10.1016/j.cub.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Oosawa F, Asakura S. Thermodynamics of the Polymerization of Protein. Academic; London: 1975. [Google Scholar]
- 8.Galkin VE, et al. Remodeling of actin filaments by ADF/cofilin proteins. Proc Natl Acad Sci USA. 2011;108(51):20568–20572. doi: 10.1073/pnas.1110109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: Implications for actin filament dynamics and cellular function. J Cell Biol. 1997;138(4):771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J, et al. Molecular origins of cofilin-linked changes in actin filament mechanics. J Mol Biol. 2013;425(7):1225–1240. doi: 10.1016/j.jmb.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough BR, Blanchoin L, Martiel JL, De la Cruz EM. Cofilin increases the bending flexibility of actin filaments: Implications for severing and cell mechanics. J Mol Biol. 2008;381(3):550–558. doi: 10.1016/j.jmb.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCullough BR, et al. Cofilin-linked changes in actin filament flexibility promote severing. Biophys J. 2011;101(1):151–159. doi: 10.1016/j.bpj.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prochniewicz E, Janson N, Thomas DD, De la Cruz EM. Cofilin increases the torsional flexibility and dynamics of actin filaments. J Mol Biol. 2005;353(5):990–1000. doi: 10.1016/j.jmb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 14.De La Cruz EM. Cofilin binding to muscle and non-muscle actin filaments: Isoform-dependent cooperative interactions. J Mol Biol. 2005;346(2):557–564. doi: 10.1016/j.jmb.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 15.Suarez C, et al. Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr Biol. 2011;21(10):862–868. doi: 10.1016/j.cub.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24(1):13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Bobkov AA, et al. Cooperative effects of cofilin (ADF) on actin structure suggest allosteric mechanism of cofilin function. J Mol Biol. 2006;356(2):325–334. doi: 10.1016/j.jmb.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 18.Pavlov D, Muhlrad A, Cooper J, Wear M, Reisler E. Actin filament severing by cofilin. J Mol Biol. 2007;365(5):1350–1358. doi: 10.1016/j.jmb.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang H, et al. Identification of cation-binding sites on actin that drive polymerization and modulate bending stiffness. Proc Natl Acad Sci USA. 2012;109(42):16923–16927. doi: 10.1073/pnas.1211078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W, Goodarzi JP, De La Cruz EM. Energetics and kinetics of cooperative cofilin-actin filament interactions. J Mol Biol. 2006;361(2):257–267. doi: 10.1016/j.jmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Kang H, Bradley MJ, Elam WA, De La Cruz EM. Regulation of actin by ion-linked equilibria. Biophys J. 2013;105(12):2621–2628. doi: 10.1016/j.bpj.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stokasimov E, McKane M, Rubenstein PA. Role of intermonomer ionic bridges in the stabilization of the actin filament. J Biol Chem. 2008;283(50):34844–34854. doi: 10.1074/jbc.M804419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galkin VE, Orlova A, Schröder GF, Egelman EH. Structural polymorphism in F-actin. Nat Struct Mol Biol. 2010;17(11):1318–1323. doi: 10.1038/nsmb.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlova A, Egelman EH. A conformational change in the actin subunit can change the flexibility of the actin filament. J Mol Biol. 1993;232(2):334–341. doi: 10.1006/jmbi.1993.1393. [DOI] [PubMed] [Google Scholar]
- 25.Chu JW, Voth GA. Coarse-grained modeling of the actin filament derived from atomistic-scale simulations. Biophys J. 2006;90(5):1572–1582. doi: 10.1529/biophysj.105.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaendtner J, De La Cruz EM, Voth GA. Actin filament remodeling by actin depolymerization factor/cofilin. Proc Natl Acad Sci USA. 2010;107(16):7299–7304. doi: 10.1073/pnas.0911675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham JS, et al. Multi-platform compatible software for analysis of polymer bending mechanics. PLoS ONE. 2014;9(4):e94766. doi: 10.1371/journal.pone.0094766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isambert H, et al. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J Biol Chem. 1995;270(19):11437–11444. doi: 10.1074/jbc.270.19.11437. [DOI] [PubMed] [Google Scholar]
- 29.Prochniewicz E, et al. Actin filament dynamics in the actomyosin VI complex is regulated allosterically by calcium-calmodulin light chain. J Mol Biol. 2011;413(3):584–592. doi: 10.1016/j.jmb.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanagida T, Nakase M, Nishiyama K, Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]
