Abstract
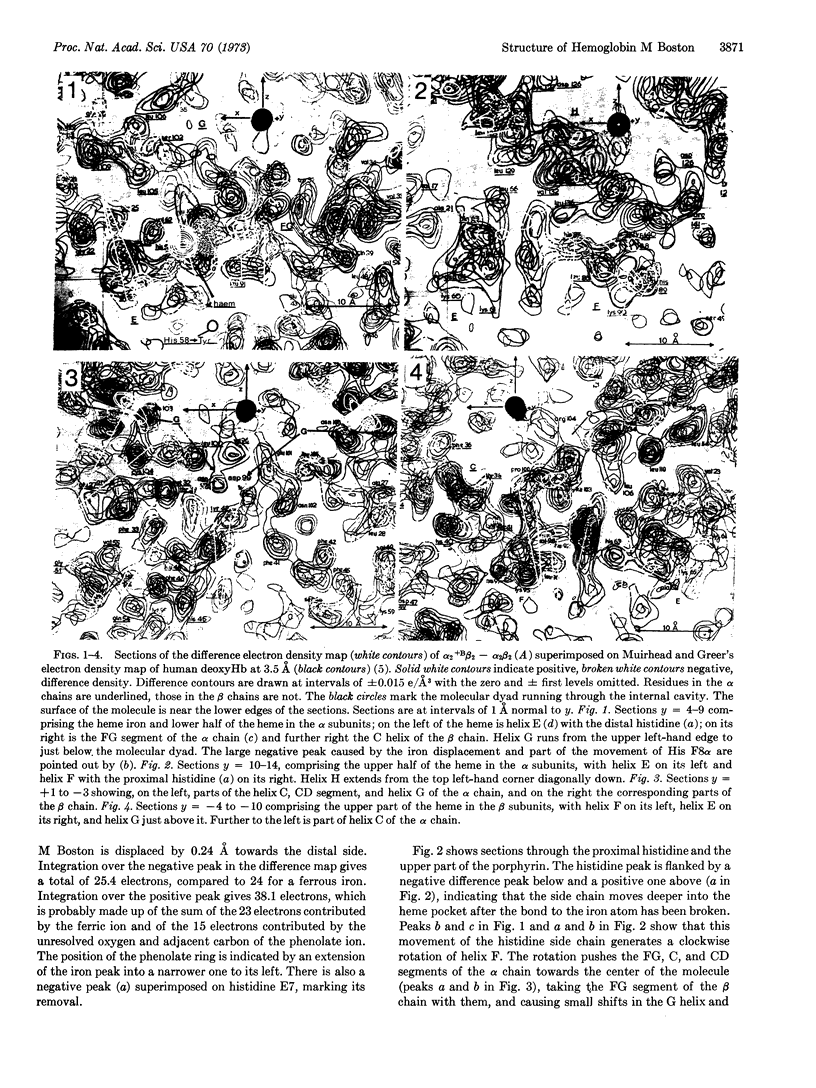
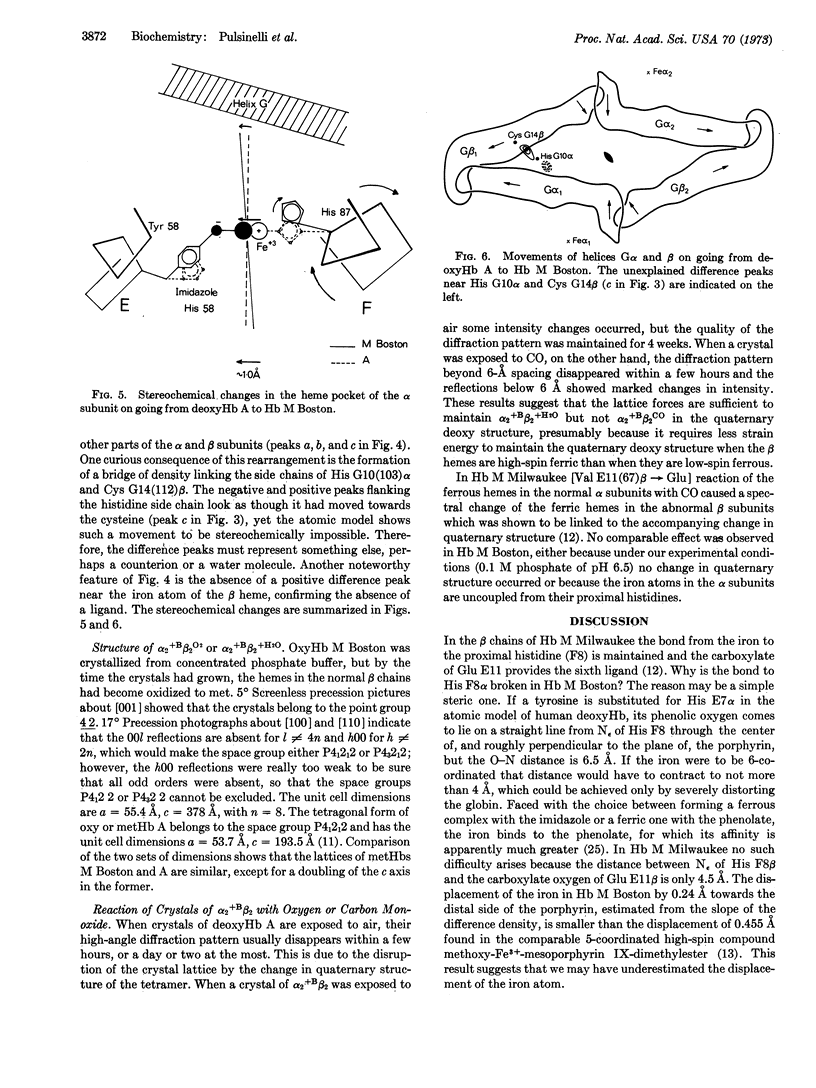
X-ray analysis of the natural valency hybrid α2+M Bostonβ2deoxy shows that the ferric iron atoms in the abnormal α subunits are bonded to the phenolate side chains of the tyrosines that have replaced the distal histidines; the iron atoms are displaced to the distal side of the porphyrin ring and are not bonded to the proximal histidines. The resulting changes in tertiary structure of the α subunits stabilize the hemoglobin tetramer in the quaternary deoxy structure, which lowers the oxygen affinity of the normal β subunits and causes cyanosis. The strength of the bond from the ferric iron to the phenolate oxygen appears to be the main factor responsible for the many abnormal properties of hemoglobin M Boston.
Keywords: x-ray analysis, molecular pathology, inherited blood diseases
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. Intermediate structure of normal human haemoglobin: methaemoglobin in the deoxy quaternary conformation. J Mol Biol. 1973 Sep 25;79(3):495–506. doi: 10.1016/0022-2836(73)90401-4. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- GERALD P. S., COOK C. D., DIAMOND L. K. Hemoglobin M. Science. 1957 Aug 16;126(3268):300–301. doi: 10.1126/science.126.3268.300. [DOI] [PubMed] [Google Scholar]
- GERALD P. S., EFRON M. L. Chemical studies of several varieties of Hb M. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1758–1767. doi: 10.1073/pnas.47.11.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERALD P. S. The electrophoretic and spectroscopic characterization of Hgb M. Blood. 1958 Oct;13(10):936–949. [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Imai K., Morimoto H., Watari H. Properties of hemoglobin M, Milwaukee-I variant and its unique characteristic. Biochim Biophys Acta. 1969 Nov 11;194(1):6–15. doi: 10.1016/0005-2795(69)90173-1. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shimizu A., Morimoto H., Watari H. Changes in EPR spectra of M-type abnormal haemoglobins induced by deoxygenation and their implications for the haem-haem interaction. Biochim Biophys Acta. 1967 Oct 23;147(2):407–409. doi: 10.1016/0005-2795(67)90426-6. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shimizu A., Yamamura Y. Properties of hemoglobin M. Unequivalent nature of the alpha and beta subunits in the hemoglobin molecule. Biochim Biophys Acta. 1968 Oct 21;168(2):262–273. doi: 10.1016/0005-2795(68)90149-9. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Greer J. Three-dimensional Fourier synthesis of human deoxyhaemoglobin at 3.5 Angstrom units. Nature. 1970 Nov 7;228(5271):516–519. doi: 10.1038/228516a0. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Ranney H. M., Bradley T. B., Jacobs A., Udem L. Hemoglobin L Ferrara in a Jewish family associated with a hemolytic state in the propositus. Blood. 1969 Aug;34(2):157–165. [PubMed] [Google Scholar]
- PERUTZ R. R., LIQUORI A. M., EIRICH F. X-ray and solubility studies of the haemoglobin of sickle-cell anaemia patients. Nature. 1951 Jun 9;167(4258):929–931. doi: 10.1038/167929a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Nature of haem-haem interaction. Nature. 1972 Jun 30;237(5357):495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Pulsinelli P. D., Ranney H. M. Structure and subunit interaction of haemoglobin M Milwaukee. Nat New Biol. 1972 Jun 28;237(78):259–263. doi: 10.1038/newbio237259a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., TenEyck L. F. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:295–310. doi: 10.1101/sqb.1972.036.01.040. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hayashi A., Shimizu A., Yamamura Y. The oxygen equilibrium of hemoglobin MSaskatoon. Biochim Biophys Acta. 1966 Sep 26;127(1):280–282. doi: 10.1016/0304-4165(66)90510-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hayashi A., Yamamura Y., Enoki Y., Tyuma I. Functional abnormality of hemoglobin M-Osaka. Biochem Biophys Res Commun. 1965 Jun 9;19(6):691–695. doi: 10.1016/0006-291x(65)90312-8. [DOI] [PubMed] [Google Scholar]