- 31.Elam WA, Kang H, De La Cruz EM. Competitive displacement of cofilin can promote actin filament severing. Biochem Biophys Res Commun. 2013;438(4):728–731. doi: 10.1016/j.bbrc.2013.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrmann E, et al. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150(2):327–338. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriyama K, Yahara I. The actin-severing activity of cofilin is exerted by the interplay of three distinct sites on cofilin and essential for cell viability. Biochem J. 2002;365(Pt 1):147–155. doi: 10.1042/bj20020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467(7316):724–728. doi: 10.1038/nature09372. [DOI] [PubMed] [Google Scholar]
- 35.Galkin VE, Orlova A, Vos MR, Schröder GF, Egelman EH. Near-atomic resolution for one state of F-actin. Structure. 2014 doi: 10.1016/j.str.2014.11.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albinsson S, Nordström I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem. 2004;279(33):34849–34855. doi: 10.1074/jbc.M403370200. [DOI] [PubMed] [Google Scholar]
- 37.Andrianantoandro E, Blanchoin L, Sept D, McCammon JA, Pollard TD. Kinetic mechanism of end-to-end annealing of actin filaments. J Mol Biol. 2001;312(4):721–730. doi: 10.1006/jmbi.2001.5005. [DOI] [PubMed] [Google Scholar]
- 38.De La Cruz EM, Roland J, McCullough BR, Blanchoin L, Martiel JL. Origin of twist-bend coupling in actin filaments. Biophys J. 2010;99(6):1852–1860. doi: 10.1016/j.bpj.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De La Cruz EM, Sept D. The kinetics of cooperative cofilin binding reveals two states of the cofilin-actin filament. Biophys J. 2010;98(9):1893–1901. doi: 10.1016/j.bpj.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong W, et al. A mechanism for actin filament severing by malaria parasite actin depolymerizing factor 1 via a low affinity binding interface. J Biol Chem. 2014;289(7):4043–4054. doi: 10.1074/jbc.M113.523365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elam WA, Kang H, De la Cruz EM. Biophysics of actin filament severing by cofilin. FEBS Lett. 2013;587(8):1215–1219. doi: 10.1016/j.febslet.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hild G, Kalmár L, Kardos R, Nyitrai M, Bugyi B. The other side of the coin: Functional and structural versatility of ADF/cofilins. Eur J Cell Biol. 2014;93(5-6):238–251. doi: 10.1016/j.ejcb.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin PJ, Gooch JT, Mannherz HG, Weeds AG. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- 44.Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- 45.Grintsevich EE, et al. Mapping the cofilin binding site on yeast G-actin by chemical cross-linking. J Mol Biol. 2008;377(2):395–409. doi: 10.1016/j.jmb.2007.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 47.Warshaw DM, Desrosiers JM, Work SS, Trybus KM. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding velocity in vitro. J Cell Biol. 1990;111(2):453–463. doi: 10.1083/jcb.111.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeune-Smith Y, Hess H. Engineering the length distribution of microtubules polymerized in vitro. Soft Matter. 2010;6(8):1778–1784. [Google Scholar]
- 49.Li X, et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods. 2013;10(6):584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142(3):334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 51.Behrmann E, et al. Real-space processing of helical filaments in SPARX. J Struct Biol. 2012;177(2):302–313. doi: 10.1016/j.jsb.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egelman EH. Reconstruction of helical filaments and tubes. Methods Enzymol. 2010;482:167–183. doi: 10.1016/S0076-6879(10)82006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grigorieff N. FREALIGN: High-resolution refinement of single particle structures. J Struct Biol. 2007;157(1):117–125. doi: 10.1016/j.jsb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Henderson R, et al. Outcome of the first electron microscopy validation task force meeting. Structure. 2012;20(2):205–214. doi: 10.1016/j.str.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular and Cell Biology: Part C. Gulf Professional Publishing; Oxford: 2002. [Google Scholar]
- 56.Haarer B, Viggiano S, Hibbs MA, Troyanskaya OG, Amberg DC. Modeling complex genetic interactions in a simple eukaryotic genome: Actin displays a rich spectrum of complex haploinsufficiencies. Genes Dev. 2007;21(2):148–159. doi: 10.1101/gad.1477507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 58.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taxis C, Knop M. System of centromeric, episomal, and integrative vectors based on drug resistance markers for Saccharomyces cerevisiae. Biotechniques. 2006;40(1):73–78. doi: 10.2144/000112040. [DOI] [PubMed] [Google Scholar]
- 60.Michelot A, et al. Actin filament elongation in Arp2/3-derived networks is controlled by three distinct mechanisms. Dev Cell. 2013;24(2):182–195. doi: 10.1016/j.devcel.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu S-L, Needham KM, May JR, Nolen BJ. Mechanism of a concentration-dependent switch between activation and inhibition of Arp2/3 complex by coronin. J Biol Chem. 2011;286(19):17039–17046. doi: 10.1074/jbc.M111.219964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.